Abstract
Background
Cancer is a disease that mostly affects older adults. Treatment adherence is crucial to obtain optimal outcomes such as cure or improvement in quality of life. Older adults have numerous comorbidites as well as cognitive and sensory impairments that may affect adherence. The aim of this systematic review was to examine factors that influence adherence to cancer treatment in older adults with cancer.
Patients and Methods
Systematic review of the literature published between inception of the databases and February 2013. English, Dutch, French and German-language articles reporting cross-sectional or longitudinal, intervention or observational studies of cancer treatment adherence were included. Data sources included MEDLINE, EMBASE, PsychINFO, Cumulative Index to Nursing and Allied Health (CINAHL), Web of Science, ASSIA, Ageline, Allied and Complementary Medicine (AMED), SocAbstracts and the Cochrane Library. Two reviewers reviewed abstracts and abstracted data using standardized forms. Study quality was assessed using the Mixed Methods Appraisal Tool 2011.
Results
Twenty-two manuscripts were identified reporting on 18 unique studies. The quality of most studies was good. Most studies focused on women with breast cancer and adherence to adjuvant hormonal therapy. More than half of the studies used data from administrative or clinical databases or chart reviews. The adherence rate varied from 52% to 100%. Only one qualitative study asked older adults about reasons for non-adherence. Factors associated with non-adherence varied widely across studies.
Conclusion
Non-adherence was common across studies but little is known about the factors influencing non-adherence. More research is needed to investigate why older adults choose to adhere or not adhere to their treatment regimens taking into account their multimorbidity.
Keywords: systematic review, geriatric oncology, non-adherence, cancer treatment, aged
introduction
Cancer is a disease that mostly affects older adults. It is estimated that 70% of all incident cases and over 82% of deaths due to cancer occur in persons aged ≥60 years in Canada [1]. This is similar to other Western developed countries [1, 2]. With an aging population, there will be a significant increase in the number of older adults being diagnosed with cancer [1, 2].
Treatment adherence is defined by the World Health Organization (WHO) (2003) as “the extent to which a person's behaviour—taking medication, following a diet and/or executing lifestyle changes, corresponds with agreed recommendation from a health care provider” [3]. Cancer treatment adherence is crucial to obtain optimal health outcomes, such as cure or improvement in quality of life. Cancer medication non-adherence has been shown to lead to decreased survival [4–7], higher recurrence/treatment failure rates [8–10] and health care costs [4–9, 11, 12]. Adherence is a multidimentional phenomenon, and according to the WHO, is influenced by patient-related factors, therapy-related factors, condition-related factors, health system factors and social economic factors [3].
In addition to cancer, older persons often have other medical conditions. In 2006, 88% of Canadian older adults had at least one medical condition, and 65% had two or more conditions [13]. With increasing age, the number of chronic conditions increases. For the treatment of these chronic conditions, older adults usually take multiple medications. Older adults take, on average, 6.5 medications per day [14]. Multimorbidity in the older population increases treatment complexity (e.g. conflicting treatments, drug interactions) [15–17]. An increasing number of prescribed medications are associated with decreasing medication adherence in the general older population as well as in older adults who are prescribed oral chemotherapy and/or hormonal therapy [18–23]. Research findings suggest that in the general older population, up to 50% are non-adherent to medication recommendations [19, 24], which can consequently have serious complications for the health status of an older adult.
Although there have been narrative/expert reviews of adherence to medication in the general older adult population [19], and several narrative and systematic reviews of adherence to oral antineoplastic agents for cancer patients across age groups [18, 25–32], there has been no systematic review of the factors influencing adherence to all forms of active cancer treatment that focused specifically on older adults with cancer. Furthermore, most of the reports of these reviews did not specify the search strategy, inclusion and exclusion criteria, the results of the search strategy, setting and sample of studies, or did not assess the quality of included studies, and it is not clear if the data abstraction for the review was done by one or more researchers. Moreover, many included only studies published in English while ignoring studies published in other languages. Therefore, the objective of this systematic review was to synthesize all studies to address the research question ‘What factors influence adherence to active cancer treatment in older adults aged 65 and over diagnosed with cancer?’
