Abstract
Background
Existing epidemiological evidence is controversial regarding the possible associations between coffee consumption and risk of prostate cancer (PCa) by aggressiveness of the disease.
Materials and methods
We conducted a random-effects dose–response meta-analysis to assess the relationships between coffee consumption and nonaggressive, aggressive and fatal PCa risk. Studies were identified by a search of Medline and Embase databases to 15 July 2013. We carried out separate analyses by grade (Gleason score: low-grade, high-grade) and stage (TNM staging system: localized, advanced) of the tumors. Nonaggressive tumors were defined as low-grade or localized, while aggressive tumors were defined as high-grade or advanced.
Results
Eight studies (three case–control and five cohort) were included in this meta-analysis. Gleason 7 tumors were classified as high-grade in one study, while in another study, Gleason 7(4 + 3) tumors were classified as high-grade and Gleason 7(3 + 4) as low-grade. In the remaining four studies, Gleason 7 tumors were excluded from the analyses or analyzed separately. The pooled relative risk (RR) for a consumption increment of 3 cups/day was 0.97 [95% confidence interval (CI) 0.92–1.03] for low-grade PCa (n = 6), 0.97 (95% CI 0.94–0.99) for localized PCa (n = 6), 0.89 (95% CI 0.78–1.00) for high-grade PCa (n = 6), 0.95 (95% CI 0.85–1.06) for advanced PCa (n = 6) and 0.89 (95% CI 0.82–0.97) for fatal PCa (n = 4). No evidence of publication bias was observed. Heterogeneity was absent or marginal (I2 range = 0–26%), with the only exception of the analysis on advanced PCa, where moderate heterogeneity was observed (I2 = 60%). When restricting the analyses only to those studies that defined high-grade tumors as Gleason 8–10, the inverse association became slightly stronger [RR: 0.84 (95% CI 0.72–0.98); n = 4].
Conclusions
Results from this dose–response meta-analysis suggest that coffee consumption may be inversely associated with the risk of fatal PCa. No clear evidence of an association with PCa incidence was observed.
Keywords: coffee, dose–response meta-analysis, epidemiology, prostate cancer
introduction
Coffee is one of the most common beverages worldwide, while prostate cancer (PCa) is the most frequent cancer malignancy among men and the third leading cause of cancer death in men in the developed world [1]. Thus, even small effects of coffee consumption on PCa risk could have significant public health consequences. In fact, coffee has been observed to increase levels of adiponectin [2–5], an insulin sensitizer, which is in turn associated with lower concentrations of both plasma insulin and insulin-like growth factor 1 (IGF-1) [6]. Additionally, both coffee and adiponectin have been observed to be associated with lower oxidative stress [7–9] and increased sex hormone-binding globulin (SHBG) concentrations [10, 11]. Changes in insulin, IGF-1, SHBG and oxidative stress have also been showed to be associated with the development and progression of PCa [12–16]. A relation between coffee consumption and PCa risk is therefore biologically plausible.
Existing epidemiological evidence is controversial regarding the associations between coffee consumption and PCa risk by aggressiveness of the disease (nonaggressive, aggressive and fatal). In fact, some epidemiological studies observed inverse associations limited to nonaggressive PCa [17, 18], while other studies found inverse associations with aggressive or fatal PCa only [19–21]. The two available meta-analyses on coffee and PCa incidence summarized the existing evidence on total PCa incidence only [22, 23]. In addition, results from studies on the association between coffee consumption and fatal PCa risk have not yet been summarized.
To clarify potential associations between coffee consumption and risk of nonaggressive, aggressive and fatal PCa, we carried out a dose–response meta-analysis of case–control and cohort studies. We also examined the possibility of nonlinear associations.
materials and methods
search strategy
We carried out a literature search for studies in humans to 15 July 2013 using the Medline and Embase databases using the following search query: [(coffee or beverages) and (PCa or prostatic neoplasms or prostate neoplasms)]. No language restrictions were imposed. Furthermore, we reviewed the references lists from retrieved articles for additional relevant studies.
selection criteria
Studies were included in the meta-analysis if they met the following criteria: (i) the exposure of interest was coffee consumption; (ii) the outcome of interest was incident PCa (analyzed by aggressiveness) or fatal PCa; (iii) RRs with corresponding 95% confidence intervals (CIs), number of cases and person-years (or number of noncases) per coffee consumption category were reported; (iv) the analyses were adjusted for smoking status, since smoking has been observed to be a risk factor for PCa in some studies [24–26], and it is also associated with higher coffee consumption levels.
data extraction
Data extracted from each study included: last name of the first author, publication year, country were the study was carried out and study period, number of cases and cohort size (or alternatively number of controls), criteria for classification of incident PCa cases according to their aggressiveness, variables adjusted for in the multivariable analysis and RRs with corresponding 95% CIs for each category of coffee consumption. We extracted the RR that reflected the greatest degree of adjustment for potential confounding variables. Two authors (AD and NO) independently retrieved the data. Disagreements were resolved by consensus.
classification of incident PCa cases according to their aggressiveness
We pooled RRs from studies that adopted similar criteria for the classification of incident PCa cases; this was done in order to analyze homogenous outcomes and to examine potential differences in the associations between coffee consumption and PCa risk by aggressiveness of the disease at diagnosis. In particular, we analyzed separately low-grade from high-grade PCa (classified using Gleason score) and localized from advanced PCa (classified using TNM staging system). To do so, we had to reanalyze the study by Discacciati et al. [18], which used a composite classification criterion that mixed together Gleason score, TNM staging system and prostate-specific antigen. We reanalyzed that study using Gleason score only [2–6 (low-grade) versus 8–10 (high-grade)] and TNM staging system only [T1-2, N0 and M0 (localized) versus T3-4, N1 or M1 or PCa death (advanced)] as the criteria for PCa classification (see supplementary Table S1, available at Annals of Oncology online).
