Abstract
Background
Low bispectral index values frequently reflect EEG suppression and have been associated with postoperative mortality. This study investigated whether intraoperative EEG suppression was an independent predictor of 90 day postoperative mortality and explored risk factors for EEG suppression.
Methods
This observational study included 2662 adults enrolled in the B-Unaware or BAG-RECALL trials. A cohort was defined with >5 cumulative minutes of EEG suppression, and 1:2 propensity-matched to a non-suppressed cohort (≤5 min suppression). We evaluated the association between EEG suppression and mortality using multivariable logistic regression, and examined risk factors for EEG suppression using zero-inflated mixed effects analysis.
Results
Ninety day postoperative mortality was 3.9% overall, 6.3% in the suppressed cohort, and 3.0% in the non-suppressed cohort {odds ratio (OR) [95% confidence interval (CI)]=2.19 (1.48–3.26)}. After matching and multivariable adjustment, EEG suppression was not associated with mortality [OR (95% CI)=0.83 (0.55–1.25)]; however, the interaction between EEG suppression and mean arterial pressure (MAP) <55 mm Hg was [OR (95% CI)=2.96 (1.34–6.52)]. Risk factors for EEG suppression were older age, number of comorbidities, chronic obstructive pulmonary disease, and higher intraoperative doses of benzodiazepines, opioids, or volatile anaesthetics. EEG suppression was less likely in patients with cancer, preoperative alcohol, opioid or benzodiazepine consumption, and intraoperative nitrous oxide exposure.
Conclusions
Although EEG suppression was associated with increasing anaesthetic administration and comorbidities, the hypothesis that intraoperative EEG suppression is a predictor of postoperative mortality was only supported if it was coincident with low MAP.
Clinical trial registration
NCT00281489 and NCT00682825.
Keywords: anaesthesia, general; comorbidity; deep sedation; electroencephalography; risk assessment
Editor's key points.
Previous studies have suggested a link between deep anaesthesia and mortality.
The authors studied this association using data from two previous studies.
A multivariate analysis did not show an association between >5 min of EEG suppression and mortality.
EEG suppression and coincident hypotension were however strongly associated with mortality.
It is estimated that between 2% and 5% of surgical inpatients die within 90 days of their operations.1–4 However, it is unknown to what extent intraoperative management contributes directly to this mortality. Several studies have shown an association between low intraoperative bispectral index (BIS) values and postoperative mortality.2,5–8 The BIS is a proprietary processed EEG index, ranging from high values (approaching 100) when patients are awake to low values (approaching 0) during very deep general anaesthesia. Low BIS values frequently reflect epochs of isoelectric EEG punctuated by bursts of activity, a pattern described as EEG burst suppression.9 EEG burst suppression has also been observed in pathological states, including traumatic brain injury, coma, severe hypothermia, hypoxia, hypoglycaemia, or childhood encephalopathies.10–13 Computer models indicate that burst suppression results from depleted intracellular ATP and extracellular calcium stores and is associated with depressed neuronal metabolism.14,15 As such, burst suppression could directly reflect important neurobiological processes. The potential clinical relevance of EEG suppression was made salient in a study in medical intensive care patients which found that burst suppression was independently associated with a doubling of mortality at 6 months.16 In this respect, avoiding the potential harm of intraoperative EEG suppression by reducing anaesthetic agents might be worthy of further investigation.
Analysis into postoperative outcomes after low intraoperative BIS values have prompted an ongoing debate about the potential of relatively excessive anaesthetic administration, within a clinically relevant range, to be directly injurious and to increase postoperative mortality. Many of these studies have been dispraised for incompletely capturing confounding factors.17–19 Preliminary research has shown that patients are more likely to exhibit EEG suppression during general anaesthesia with propofol when they were elderly, male, or had coronary artery disease.20 The primary goal of this study was to investigate whether intraoperative EEG suppression is an independent predictor of 90 day postoperative mortality in patients at risk for intraoperative awareness who underwent general anaesthesia with volatile anaesthetics. A secondary goal was to explore risk factors for intraoperative EEG suppression.
Methods
Patient population
This study includes subjects from the B-Unaware (NCT00281489) and BAG-RECALL (NCT00682825) clinical trials, which were designed to test whether an anaesthetic protocol based on BIS guidance was superior to a protocol based on end-tidal anaesthetic concentration (ETAC) alerts in preventing intraoperative awareness.21,22 These trials included patients >18 yr old receiving volatile anaesthetics, who were at high risk for intraoperative awareness as defined by having at least one of the following risk factors: preoperative long-term use of anticonvulsants, opioids, benzodiazepines, or cocaine; cardiac ejection fraction <40%; history of anaesthesia awareness; history of difficult intubation or anticipated difficult intubation; ASA physical status class IV or V; aortic stenosis; end-stage lung disease; marginal exercise tolerance not resulting from musculoskeletal dysfunction; pulmonary hypertension; planned open-heart surgery; and daily alcohol consumption. The B-Unaware trial enrolled 2000 patients at Washington University in St Louis between 2004 and 2006. The BAG-RECALL trial enrolled 6041 patients between 2008 and 2010 at Washington University in St Louis and the University of Chicago and Manitoba (Winnipeg, Manitoba, Canada). Patients were randomized to receive general anaesthesia dosed by either ETAC or BIS values. Under the ETAC protocol, an alarm sounded when the patient's ETAC went outside the target range of 0.7–1.3 age-adjusted minimum alveolar concentration23 (MAC), and providers were blinded to their patient's BIS values. In the BIS group, an alarm sounded when BIS values went outside the target range of 40–60, and ETAC values were available to practitioners.