materials and methods
search strategy and selection criteria
This review was based on a systematic, comprehensive search of 10 databases from inception of each database until February 2013, including the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health (CINAHL), Allied and Complementary Medicine (AMED), Psych-INFO, Ageline, Sociological Abstracts, Web of Science, and Applied Social Sciences Index and Abstracts (ASSIA) databases. Eligible studies were searched using key words/medical subject headings (MeSHs) such as medication adherence, guideline adherence, compliance, treatment preferences, medication management, and perceptions of medication AND neoplasms/cancer AND Aged, 65 and over, elderly, older adult (see supplementary Appendix 1, available at Annals of Oncology online, for the complete search strategy used in MEDLINE). A similar search strategy was used in the remaining nine databases. In addition, we reviewed the reference lists of previous reviews to identify potentially eligible studies. The literature search was conducted by an experienced university librarian (ES).
Inclusion criteria: Publications were included if reporting on factors influencing adherence to any active cancer treatment (i.e. chemotherapy, surgery, radiation therapy, hormonal therapy and therapy with molecular-targeted agents and any combinations of these treatments) in older patients aged ≥65, being diagnosed with cancer. Study designs could include cross-sectional, prospective, controlled interventional or observational studies, or qualitative studies that assessed the factors influencing cancer treatment adherence of older adults (≥65) with cancer. Articles written in English, French, Dutch and German were eligible.
Exclusion criteria: Publications focusing on cancer patients younger than 65 years of age, editorials and review articles were ineligible. However, if a study included participants with a mean/median age of <65 years but reported on results for a subgroup of which the mean/median age is ≥65, the publication was considered eligible for inclusion.
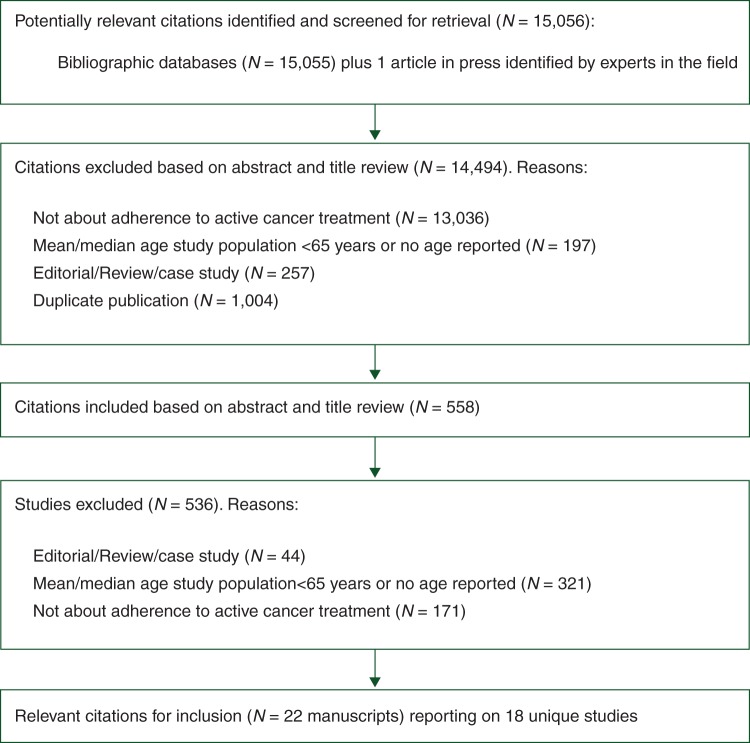
The studies were selected in a two-step process (Figure 1). First, an initial selection based on titles and abstracts was completed independently by two reviewers (MP and HAT). In case of uncertainty, the abstract was included for full-text review (including abstracts that were addressing adherence but no age for the study population was reported). Second, the full-text articles were retrieved and reviewed independently by the same reviewers. In case of disagreement between the two reviewers or uncertainty, the other members of the research team were provided the full-text article for consensus decision-making. For all articles that referred to additional publications for more details on study methods, those publications were retrieved and reviewed to complement the data abstraction and quality assessment of the eligible study publication. In articles, where no age for the study population was reported in the full text, the study authors were then contacted to obtain the details on the study age. If no response was received after at least three attempts, the article was not included in the final selection as no paper indicated that the study population were older adults.