In the present study, low-grade and localized tumors were defined as nonaggressive, while high-grade and advanced tumors cases were defined as aggressive.
statistical analysis
We repeated the following statistical analyses for all the outcomes considered in the present study, namely low-grade, high-grade, localized, advanced and fatal PCa.
We carried out a random-effect dose–response meta-analysis using the method proposed by Greenland and Longnecker [27] and Orsini et al. [28], which takes into account the correlation between the log RR estimates across categories of coffee consumption. We also explored the possibility of nonlinear relationships by modeling coffee consumption using restricted cubic splines with three knots (i.e. two spline transformations) at fixed percentiles (25%, 50% and 75%) of coffee distribution. A P-value for nonlinearity was calculated by testing against the null hypothesis that the coefficient of the second spline transformation was equal to zero [29]. The median coffee consumption for each specific category was assigned to each corresponding log RR estimate. If the median consumption was not reported in the article, we used the midpoint between the upper and lower boundary. If the lowest category was open-ended, its lower boundary was set to zero. If the upper boundary of the highest category was left unspecified, we assumed the category to be of the same amplitude as the preceding one. Statistical heterogeneity across studies was assessed using the Q and I2 statistics [30]. An I2 statistic <30% indicated no or marginal between-study heterogeneity, 30%–75% moderate heterogeneity and >75% considerable heterogeneity. Publication bias was evaluated by means of Egger's regression test [31]. We also carried out subgroup meta-analyses by type of study design (case–control versus cohort).
All reported P-values are two sided. All statistical analyses were carried out using Stata release 12.1 (StataCorp, College Station, TX).
results
literature search
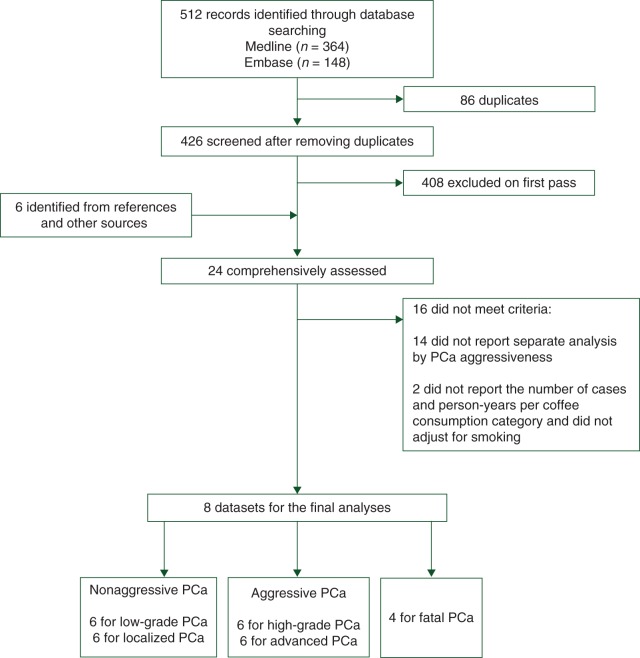
A flowchart of the identification of relevant studies is presented in Figure 1. We identified a total of 512 articles by searching the Medline and Embase databases. After removing the duplicates (n = 86), a total of 426 articles were left. We excluded 408 articles after review of the title or abstract, while 6 additional articles were identified from reference lists or other sources. Of the remaining 24 articles, 16 were excluded because did not report results for nonaggressive and aggressive incident PCa separately, while 2 articles on fatal PCa were excluded because did not adjust for smoking and, additionally, did not present the number of cases and person-years per coffee consumption category. Overall, eight articles met the inclusion criteria and were therefore available for the analyses of incident PCa by aggressiveness of the disease [17–21, 32–34]. Four studies were available for the meta-analysis on fatal PCa [17, 18, 20, 21].
Figure 1.
Flowchart of selection of studies for inclusion in the meta-analysis on coffee consumption and prostate cancer risk. PCa, prostate cancer.