In this substudy, we included patients whose surgeries lasted at least 30 min and whose intraoperative EEG suppression was recorded and available for at least half of the case's duration. Suppression ratio (SR) values were excluded from analysis if the BIS electrode's indicated signal quality index was <50. SR was not a primary target in this study's parent trials and hence was not recorded electronically for many cases (Fig. 1). We included intraoperative data from the most recent surgery if a patient had multiple operations in one or both studies. Our final sample included 2662 patients.
Fig 1.
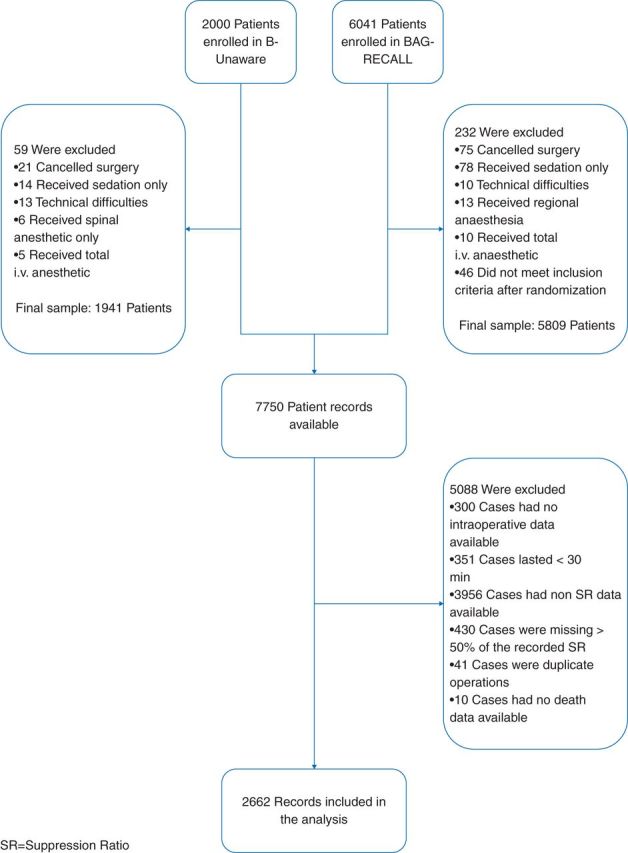
Study enrolment.
Ethics
Both parent trials (B-Unaware and BAG-RECALL) received regulatory approval from institutional review boards at all participating institutions, and both studies specified at registration that associations between anaesthetic depth and postoperative mortality would be explored in secondary studies (NCT00682825, NCT00281489).
Outcome measures and data collection
SR is a variable calculated by the BIS monitor® (Covidien, Boulder, CO, USA) as the per cent of the previous 63 s during which the EEG was suppressed.24 BIS and SR values were recorded electronically using BIS Quatro® (Covidien) forehead electrodes and a BIS XP® (Covidien) processing module. For patients included in this substudy, ETAC and SR values were recorded using Trendface Solo software (ixellence, Wildau, Germany).
Data were processed using Matlab® 7.14 R2012a (The MathWorks Inc., Natick, MA, USA). ETAC and SR measurements were resampled to intervals of one measurement per minute by retaining the first value of every minute, and ETAC values were converted to MAC equivalents using the formulae provided by Nickalls.23 Anaesthetic concentrations were considered missing when ETAC fell outside the range of 0.1–4 MAC equivalents. To evaluate the relationship between EEG suppression and mortality, we estimated the total time during which a patient's EEG was suppressed by summing each case's fractional SR values and multiplying them by 100. Leslie and colleagues7 analysed patients according to whether or not BIS was <40 for at least 5 min. Based on this approach, we defined a ‘suppressed group’ of patients whose EEGs were suppressed for at least 5 cumulative (but not necessarily contiguous) minutes; the ‘non-suppressed group’ included the remaining patients.
Perioperative data were retrieved from medical files. Postoperative mortality dates were ascertained from the US Social Security Death Index and by contacting patients and their families in Canada to establish vital status.