Figure 1.
Flow chart of study selection.
Data abstraction
We have used the PRISMA statement for guiding the data abstraction and reporting of this systematic review [33]. Data were abstracted using the data abstraction form that had been developed for this systematic review by the research team. Data abstraction was completed independently by the same reviewers, who carried out the article selection (MP and HAT). The abstracted information included study design, aim of study, location of study, sampling method and sample size, response rate, source of data, characteristics of included study participants including age, sex, cancer type, cancer stage, setting (country), date of diagnosis, comorbid conditions, cancer treatment (surgery, chemotherapy, radiation, hormonal treatment, targeted therapy/biological agents), definition of treatment adherence, factors influencing the cancer treatment adherence and details of statistical analysis. If any aspect of the study design and conduct was unclear, the study authors were contacted. A meta-analysis was not possible as studies were heterogeneous with respect to adherence definitions, cancer treatments, study populations, methods and outcomes.
Although the International Society for Pharmacoeconomics and Outcomes Research workgroup Medication Compliance and Persistence in 2008 published definitions for both adherence/compliance (synonyms) and persistence [34], in this review we chose to use the definitions of adherence/persistence as provided by the study authors in the manuscript, as many of the included studies were published before these definitions and might have used these terms interchangeably.
quality assessment
Both qualitative and quantitative studies were included. Pluye et al. [35] have developed a scoring system to assess the methodological quality of each individual study called the Mixed Methods Assessment Tool (MMAT) that can be used for mixed methods research and mixed studies reviews (MSRs). The authors tested the reliability and efficacy of this system and found that agreement between reviewers was moderate to excellent for the MMAT criteria and it was easy to use [35]. The 2011 MMAT scoring system contains five types of mixed methods study components or primary studies in a MSR context each with its own set of methodological quality criteria based on the existing published criteria. For each item, the answer categories were ‘yes’, ‘no’, ‘can't tell’ followed by comments. The five types of mixed methods study components or primary studies included in the MMAT are (i) qualitative; (ii) quantitative, randomized, controlled trials; (iii) quantitative non-randomized; (iv) quantitative descriptive and (v) mixed methods. Two reviewers (MP and HU) scored the quality of included studies independently. No study was excluded based on the quality assessment.
results
We screened 15 056 titles and abstracts for eligibility in the first step, from which we selected 558 for full-text review (see Figure 1, for an overview of the selection and the reasons for exclusions). In total, 21 manuscripts were included in this review [36–57] reporting on 18 unique studies. In two manuscripts, authors used data from the same clinical trial [51, 54]. In two other manuscripts, authors used data from the same prospective observational cohort study [41, 47]. One author published three manuscripts using the same clinical chart database, but the populations were not completely overlapping (different age inclusion criteria and time periods of the data collected) [42–44]. Thus, a total of 18 unique studies were included. All included manuscripts were written in English. The percentage identified below refers to the percentage of the total of 18 studies in the result sections.
quality assessment
The quality was good for most studies, see supplementary Table S1, available at Annals of Oncology online. Ten studies (56%) used data from several administrative and clinical databases or chart reviews [36, 37, 39, 40, 42–44, 46, 49, 50, 52, 53, 55]. In three studies (17%), data from clinical trials were used [45, 48, 51, 54]. In three other studies (17%), data were collected using prospective observational studies [38, 41, 47, 56]. One study (6%) used a retrospective observational study design [57]. One study used a qualitative study design [53]. Of those eight studies that did not use administrative databases/clinical databases/chart reviews, only three studies reported the response rate [38, 41, 47, 53], and thus the extent of selection bias cannot be evaluated for the majority of studies. For the prospective observational studies, the method of how the follow-up was conducted was described for all studies. For six studies (33%), it was not clear how much missing data there were and/or how the investigators dealt with the missing data in the analyses [37, 38, 40, 45, 46, 57]. For three studies (17%), the data analysis methods were not described in sufficient detail [48, 53, 56].
characteristics of included studies
The characteristics of the included studies are described in supplementary Table S2, available at Annals of Oncology online. Of the 18 studies included, 11 (61%) were conducted in the United States, two in Switzerland (6%), one in the UK (6%), one in Germany (6%), one in Ireland (6%), one in France (6%) and one in Hong Kong (6%).