study characteristics
The dose–response meta-analysis on incident PCa includes eight epidemiological studies (three case–control [21, 32, 33] and five cohort studies [17–20, 34]), published between 2011 and 2013. Of the three case–control studies, one was conducted in Italy [32], one in the United States [33] and one in Sweden [21]. Of the five cohort studies, two were conducted in the United States [17, 20], one in the United Kingdom [19], one in Sweden [18] and one in Japan [34]. Four of the eight available studies repeated the analyses using both Gleason score only and TNM staging system only for the classification of incident PCa cases [18, 20, 21, 33], two studies used Gleason score only [19, 32] and two studies used TNM staging system only [17, 34]. In particular, one study included Gleason 7 cases among high-grade tumors [32], another study classified Gleason (3 + 4) cases as low-grade and Gleason (4 + 3) cases as high-grade [33], while the remaining four studies excluded Gleason 7 cases from the analyses of low-grade and high-grade tumors [18–21]. Advanced PCa cases were defined as (T3-4, N1 or M1 or PCa death) in three studies [17, 18, 20] and as (T4, N1 or M1 or PCa death) in one study [21]. Two studies did not report details on the TNM classification criteria [33, 34]. Most of the studies provided RR estimates adjusted for age (n = 8), smoking status (n = 8), body mass index (BMI) (n = 7), family history of PCa (n = 6), energy intake (n = 6) and physical activity (PA) (n = 5). Race was adjusted for in all the studies conducted in the United States (n = 3). Combined, those eight studies involved a total of 406 718 subjects and 5733 cases of low-grade PCa (n = 6), 25 188 cases of localized PCa (n = 6), 1965 cases of high-grade PCa (n = 6) and 5724 cases of advanced PCa (n = 6) (see supplementary Tables S2 and S3, available at Annals of Oncology online).
The dose–response meta-analysis on fatal PCa includes four epidemiological studies (one case–control [21] and three cohort studies [17, 18, 20]), which were published between 2011 and 2013. The case–control study was conducted in Sweden [21], while among the three cohort studies, two were conducted in the United States [17, 20] and one in Sweden [18]. All the four studies adjusted for age, smoking status BMI and energy intake. Three studies adjusted also for family history of PCa and PA. Overall, those four studies included 382 327 study participants and 2381 fatal PCa cases (see supplementary Table S4, available at Annals of Oncology online).
dose–response meta-analysis for nonaggressive PCa
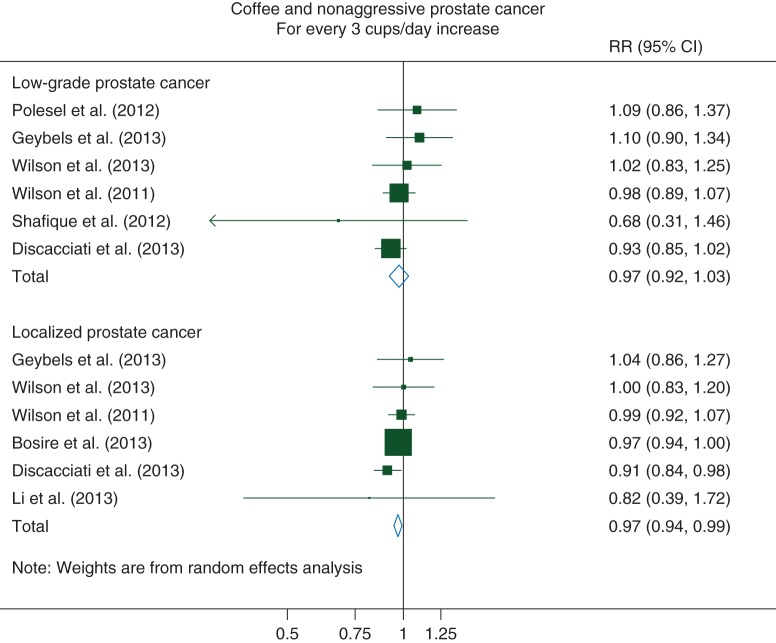
For low-grade PCa, we observed a non-statistically significant inverse linear association with coffee consumption: for every 3 cups/day increase, the pooled RR was 0.97 (95% CI 0.92–1.03) (Pnonlinearity = 0.12). There was no evidence of statistical heterogeneity among the studies [I2 = 0% (95% CI 0%–75%)] or of publication bias (P = 0.63) (Figure 2).
Figure 2.
Relative risks of nonaggressive prostate cancer for every 3 cups/day increase in coffee consumption. RR, relative risk; CI, confidence interval.
For localized PCa, we observed a statistically significant decreased 3% risk for every 3 cups/day increase in coffee consumption [RR: 0.97 (95% CI 0.94–0.99)] (Pnonlinearity = 0.10). No evidence of between-study heterogeneity [I2 = 0% (95% CI 0%–75%)] or of publication bias was observed (P = 0.94) (Figure 2).