Statistics
Differences in patient characteristics and comorbidities between the suppressed and non-suppressed groups were evaluated with Student's t-test and χ2 test as appropriate. Normality of continuous variables was verified with one-sided Kolmogorov–Smirnov tests before parametric statistics were applied. We evaluated the unadjusted association between EEG suppression and postoperative mortality using a univariable logistic regression. To account for significant differences in baseline characteristics between the suppressed and non-suppressed group, we propensity matched each suppressed case with up to two non-suppressed controls based on their patient characteristics and comorbid covariates (Table 1).25 We performed a 2:1 nearest neighbour matching with replacement to allow every suppressed case to be matched to at least one control, and a calliper allowed matches up to a distance of 0.2 standard deviations of the predicted propensity score.26,27 To account for controls matched to multiple cases, we generated weights to deflate the sample to its original size. The paired t-tests and χ2 tests were used to evaluate differences before and after matching, and variance ratios and absolute values of the standardized differences in means were used to evaluate post-match balance between the two matched groups.28
Table 1.
Characteristics and absolute standardized mean difference effect sizes (d) between EEG suppressed (EEG-S) and non-suppressed (Non-S) groups on all baseline covariates before and after propensity score matching. M, mean; n, number; sd, standard deviation. *Compared with patients with burst suppression
| Covariate | All patients (2662) |
Unmatched |
Propensity score matched |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EEG-S (756) |
Non-S (1906) |
d | P-value* | Non-S (926) |
d | P-value* | ||||||
| M or n | sd or % | M or n | sd or % | M or n | sd or % | M or n | sd or % | |||||
| Age | 60.8 | 13.7 | 64.3 | 12.8 | 59.4 | 13.8 | 0.36 | 0.03 | 64.1 | 12.6 | 0.13 | 0.73 |
| BMI | 30.0 | 7.7 | 28.8 | 6.6 | 30.5 | 8.1 | 0.22 | 0.00 | 28.7 | 6.3 | 0.09 | 0.90 |
| Male gender | 1632 | 61.3 | 447 | 59.1 | 1185 | 62.2 | 0.06 | 0.16 | 541 | 58.5 | 0.05 | 0.78 |
| White race | 2324 | 87.3 | 645 | 85.3 | 1679 | 88.1 | 0.08 | 0.06 | 791 | 85.5 | 0.05 | 0.94 |
| ASA | <0.001 | 0.0 | 0.14 | |||||||||
| I | 27 | 1.0 | 0 | 0.0 | 27 | 1.4 | 2 | 0.3 | ||||
| II | 429 | 16.1 | 54 | 7.1 | 375 | 19.7 | 81 | 8.8 | ||||
| III | 1089 | 40.9 | 279 | 36.9 | 810 | 42.5 | 306 | 33.0 | ||||
| IV | 1117 | 42.0 | 423 | 56.0 | 694 | 36.4 | 537 | 57.9 | ||||
| Planned heart surgery | 1316 | 49.4 | 500 | 66.1 | 816 | 42.8 | 0.47 | <0.001 | 616 | 66.5 | 0.15 | 0.86 |
| Past medical history | 0.0 | |||||||||||
| Aortic stenosis | 245 | 9.2 | 95 | 12.6 | 150 | 7.9 | 0.16 | <0.001 | 111 | 12.0 | 0.03 | 0.71 |
| Cerebrovascular disease | 154 | 5.8 | 68 | 9.0 | 86 | 4.5 | 0.19 | <0.001 | 64 | 6.9 | 0.16 | 0.12 |
| Cancer | 519 | 19.5 | 92 | 12.2 | 427 | 22.4 | 0.26 | <0.001 | 120 | 13.0 | 0.12 | 0.63 |
| Chronic obstructive pulmonary disease | 451 | 16.9 | 140 | 18.5 | 311 | 16.3 | 0.06 | 0.19 | 168 | 18.1 | 0.04 | 0.83 |
| Congestive heart failure | 398 | 15.0 | 142 | 18.8 | 156 | 13.4 | 0.15 | 0.00 | 164 | 17.7 | 0.09 | 0.58 |
| Coronary artery disease | 1287 | 48.3 | 428 | 56.6 | 859 | 45.1 | 0.23 | <0.001 | 523 | 56.5 | 0.09 | 0.96 |
| End-stage lung disease | 30 | 1.1 | 14 | 1.9 | 16 | 0.8 | 0.10 | 0.04 | 21 | 2.3 | 0.04 | 0.51 |
| Ejection fraction <40% | 224 | 8.4 | 72 | 9.5 | 152 | 8.0 | 0.06 | 0.22 | 78 | 8.5 | 0.03 | 0.45 |
| Diabetes | 658 | 24.7 | 210 | 27.8 | 448 | 23.5 | 0.10 | 0.02 | 255 | 27.6 | 0.02 | 0.93 |
| Dysrhythmia | 290 | 10.9 | 118 | 15.6 | 172 | 9.0 | 0.21 | <0.001 | 138 | 15.0 | 0.12 | 0.71 |
| Hypertension | 1788 | 67.2 | 546 | 72.2 | 1242 | 65.2 | 0.15 | <0.001 | 672 | 72.6 | 0.04 | 0.88 |
| Marginal exercise tolerance | 1332 | 50.0 | 338 | 44.7 | 994 | 52.2 | 0.15 | 0.00 | 409 | 44.2 | 0.06 | 0.83 |
| Peripheral vascular occlusive disease | 383 | 14.4 | 134 | 17.7 | 249 | 13.1 | 0.13 | 0.00 | 160 | 17.3 | 0.06 | 0.83 |
| Pulmonary hypertension | 93 | 3.5 | 33 | 4.4 | 60 | 3.1 | 0.07 | 0.13 | 37 | 4.0 | 0.06 | 0.68 |
| Sleep apnoea | 288 | 10.8 | 80 | 10.6 | 208 | 10.9 | 0.01 | 0.84 | 94 | 10.1 | 0.03 | 0.76 |
| Regular alcohol use (daily) | 353 | 13.3 | 75 | 9.9 | 278 | 14.6 | 0.14 | 0.00 | 97 | 10.5 | 0.08 | 0.69 |
| Regular anticonvulsant use | 147 | 5.5 | 40 | 5.3 | 107 | 5.6 | 0.01 | 0.78 | 48 | 5.2 | 0.03 | 0.95 |