dose–response meta-analysis for aggressive PCa
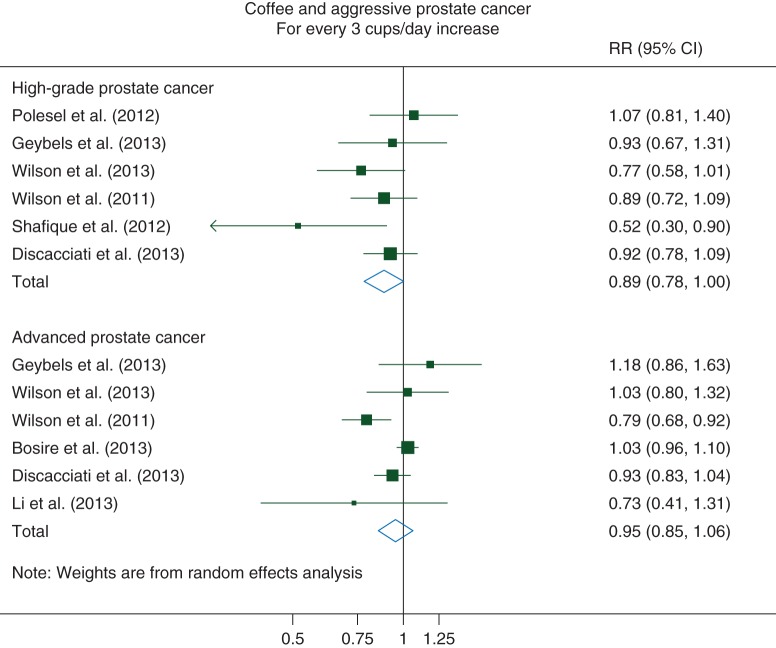
For high-grade PCa, we observed a borderline statistically significant pooled RR of 0.89 (95% CI 0.78–1.00) for every 3 cups/day increase in coffee consumption (Pnonlinearity = 0.89). No evidence of substantial between-study heterogeneity [I2 = 26% (95% CI 0%–69%)] or of publication bias (P = 0.26) was observed (Figure 3).
Figure 3.
Relative risks of aggressive prostate cancer for every 3 cups/day increase in coffee consumption. RR, relative risk; CI, confidence interval.
For advanced PCa, we observed a nonstatistically significant linear inverse association with coffee consumption, with a 5% decreased risk [RR: 0.95 (95% CI 0.85–1.06)] for every 3 cups/day in coffee consumption (Pnonlinearity = 0.18). Moderate heterogeneity among the study was observed [I2 = 60% (95% CI 2%–84%)], but no evidence of publication bias (P = 0.54) (Figure 3).
dose–response meta-analysis for fatal PCa
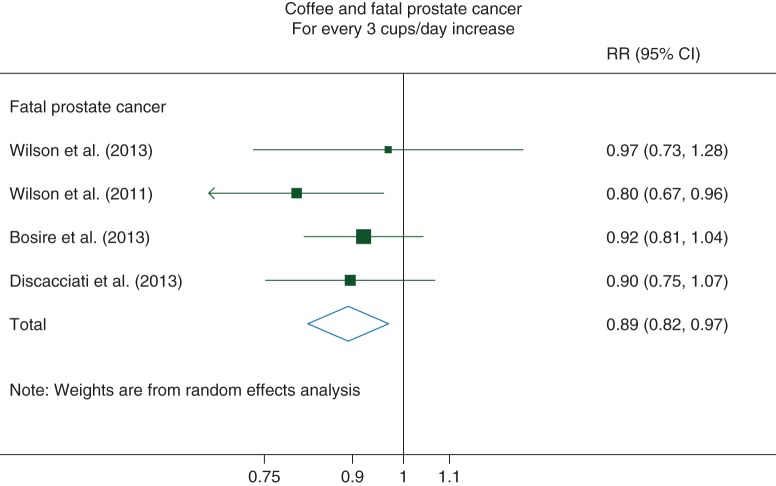
For fatal PCa, we observed a statistically significant 11% reduced PCa mortality [RR: 0.89 (95% CI 0.82–0.97)] for every 3 cups/day increase in coffee consumption (Pnonlinearity = 0.36). No evidence of between-study heterogeneity [I2 = 0% (95% CI 0%–85%)] or of publication bias was observed (P = 0.58) (Figure 4).
Figure 4.
Relative Risks of fatal prostate cancer for every 3 cups/day increase in coffee consumption. RR, relative risk; CI, confidence interval.
subgroup and sensitivity analyses
The association between coffee consumption and the five different outcomes by type of study design are presented in Table 1. Cohort studies always showed a stronger inverse association between coffee consumption and the different outcomes when compared with case–control studies. In particular, among cohort studies on nonaggressive PCa, every 3 cups/day increase in coffee consumption were associated with a 5% decreased risk of low-grade PCa [RR: 0.95 (95% CI 0.89–1.01); n = 3] and a 3% decreased risk of localized PCa [RR: 0.97 (95% CI 0.95–0.99); n = 4]. Among the cohort studies on aggressive PCa, a 15% decreased risk of high-grade PCa [RR: 0.85 (95% CI 0.69–1.04); n = 3] and a 9% decreased risk of advanced PCa [RR: 0.91 (95% CI 0.80–1.05); n = 4] were observed for every 3 cups/day in coffee increase. Finally, the cohort studies on fatal PCa showed a 12% reduced PCa mortality risk [RR: 0.88 (95% CI 0.81–0.97); n = 3] for every 3 cups/day increase in coffee consumption.
Table 1.
Summary estimates of the dose–response association between coffee consumption and prostate cancer risk
| No. of studies | Pooled RR (95% CI)a | Heterogeneity |
||||
|---|---|---|---|---|---|---|
| Q | df | P-value | I2 (%) | |||
| Nonaggressive PCa | ||||||
| Low-grade | 6 | 0.97 (0.92–1.03) | 4.5 | 5 | 0.48 | 0 |
| Case–control studies | 3 | 1.07 (0.95–1.20) | 0.3 | 2 | 0.87 | 0 |
| Cohort studies | 3 | 0.95 (0.89–1.01) | 1.3 | 2 | 0.48 | 0 |
| Localized | 6 | 0.97 (0.94–0.99) | 3.6 | 5 | 0.61 | 0 |
| Case–control studies | 2 | 1.02 (0.89–1.17) | 0.1 | 1 | 0.76 | 0 |
| Cohort studies | 4 | 0.97 (0.95–0.99) | 2.9 | 3 | 0.41 | 0 |
| Aggressive PCa | ||||||
| High-grade | 6 | 0.89 (0.78–1.00) | 6.8 | 5 | 0.24 | 26 |
| Case–control studies | 3 | 0.91 (0.75–1.15) | 2.8 | 2 | 0.25 | 29 |
| Cohort studies | 3 | 0.85 (0.69–1.04) | 3.8 | 2 | 0.15 | 48 |
| Advanced | 6 | 0.95 (0.85–1.06) | 12.5 | 5 | 0.03 | 60 |
| Case–control studies | 2 | 1.08 (0.89–1.32) | 0.5 | 1 | 0.50 | 0 |
| Cohort studies | 4 | 0.91 (0.80–1.05) | 12.5 | 3 | 0.01 | 73 |
| Fatal PCa | 4 | 0.89 (0.82–0.97) | 2.0 | 3 | 0.58 | 0 |
| Case–control studies | 1 | 0.97 (0.73–1.28) | — | — | — | — |
| Cohort studies | 3 | 0.88 (0.81–0.97) | 1.6 | 2 | 0.45 | 0 |
aFor every 3 cups/day increase in coffee consumption.
PCa, prostate cancer; RR, relative risk; CI, confidence interval; df, degrees of freedom.
Concerns may arise regarding the classification of Gleason 7 PCa cases, given the heterogeneity of those tumors and the different outcomes observed for Gleason 3 + 4 when compared with Gleason 4 + 3 tumors [35]. In a sensitivity analysis, we pooled the RRs only from those studies that defined low-grade tumors as Gleason 2–6 (n = 5) and high-grade tumors as Gleason 8–10 (n = 4). The observed associations became slightly stronger, especially for high-grade PCa: RRs were 0.96 (95% CI 0.91–1.02) (low-grade) and 0.84 (95% CI 0.72–0.98) (high-grade) for every 3 cups/day increase in coffee consumption.
In the analysis of localized PCa, one single study contributed with nearly 75% of all the PCa cases [17]. Leaving that study out of the analysis, however, did not appreciably change the pooled RR point estimate [RR: 0.96 (95% CI 0.91–1.01)], for every 3 cups/day increase in coffee consumption).
discussion
Findings from this meta-analysis suggest that coffee consumption might be inversely associated with risk of fatal PCa. No clear evidence of an inverse association between coffee consumption and PCa incidence was found, although slightly stronger associations were observed among cohort studies for both nonaggressive and aggressive PCa.
This is the first meta-analysis to explore the association between coffee consumption and incidence of PCa by aggressiveness of the disease. Two meta-analyses on coffee consumption and incidence of total PCa have been carried out so far, both of which reported only a pooled RR for high versus low coffee intake [22, 23]. The meta-analysis that included both case–control (n = 8) and cohort studies (n = 4) observed a 16% increased risk of total PCa [RR: 1.16 (95% CI 1.01–1.33)], but the pooled RR from cohort studies only did not reach statistical significance [RR: 1.06 (95% CI 0.83–1.35)] [22]. The authors of that study observed strong evidence of publication bias (P < 0.001), which we did not observe. In contrast, the meta-analysis that included only cohort studies (n = 5) observed a statistically significant 21% reduced risk of total PCa [RR: 0.79 (95% CI 0.61–0.98)], which is compatible with our findings [23]. Noteworthy, those two previously published meta-analyses have only two cohort studies in common; differences between the two meta-analyses in terms of literature search strategies and inclusion criteria of the single studies could explain, at least partially, the different results.
The higher heterogeneity that we observed among studies on aggressive PCa, when compared with studies on nonaggressive PCa, was similarly observed in a previous meta-analysis on BMI and incidence of PCa [36] and in a recent meta-analysis on diabetes mellitus and PCa risk [37]. Recall bias might, at least partly, explain the stronger inverse associations observed among cohort studies when compared with case–control studies. In fact, if drinking much coffee is perceived as unhealthy, subjects diagnosed with PCa (cases) might tend to overestimate their past coffee consumption when compared with noncases. This might lead, among case–control studies, to weaker or even positive observed associations.
To the best of our knowledge, the present study is the first meta-analysis that summarized the association between coffee consumption and risk of fatal PCa. Two small cohort studies on fatal PCa were not included in the present study since did not report the number of cases and person-years per coffee consumption category and did not adjust for smoking status [38, 39]. One of those studies, based on 93 PCa deaths, observed a RR of 0.70 (95% CI 0.38–1.30) for men drinking ≥2 cups/day compared with nondrinkers [39]. In the other study, including 149 PCa deaths, the authors found no evidence of an association between coffee and fatal PCa death: the RRs were 0.8 (95% CI 0.6–1.2) and 1.0 (95% CI 0.6–1.6) for men who drank 3–4 and ≥5 cups of coffee per day, respectively, when compared with those who drank <3 cups/day [38]. Given the wide confidence intervals, the results from those two studies are not incompatible with ours.