| Regular benzodiazepine use | 339 | 12.7 | 84 | 11.1 | 255 | 13.4 | 0.07 | 0.12 | 102 | 11.0 | 0.05 | 0.97 |
| Regular opiate use | 515 | 19.3 | 98 | 13.0 | 417 | 21.9 | 0.23 | <0.001 | 115 | 12.4 | 0.06 | 0.75 |
Survival information beyond the 90 day postoperative period was available for 2420 (90.9%) patients. To evaluate the potentially longer-term effects of EEG suppression, we used the Kaplan–Meier analyses to determine the association between EEG suppression and time to mortality up to 1 yr after surgery. A conditional multivariable logistic regression was performed using data from the matched patient cohort to evaluate the independent effect of EEG suppression on 90 day mortality. Covariates were defined a priori by clinical relevance or significance in prior research studies. They included age (continuous), gender, ASA physical status score (dichotomous, defined as IV vs I–III), number of comorbidities (continuous), planned heart surgery, history of congestive heart failure or malignancy (all dichotomous), duration of low mean arterial pressure (MAP) (<55 mm Hg), and an interaction term between low MAP and EEG suppression group.8 All variables were force-entered into the model in a single step. Model goodness of fit was assessed with the log-likelihood ratio.
Finally, we constructed a non-linear mixed effects model using the complete, unmatched patient sample to evaluate the strength of association between several candidate risk factors and intraoperative burst suppression. Because most (∼90%) SR measurements were zero, we included a two-part piecewise likelihood function in the model.29 When SR=0, a logistic likelihood function was used to compute a set of coefficients describing a patient's odds of developing any amount of EEG suppression. When SR >0, a general γ distribution was assumed and a second set of coefficients were computed to describe the association between the included factors and the level of SR. The included risk factors were defined a priori based on prior research studies or clinical relevance and included age (continuous), sex (dichotomous), ASA physical status score (dichotomous, defined as IV vs I–III), number of comorbidities (continuous), histories of coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, malignancy, and regular preoperative alcohol, opioid, or benzodiazepine use (all dichotomous).20,30 We controlled for anaesthetic factors including ETAC in 0.1 MAC units (continuous), and whether the patient received >2 mg midazolam equivalents (dichotomous), >50 mg morphine equivalents (dichotomous), or any amount of nitrous oxide (dichotomous). To reduce pharmacokinetic confounding, intraoperative data included in this model were restricted to epochs when ETAC was within ±0.05 MAC for the preceding 10 min. A random effect was included as part of the intercept to allow for variation between patients. Statistical analyses were performed in SAS® software version 9.3 (SAS Institute Inc., Cary, NC, USA), and R version 2.10 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
This analysis included 2662 unique patients (Fig. 1) who provided 10 216 h of recorded intraoperative parameters. Most surgeries were performed by cardiothoracic (60.5%), general (14.8%), or urological (6.1%) services. Seven hundred and fifty-six (28.4%) patients experienced >5 cumulative minutes of suppressed EEG and comprised the suppressed group. The median duration of EEG suppression in the suppressed group was 15.3 min (range: 5.0–235.6 min), compared with 0.2 min (range: 0.0–5.0 min) in the non-suppressed group. Patients in the suppressed group tended to be older, had higher ASA scores, and had a higher prevalence of many comorbidities, but a 46% reduced prevalence of malignancy compared with patients in the non-suppressed group (12.2% vs 22.4%, P<0.001; Table 1). Patients in the suppressed group were less likely to regularly use sedatives, including alcohol, benzodiazepines, or opioids.
Mortality analyses
The 90 day all-cause mortality rate in our study cohort was 3.9% (105 of 2662). Mortality at 90 days was 6.3% (48 of 756) in the suppressed group, 3.0% (57 of 1906) in the non-suppressed group (P<0.001), and 5.5% (51 of 926) in the matched cohort (P=0.456). Before adjusting for confounders, patients in the suppressed group had 2.19 (95% CI: 1.48–3.26) times higher odds of dying up to 90 days after surgery than those in the non-suppressed group.