Strengths of this meta-analysis, in addition to the use of a dose–response approach, are the assessment of potential nonlinear relationships and the separate analyses by aggressiveness of the disease using homogeneous classification criteria, in order to explore possible differences in the associations by PCa aggressiveness. Noteworthy, no studies were excluded from this meta-analysis because of inconsistent classification methods, as we had the possibility to reanalyze the data from one study [18]. However, our study has several limitations too and our analyses must be interpreted in the context of the limited available data. The problem of confounding, which is inherent in all observational studies, cannot be solved at a meta-analysis level, but has to be addressed within the individual studies. Although all studies adjusted for important confounders such as age and smoking status and most of the studies adjusted for other potential confounders too, it is not possible to rule out unmeasured confounding as a partial explanation of the observed results. Residual confounding by inadequately measured covariates could also be of concern. Another limitation is misclassification of coffee consumption, which was inevitable since all the studies relied on self-reported consumption. However, data from validation studies showed that coffee consumption was reported with rather high validity. In particular, the correlations between coffee consumption assessed by questionnaires data and assessed by diet records were 0.6 and 0.7 in Swedish men [18, 21], 0.7 in Japanese men [34] and 0.8 and 0.9 in US men [17, 20]. We have no information on the methods of coffee preparation (e.g. boiled, filtered, espresso), type of coffee (caffeinated, decaffeinated) or brewing strength. However, two studies reported results for both caffeinated and decaffeinated coffee and observed no appreciable differences [17, 20]. Lastly, since in all studies included in this meta-analysis the classification of the tumors was carried out at diagnosis, we have no information about the possible upstaging of some of the cases following radical prostatectomy.
An inverse association between coffee consumption and PCa risk is biologically plausible and, in fact, several mechanisms could explain the observed inverse, albeit weak, associations. Adiponectin is an endogenous insulin sensitizer [40], which has been observed to be directly associated with coffee consumption both in clinical trials [3, 4] and in observational studies [2, 5]. Adiponectin itself has been associated to a reduced risk of PCa, as recently reviewed [41]. In addition, higher adiponectin plasma concentrations have been shown to decrease both plasma insulin and IGF-1 blood levels [6]. Insulin levels, in turn, have been observed to be directly associated with PCa mortality [42, 43], incidence [44] and recurrence [45]. Furthermore, IGF-1 levels were observed to be directly associated with PCa risk in a pooled analysis of individual patient data from 12 prospective studies [14], as well as in a more recent nested case–control study [13].
Coffee consumption was observed to be directly associated with SHBG in both men [10] and women [46–49]; consistently with this finding, adiponectin was also observed to be positively correlated with SHBG concentration [10, 11]. In a collaborative analysis of 18 prospective studies, SHBG was observed to be weakly inversely associated with PCa incidence [15].
Lastly, adiponectin has been observed to inhibit oxidative stress in human prostate carcinoma cells in a dose–response fashion [7]. In addition, coffee is an important source of antioxidants [8, 9]. Oxidative stress is a key event in the development of PCa and although no conclusive data have been presented so far to start recommending any antioxidants as chemopreventive agents [50], various dietary antioxidants have been observed to be associated with a decreased risk of advanced PCa [12, 16]. These results could explain, at least partly, the inverse associations between coffee consumption and risk of PCa incidence and mortality that we observed in the present meta-analysis.
In conclusion, results from this meta-analysis suggest that coffee consumption may be associated with a lower risk of PCa mortality.
funding
This work was supported by research grants from the Swedish Cancer Society (Cancerfonden) and KID funding (Karolinska Institutet's funding for doctoral students).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank three anonymous reviewers for useful comments.
references
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. doi:10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Imatoh T, Tanihara S, Miyazaki M, et al. Coffee consumption but not green tea consumption is associated with adiponectin levels in japanese males. Eur J Nutr. 2011;50(4):279–284. doi: 10.1007/s00394-010-0136-5. doi:10.1007/s00394-010-0136-5. [DOI] [PubMed] [Google Scholar]
- 3.Kempf K, Herder C, Erlund I, et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91(4):950–957. doi: 10.3945/ajcn.2009.28548. doi:10.3945/ajcn.2009.28548. [DOI] [PubMed] [Google Scholar]
- 4.Wedick NM, Brennan AM, Sun Q, et al. Effects of caffeinated and decaffeinated coffee on biological risk factors for type 2 diabetes: a randomized controlled trial. Nutr J. 2011;10:93. doi: 10.1186/1475-2891-10-93. doi:10.1186/1475-2891-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams CJ, Fargnoli JL, Hwang JJ, et al. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: a prospective cohort study. Diabetes Care. 2008;31(3):504–507. doi: 10.2337/dc07-1952. doi:10.2337/dc07-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakajima A, Endo H, Yoneda K, et al. Molecular mechanisms linking adiponectin receptor signalling and cancer. Open Obes J. 2010;2:43–49. doi:10.2174/1876823701002020043. [Google Scholar]
- 7.Lu J-P, Hou ZF, Duivenvoorden WC, et al. Adiponectin inhibits oxidative stress in human prostate carcinoma cells. Prostate Cancer Prostatic Dis. 2012;15(1):28–35. doi: 10.1038/pcan.2011.53. doi:10.1038/pcan.2011.53. [DOI] [PubMed] [Google Scholar]
- 8.Rautiainen S, Levitan EB, Mittleman MA, et al. Total antioxidant capacity of diet and risk of heart failure: a population-based prospective cohort of women. Am J Med. 2013;126(6):494–500. doi: 10.1016/j.amjmed.2013.01.006. doi:10.1016/j.amjmed.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Rautiainen S, Levitan EB, Orsini N, et al. Total antioxidant capacity from diet and risk of myocardial infarction: a prospective cohort of women. Am J Med. 2012;125(10):974–980. doi: 10.1016/j.amjmed.2012.03.008. doi:10.1016/j.amjmed.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Svartberg J, Midtby M, Bønaa KH, et al. The associations of age, lifestyle factors and chronic disease with testosterone in men: the Tromsø Study. Eur J Endocrinol Eur Fed Endocr Soc. 2003;149(2):145–152. doi: 10.1530/eje.0.1490145. doi:10.1530/eje.0.1490145. [DOI] [PubMed] [Google Scholar]
- 11.Yasui T, Tomita J, Miyatani Y, et al. Associations of adiponectin with sex hormone-binding globulin levels in aging male and female populations. Clin Chim Acta Int J Clin Chem. 2007;386(1–2):69–75. doi: 10.1016/j.cca.2007.08.001. doi:10.1016/j.cca.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Bardia A, Platz EA, Yegnasubramanian S, et al. Anti-inflammatory drugs, antioxidants, and prostate cancer prevention. Curr Opin Pharmacol. 2009;9(4):419–426. doi: 10.1016/j.coph.2009.06.002. doi:10.1016/j.coph.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Price AJ, Allen NE, Appleby PN, et al. Insulin-like growth factor-I concentration and risk of prostate cancer: results from the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2012;21(9):1531–1541. doi: 10.1158/1055-9965.EPI-12-0481-T. doi:10.1158/1055-9965.EPI-12-0481-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roddam AW, Allen NE, Appleby P, et al. Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med. 2008;149:461–471. doi: 10.7326/0003-4819-149-7-200810070-00006. – doi:10.7326/0003-4819-149-7-200810070-000067,W83–W88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roddam AW, Allen NE, Appleby P, et al. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100(3):170–183. doi: 10.1093/jnci/djm323. doi:10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schröder FH, van Weerden WM. Prostate cancer—chemoprevention. Eur J Cancer. 2009;45(Suppl. 1):355–359. doi: 10.1016/S0959-8049(09)70050-4. doi:10.1016/S0959-8049(09)70050-4. [DOI] [PubMed] [Google Scholar]
- 17.Bosire C, Stampfer MJ, Subar AF, et al. Coffee consumption and the risk of overall and fatal prostate cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2013;24(8):1527–1534. doi: 10.1007/s10552-013-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Discacciati A, Orsini N, Andersson S-O, et al. Coffee consumption and risk of localized, advanced and fatal prostate cancer: a population-based prospective study. Ann Oncol. 2013;24(7):1912–1918. doi: 10.1093/annonc/mdt105. doi:10.1093/annonc/mdt105. [DOI] [PubMed] [Google Scholar]
- 19.Shafique K, McLoone P, Qureshi K, et al. Coffee consumption and prostate cancer risk: further evidence for inverse relationship. Nutr J. 2012;11:42. doi: 10.1186/1475-2891-11-42. doi:10.1186/1475-2891-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson KM, Kasperzyk JL, Rider JR, et al. Coffee consumption and prostate cancer risk and progression in the Health Professionals Follow-up Study. J Natl Cancer Inst. 2011;103(11):876–884. doi: 10.1093/jnci/djr151. doi:10.1093/jnci/djr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson KM, Bälter K, Möller E, et al. Coffee and risk of prostate cancer incidence and mortality in the Cancer of the Prostate in Sweden Study. Cancer Causes Control. 2013;24(8):1575–1581. doi: 10.1007/s10552-013-0234-9. [DOI] [PubMed] [Google Scholar]
- 22.Park C-H, Myung S-K, Kim T-Y, et al. Coffee consumption and risk of prostate cancer: a meta-analysis of epidemiological studies. BJU Int. 2010;106(6):762–769. doi: 10.1111/j.1464-410X.2010.09493.x. doi:10.1111/j.1464-410X.2010.09493.x. [DOI] [PubMed] [Google Scholar]
- 23.Yu X, Bao Z, Zou J, et al. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer. 2011;11:96. doi: 10.1186/1471-2407-11-96. doi:10.1186/1471-2407-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong Z, Agalliu I, Lin DW, et al. Cigarette smoking and prostate cancer-specific mortality following diagnosis in middle-aged men. Cancer Causes Control. 2008;19(1):25–31. doi: 10.1007/s10552-007-9066-9. doi:10.1007/s10552-007-9066-9. [DOI] [PubMed] [Google Scholar]