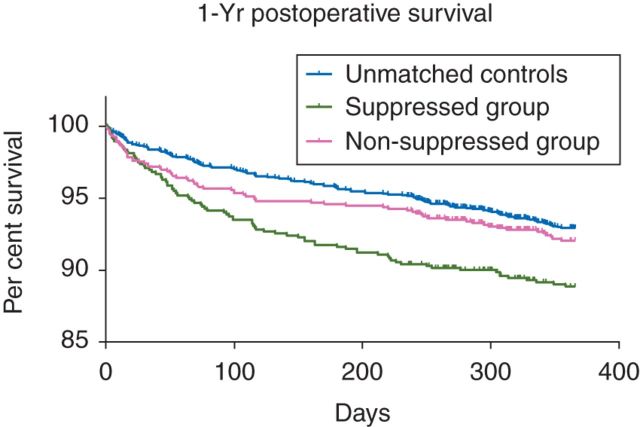
There were no significant differences in patient characteristics or prevalence of comorbidities between the two propensity-matched groups (Table 1). No absolute value of the standardized mean difference for any variable exceeded 0.25, and no variance ratio was outside the range of 0.5–2. After matching and adjusting for confounding factors, patients who experienced EEG suppression had similar odds of dying by 90 days as their non-suppressed counterparts [odds ratio (OR)=0.83, 95% confidence interval (CI)=0.55–1.25, P=0.375; Table 2]. However, patients in the suppressed group who also had low MAP had nearly three times higher odds of dying compared with their non-suppressed counterparts without low arterial pressure (OR=2.96, 95% CI: 1.34–6.52, P=0.007). Comparisons of the proportion of patients surviving up to 1 yr after surgery revealed that patients with EEG suppression had a shorter time-to-death than the non-suppressed group (log-rank χ2=14.09, df=1, P=0.0002), but there was no significant difference between the patients with EEG suppression and their matched counterparts (log-rank χ2=2.13, df=1, P=0.14; Fig. 2).
Table 2.
Multivariable predictors of 90 day postoperative mortality in the matched sample. CI, confidence interval; MAP, mean arterial pressure
| Factor | Conditional |
||
|---|---|---|---|
| Odds ratio | 95% CI | P-value | |
| EEG suppression×low MAP | 2.96 | 1.34–6.52 | 0.007 |
| Malignancy | 2.66 | 1.56–4.55 | <0.001 |
| Congestive heart failure | 1.62 | 1.10–2.37 | 0.014 |
| ASA IV (vs I–III) | 1.45 | 0.87–2.41 | 0.150 |
| Comorbidity index (#) | 1.15 | 1.03–1.28 | 0.011 |
| Age (yr) | 1.02 | 1.01–1.04 | 0.010 |
| Cardiac surgery | 0.97 | 0.59–1.60 | 0.971 |
| EEG suppression | 0.83 | 0.55–1.25 | 0.375 |
| Male gender | 0.75 | 0.53–1.06 | 0.101 |
| Low MAP (h) | 0.74 | 0.35–1.56 | 0.432 |
Fig 2.
Per cent of the study population surviving after surgery.
Predictors of burst suppression
After censoring unstable MAC values, 2356 h of data remained available for analysis in the mixed effects model (Table 3). In general, higher concentrations of volatile anaesthetics and higher doses of benzodiazepines or opioids were associated with increased burst suppression incidence and severity, and patients who received nitrous oxide had decreased incidence and severity of EEG suppression. Patients older than 60 yr had 5.31 times (OR=5.31, 95% CI: 3.81–7.41, P<0.0001) higher odds of developing EEG suppression compared with their younger counterparts, and each additional comorbidity increased their odds by 43% (OR=1.43, 95% CI: 1.29–1.70, P<0.0001). Chronic obstructive pulmonary disease was also associated with increased incidence of EEG suppression (OR=1.65, 95% CI: 1.07–2.53, P=0.023). Regular users of alcohol, benzodiazepines, and opioids had lower odds of developing EEG suppression (OR=0.53, 95% CI: 0.38–0.75, P=0.0003).
Table 3.
Anaesthetic and patient predictors of intraoperative EEG suppression. Est., estimate; CI, confidence interval; OR, odds ratio; Eq., equivalents
| Covariates | Part 1: binary model |
Part 2: generalized γ model |
||||||
|---|---|---|---|---|---|---|---|---|
| Est. | OR | 95% CI | P-value | Est. | se | 95% CI | P-value | |
| Intercept | −9.32 | <0.0001 | −2.35 | 0.12 | −2.59 to −2.12 | <0.0001 | ||
| Anaesthetic factors | ||||||||
| MAC (0.1 units) | 4.37 | 78.67 | 64.12–96.51 | <0.0001 | 2.19 | 0.06 | 2.08–2.31 | <0.0001 |
| >2 mg midazolam Eq. | 0.86 | 2.35 | 1.69–3.28 | <0.0001 | 0.23 | 0.07 | 0.10–0.36 | 0.0004 |
| >50 mg morphine Eq. | 0.63 | 1.88 | 1.35–2.63 | 0.0002 | 0.31 | 0.07 | 0.19–0.44 | <0.0001 |
| Nitrous oxide used | −0.77 | 0.46 | 0.26–0.81 | 0.0072 | −0.49 | 0.11 | −0.70 to −0.27 | <0.0001 |
| Patient factors | ||||||||
| Male gender | 0.26 | 1.29 | 0.94–1.78 | 0.1152 | 0.14 | 0.06 | 0.01–0.26 | 0.0307 |
| Age >60 yr | 1.67 | 5.31 | 3.81–7.41 | <0.0001 | 0.43 | 0.07 | 0.30–0.56 | <0.0001 |
| ASA=IV (vs I–III) | −0.13 | 0.88 | 0.61–1.28 | 0.5008 | 0.05 | 0.07 | −0.09 to 0.20 | 0.4709 |
| Comorbidity index (#) | 0.39 | 1.48 | 1.29–1.70 | <0.0001 | 0.10 | 0.03 | 0.04–0.15 | 0.0004 |
| Coronary artery disease | −0.28 | 0.75 | 0.51–1.11 | 0.1564 | −0.02 | 0.08 | −0.17 to 0.13 | 0.7907 |
| Congestive heart failure | 0.26 | 1.30 | 0.82–2.08 | 0.2661 | 0.12 | 0.09 | −0.06 to 0.30 | 0.1989 |
| Chronic obstructive pulmonary disease | 0.50 | 1.65 | 1.07–2.53 | 0.0226 | 0.19 | 0.08 | 0.03–0.36 | 0.0229 |
| Malignancy | −0.49 | 0.61 | 0.40–0.96 | 0.0308 | −0.27 | 0.09 | −0.44 to −0.10 | 0.0018 |
| Regular preoperative alcohol, opiate, or benzodiazepine use | −0.53 | 0.59 | 0.42–0.84 | 0.0029 | −0.12 | 0.07 | −0.26 to 0.01 | 0.0793 |
Discussion
The major reason motivating this study was an ongoing debate about the potential of relatively excessive anaesthetic administration, within a clinically relevant range, to be directly injurious and to increase postoperative mortality. This study found that when considered in the absence of other clinical factors, intraoperative EEG suppression is strongly associated with postoperative mortality. However, the significant bivariate association between EEG suppression and mortality was attenuated through matching and multivariate analysis. These results do not support the hypothesis that relatively excessive anaesthetic administration, as reflected by intraoperative EEG suppression, is an independent predictor of 90 day postoperative mortality. However, EEG suppression may contribute to postoperative mortality in conjunction with low MAP during the same general anaesthesia.
Unsurprisingly, EEG suppression was related to increasing anaesthetic administration. Interestingly, there was also an independent relationship between certain patient morbidities and EEG suppression. This suggests, as others have found, that intraoperative EEG suppression is a marker of patient frailty, albeit a weak marker. We chose to examine the link between EEG suppression and mortality, rather than between a particular processed EEG index and mortality, as most intraoperative EEG monitors record EEG suppression. We therefore felt that the results of this study would have relevance for any EEG-based intraoperative brain monitor. Although the BIS algorithm is proprietary, we do know that at lower BIS values, the index is inversely correlated with the extent of EEG suppression.31,32 The results of this study are therefore consistent with the findings of several other observational studies that have not found an independent link between cumulative duration of low BIS values and postoperative mortality.6,8,33
The strong univariate association between EEG suppression and mortality is striking and, if viewed uncritically, could lead to a possibly erroneous conclusion that intraoperative EEG suppression causes mortality. This emphasizes the importance of appropriate statistical adjusting techniques (e.g. propensity matching) and inclusion of known important confounders when assessing candidate associations between perioperative variables and postoperative outcomes in non-randomized studies. However, the propensity scoring techniques used here, while effective, may not have been fully inclusive of all relevant covariates. It is possible that a confounding variable may be responsible for the significance of the EEG suppression–low MAP interaction, as the presence of both conditions may be an indicator of ‘sickness’.