- 25.Huncharek M, Haddock KS, Reid R, et al. Smoking as a risk factor for prostate cancer: a meta-analysis of 24 prospective cohort studies. Am J Public Health. 2010;100(4):693–701. doi: 10.2105/AJPH.2008.150508. doi:10.2105/AJPH.2008.150508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohrmann S, Linseisen J, Allen N, et al. Smoking and the risk of prostate cancer in the European Prospective Investigation into Cancer and Nutrition. Br J Cancer. 2013;108(3):708–714. doi: 10.1038/bjc.2012.520. doi:10.1038/bjc.2012.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 28.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation from summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 29.Orsini N, Li R, Wolk A, et al. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. doi: 10.1093/aje/kwr265. doi:10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. doi:10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. doi:10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polesel J, Zucchetto A, Talamini R, et al. Re: coffee consumption and prostate cancer risk and progression in the health professional follow-up study. J Natl Cancer Inst. 2012;104(21):1684–1686. doi: 10.1093/jnci/djs383. author reply 1686 doi:10.1093/jnci/djs383. [DOI] [PubMed] [Google Scholar]
- 33.Geybels MS, Neuhouser ML, Stanford JL. Associations of tea and coffee consumption with prostate cancer risk. Cancer Causes Control. 2013;24(5):941–948. doi: 10.1007/s10552-013-0170-8. doi:10.1007/s10552-013-0170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Q, Kakizaki M, Sugawara Y, et al. Coffee consumption and the risk of prostate cancer: the ohsaki cohort study. Br J Cancer. 2013;108(11):2381–2389. doi: 10.1038/bjc.2013.238. doi:10.1038/bjc.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stark JR, Perner S, Stampfer MJ, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27(21):3459–3464. doi: 10.1200/JCO.2008.20.4669. doi:10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Discacciati A, Orsini N, Wolk A. Body mass index and incidence of localized and advanced prostate cancer–a dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(7):1665–1671. doi: 10.1093/annonc/mdr603. doi:10.1093/annonc/mdr603. [DOI] [PubMed] [Google Scholar]
- 37.Xu H, Jiang H, Ding G, et al. Diabetes mellitus and prostate cancer risk of different grade or stage: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2013;99(3):241–249. doi: 10.1016/j.diabres.2012.12.003. doi:10.1016/j.diabres.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Hsing AW, McLaughlin JK, Schuman LM, et al. Diet, tobacco use, and fatal prostate cancer: results from the lutheran brotherhood cohort study. Cancer Res. 1990;50(21):6836–6840. [PubMed] [Google Scholar]
- 39.Phillips RL, Snowdon DA. Association of meat and coffee use with cancers of the large bowel, breast, and prostate among Seventh-Day Adventists: preliminary results. Cancer Res. 1983;43(Suppl. 5):2403s–2408s. [PubMed] [Google Scholar]
- 40.Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91(1):258S–261S. doi: 10.3945/ajcn.2009.28449C. doi:10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izadi V, Farabad E, Azadbakht L. Serum adiponectin level and different kinds of cancer: a review of recent evidence. ISRN Oncol. 2012;2012:982769. doi: 10.5402/2012/982769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hammarsten J, Högstedt B. Hyperinsulinaemia: a prospective risk factor for lethal clinical prostate cancer. Eur J Cancer. 2005;41(18):2887–2895. doi: 10.1016/j.ejca.2005.09.003. doi:10.1016/j.ejca.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 2008;9(11):1039–1047. doi: 10.1016/S1470-2045(08)70235-3. doi:10.1016/S1470-2045(08)70235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hsing AW, Chua S, Jr, Gao YT, et al. Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst. 2001;93(10):783–789. doi: 10.1093/jnci/93.10.783. doi:10.1093/jnci/93.10.783. [DOI] [PubMed] [Google Scholar]
- 45.Lehrer S, Diamond EJ, Stagger S, et al. Increased serum insulin associated with increased risk of prostate cancer recurrence. Prostate. 2002;50(1):1–3. doi: 10.1002/pros.10026. doi:10.1002/pros.10026. [DOI] [PubMed] [Google Scholar]
- 46.Ferrini RL, Barrett-Connor E. Caffeine intake and endogenous sex steroid levels in postmenopausal women. The Rancho Bernardo Study. Am J Epidemiol. 1996;144(7):642–644. doi: 10.1093/oxfordjournals.aje.a008975. doi:10.1093/oxfordjournals.aje.a008975. [DOI] [PubMed] [Google Scholar]
- 47.Kotsopoulos J, Eliassen AH, Missmer SA, et al. Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer. 2009;115(12):2765–2774. doi: 10.1002/cncr.24328. doi:10.1002/cncr.24328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.London S, Willett W, Longcope C, et al. Alcohol and other dietary factors in relation to serum hormone concentrations in women at climacteric. Am J Clin Nutr. 1991;53(1):166–171. doi: 10.1093/ajcn/53.1.166. [DOI] [PubMed] [Google Scholar]
- 49.Nagata C, Kabuto M, Shimizu H. Association of coffee, green tea, and caffeine intakes with serum concentrations of estradiol and sex hormone-binding globulin in premenopausal Japanese women. Nutr Cancer. 1998;30(1):21–24. doi: 10.1080/01635589809514635. doi:10.1080/01635589809514635. [DOI] [PubMed] [Google Scholar]
- 50.Thapa D, Ghosh R. Antioxidants for prostate cancer chemoprevention: challenges and opportunities. Biochem Pharmacol. 2012;83(10):1319–1330. doi: 10.1016/j.bcp.2011.12.027. doi:10.1016/j.bcp.2011.12.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.