One limitation of this study is that there is no physiological justification for the 5 min suppression threshold used in the propensity match. However, using a historically precedented threshold improves comparison with previous research.7 Additionally, although selected a priori, this threshold conveniently subdivided the subject pool without making any single group overly small. The result regarding nitrous oxide demonstrates that caution is warranted in interpreting SR as an accurate barometer of excessive anaesthetic depth. The addition of nitrous oxide is likely to deepen anaesthesia, but in this study, nitrous oxide was associated with a decrease in SR. An additional limitation of this study is that its observational methodology precluded us from accounting for all confounders, including temperature, which was not recorded in this study's parent trials. Even rigorous statistical approaches cannot ensure that the experimental and the control groups are equivalent apart from the exposure, which in this study was EEG suppression. Only a study that randomizes patients to EEG suppression (or its avoidance) as a therapeutic intervention can provide a more compelling answer to the hypothesis that intraoperative EEG suppression increases postoperative mortality. After the matching, the lack of an association between EEG suppression and mortality could be a false-negative finding. However, the CI for the propensity-matched OR suggests that even if there is a causal link between EEG suppression and mortality, it is likely to be a weak association. Although the findings of this study are conflicting in relation to the link between intraoperative EEG suppression and mortality reported by former trials, there are other important outcomes that this study did not evaluate.5–7 These include longer-term postoperative mortality, quality of postoperative recovery, postoperative morbidity, and postoperative cognition.
If intraoperative EEG suppression had a direct association with postoperative mortality, one would expect the largest mortality differences to occur rapidly after surgery. It is possible that 90 days are too long a period after surgery to find a significant difference. However, in this sample, the 90 day mortality rate was necessary to ensure enough events to perform a multivariate analysis without over-fitting. Future studies may consider investigating the association between EEG suppression endpoints such as delirium or postoperative cognitive changes to further explore any long-term effects of EEG suppression.
The findings of this study do not support the contention that volatile anaesthetic dose should be limited within a clinically relevant range in order to avoid intraoperative EEG suppression to decrease postoperative mortality. There is no compelling reason to desist from the practice of inducing EEG suppression when it is thought to be clinically indicated (e.g. during certain brain surgeries). However, even if EEG suppression is not associated with direct harm, for the most part, it is likely to reflect unnecessarily deep anaesthesia and should be further investigated with a randomized controlled trial.
Authors' contributions
M.W.: data acquisition and processing, study conception and design, statistical analysis, and writing the manuscript. A.B.A.: data processing, study conception and design, statistical analysis, and writing the manuscript. S.G.: data acquisition and processing. D.H.: collecting mortality data and manuscript revising. N.L.: statistical analysis and manuscript revising. A.V.: data acquisition and processing, study design, input statistical analysis, and manuscript revising. E.J.: study design and manuscript revising. M.A.: acquisition of data, study conception and design, statistical analysis, and writing the manuscript. H.K.: idea for study, acquisition of data, study conception and design, statistical analysis, and manuscript revising.
Declaration of interest
None declared.
Funding
This publication was made possible by grant number CFM-08/15/2007 to M.A. by the Foundation for Anesthesia Education and Research and the American Society of Anesthesiologists, a grant from the Winnipeg Regional Health Authority and the University of Manitoba Department of Anesthesia to E.J., grant number UL1 TR000448 and TL1 TR000449 from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, a grant from the Barnes–Jewish Hospital Foundation (to M.A.), and departmental support by the Department of Anesthesiology at Washington University in St Louis and the Department of Anesthesiology at University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FAG, Boersma E. Postoperative mortality in the Netherlands. Anesthesiology. 2010;112:1105–15. doi: 10.1097/ALN.0b013e3181d5f95c. [DOI] [PubMed] [Google Scholar]
- 2.Kertai MD, Pal N, Palanca BJ, et al. Association of perioperative risk factors and cumulative duration of low bispectral index with intermediate-term mortality after cardiac surgery in the B-Unaware Trial. Anesthesiology. 2010;112:1116–27. doi: 10.1097/ALN.0b013e3181d5e0a3. [DOI] [PubMed] [Google Scholar]
- 3.Damhuis RAM, Wijnhoven BPL, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90-day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99:1149–54. doi: 10.1002/bjs.8813. [DOI] [PubMed] [Google Scholar]
- 4.Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380:1059–65. doi: 10.1016/S0140-6736(12)61148-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 6.Lindholm M-L, Träff S, Granath F, et al. Mortality within 2 years after surgery in relation to low intraoperative bispectral index values and preexisting malignant disease. Anesth Analg. 2009;108:508–12. doi: 10.1213/ane.0b013e31818f603c. [DOI] [PubMed] [Google Scholar]
- 7.Leslie K, Myles PS, Forbes A, Chan MT. The effect of bispectral index monitoring on long-term survival in the B-aware trial. Anesth Analg. 2010;110:816–22. doi: 10.1213/ANE.0b013e3181c3bfb2. [DOI] [PubMed] [Google Scholar]
- 8.Kertai MD, Palanca BJ, Pal N, et al. Bispectral index monitoring, duration of bispectral index below 45, patient risk factors, and intermediate-term mortality after noncardiac surgery in the B-Unaware Trial. Anesthesiology. 2011;114:545–56. doi: 10.1097/ALN.0b013e31820c2b57. [DOI] [PubMed] [Google Scholar]
- 9.Swank RL. Synchronization of spontaneous electrical activity of cerebrum by barbiturate narcosis. J Neurophysiol. 1949;12:161–72. doi: 10.1152/jn.1949.12.3.161. [DOI] [PubMed] [Google Scholar]
- 10.Hayashida M, Sekiyama H, Orii R, et al. Effects of deep hypothermic circulatory arrest with retrograde cerebral perfusion on electroencephalographic bispectral index and suppression ratio. J Cardiothorac Vasc Anesth. 2007;21:61–7. doi: 10.1053/j.jvca.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–50. doi: 10.1056/NEJMra0808281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stecker MM. Neurophysiology of surgical procedures for repair of the aortic arch. J Clin Neurophysiol. 2007;24:310–5. doi: 10.1097/WNP.0b013e31811ea855. [DOI] [PubMed] [Google Scholar]
- 13.Husain AM. Electroencephalographic assessment of coma. J Clin Neurophysiol. 2006;23:208–20. doi: 10.1097/01.wnp.0000220094.60482.b5. [DOI] [PubMed] [Google Scholar]
- 14.Ching S, Purdon PL, Vijayan S, Kopell NJ, Brown EN. A neurophysiological-metabolic model for burst suppression. Proc Natl Acad Sci USA. 2012;109:3095–100. doi: 10.1073/pnas.1121461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amzica F. Basic physiology of burst-suppression. Epilepsia. 2009;50(Suppl. 1):38–9. doi: 10.1111/j.1528-1167.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 16.Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, Ely EW. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med. 2008;36:3171–7. doi: 10.1097/CCM.0b013e318186b9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen NH. Anesthetic depth is not (yet) a predictor of mortality! Anesth Analg. 2005;100:1–3. doi: 10.1213/01.ANE.0000147507.23991.47. [DOI] [PubMed] [Google Scholar]
- 18.Kheterpal S, Avidan MS. ‘Triple low’ murderer, mediator, or mirror. Anesthesiology. 2012;116:1–3. doi: 10.1097/ALN.0b013e31825681e7. [DOI] [PubMed] [Google Scholar]
- 19.Yu H, Liu B. Is ‘triple low’ of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia an independent predictor for postoperative mortality? Anesthesiology. 2012;118:225–6. doi: 10.1097/ALN.0b013e318278caf9. [DOI] [PubMed] [Google Scholar]
- 20.Besch G, Liu N, Samain E, et al. Occurrence of and risk factors for electroencephalogram burst suppression during propofol–remifentanil anaesthesia. Br J Anaesth. 2011;107:749–56. doi: 10.1093/bja/aer235. [DOI] [PubMed] [Google Scholar]
- 21.Avidan MS, Zhang L, Burnside BA, et al. Anesthesia awareness and the bispectral index. N Engl J Med. 2008;358:1097–108. doi: 10.1056/NEJMoa0707361. [DOI] [PubMed] [Google Scholar]
- 22.Avidan M, Jacobsohn E, Glick D, et al. Prevention of intraoperative awareness in a high-risk surgical population. N Engl J Med. 2011;365:591–600. doi: 10.1056/NEJMoa1100403. [DOI] [PubMed] [Google Scholar]
- 23.Nickalls RWD. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br J Anaesth. 2003;91:170–4. doi: 10.1093/bja/aeg132. [DOI] [PubMed] [Google Scholar]
- 24.Aspect Medical Systems. BIS VISTA™ Monitoring System Operating Manual. 2008. Available from http://www.covidien.com/imageServer.aspx/doc225593.00.pdf?contentID=24263&contenttype=application/pdf. (accessed 29 November 2013)
- 25.Ho DE, Imai K, King G, Stuart EA. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. [Google Scholar]
- 26.Thoemmes F. Propensity score matching in SPSS. arXiv. 2012 1201.6385. [Google Scholar]
- 27.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Strawderman RL, Johnson BA, O'Quigley JM. Analyzing repeated measures semi-continuous data, with application to an alcohol dependence study. Stat Methods Med Res. 2012:1–20. doi: 10.1177/0962280212443324. [DOI] [PubMed] [Google Scholar]
- 30.Whitlock EL, Villafranca AJ, Lin N, et al. Relationship between bispectral index values and volatile anesthetic concentrations during the maintenance phase of anesthesia in the B-Unaware trial. Anesthesiology. 2011;115:1209–18. doi: 10.1097/ALN.0b013e3182395dcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bruhn J, Bouillon TW, Shafer SL. Bispectral index (BIS) and burst suppression: revealing a part of the BIS algorithm. J Clin Monit Comput. 2000;16:593–6. doi: 10.1023/A:1012216600170. [DOI] [PubMed] [Google Scholar]
- 32.Koitabashi T. Integration of suppression ratio in the bispectral index. J Anesth. 2004;18:141–3. doi: 10.1007/s00540-003-0217-1. [DOI] [PubMed] [Google Scholar]
- 33.Sessler DI, Sigl JC, Kelley SD, et al. Hospital stay and mortality are increased in patients having a ‘triple low’ of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology. 2012;116:1195–203. doi: 10.1097/ALN.0b013e31825683dc. [DOI] [PubMed] [Google Scholar]