The sample size of the included studies ranged from 25 [55] to 22 160 patients [49].
Most studies (61%) included participants with breast cancer [36–38, 41–44, 46, 47, 49–52, 54, 57]; other studies included colon cancer [39], head and neck cancer [40], bladder cancer [45], carcinoma of the oral cavity [48], prostate cancer [55] or a mixed population [53, 56]. The majority (56%) focused on examining (non-)adherence/ (non-)persistence to adjuvant hormonal therapy [37, 38, 41–44, 46, 47, 49, 50, 52, 57], adjuvant chemotherapy/molecular targeted therapy [36, 39, 51, 54], radiation treatments [40, 45], chemotherapy/molecular targeted therapy in the context of advanced disease [53], chemotherapy/molecular targeted therapy for both adjuvant and advanced disease[56], hormone treatment in the context of advanced disease [55] and a combination of chemotherapy and surgery [48].
In 10 studies (non-)adherence was studied [36, 38, 39, 41, 45, 47, 48, 52, 55–57], in two studies non-persistence [37, 49] and in two other studies [46, 51, 54] both (non-)adherence and (non-) persistence were studied. In three studies, treatment completion/discontinuation/non-use was studied (without defining it as either as non-adherence or non-persistence) [40, 50, 53]. One study had three different publications [42–44], in which a different aspect of adherence and persistence was studied in each.
how were non-adherence and non-persistence defined?
The definition of non-adherence varied substantially between studies, ranging from having received less than four cycles of anthracycline chemotherapy [36], having received less than five cycles of chemotherapy within 9 months of diagnosis [39], missing one or more medication injections [55], self-reported intake of the medication [38, 41, 47], a medication possession rate (MPR) of <80% [46, 49, 52, 57], <80% of doses expected recorded by the microelectronic monitoring system (MEMS) [51], <80% of doses expected recorded in medication calendars [54] and <100% of expected doses recorded in medication diaries [56]. The rate of adherence varied between 52% [40] and 100% [57].
Similarly, the definition of non-persistence also varied greatly between studies ranging from having 45 days of gap between refills [49], discontinuation of >60 days [50], ≥90 days between refills [46], having 180 consecutive days of no tamoxifen supply after the first prescription [37], taking the medication <36 months [44], taking the treatment <5 years [43], coming off therapy without treatment completion [51] and being prescribed at least six cycles of it should be at least one of the three cyclophosphamide methotrexate 5-fluorouracil drugs [54]. The persistence rate varied between 51% [50] and 91.7% [43].
which factors are associated with treatment (non-)adherence and (non-)persistence in older adults with cancer?
We used the WHO classification of five factors influencing adherence to describe the diverse range of factors that were examined in the 18 studies included in this review (Table 1).
Table 1.
Factors associated with adherence and persistence to active cancer treatment in older adults diagnosed with cancer
| Author, publication date (reference) | Which cancer treatments were included in the study? | Definition of non-adherence used or adherence/non-persistence used or persistence | Measurement of non-adherence/ non-persistence | Adherence /persistence rate | Statistical analysis used to examine factors associated with non-adherence /non-persistence | Which variables were included in the statistical analysis? | Factors associated with non-adherence (−) or higher adherence (+) | Factors associated with non-persistence (−) or higher persistence (+) |
|---|---|---|---|---|---|---|---|---|
| Barcenas et al. [36] | Chemotherapy | Non-adherence: having received one to three cycles of anthracyclines. Adherent: having received four or more cycles of anthracyclines | Claim codes in the administrative databases | Adherence rate: 83% | Logistic regression analysis | Age at diagnosis, race, marital status, educational level, poverty level, SEER region, year of diagnosis, lymph node involvement, tumour size, tumour grade, PR and ER receptor status, surgery type, Charlson comorbidity index, radiation therapy and number of hospitalizations | Age >75 years (−), black race (−), unmarried status (−), two different SEER-regions (−), those diagnosed in 2000 or earlier (−), number of hospitalizations (more hospitalizations had larger impact on non-adherence) (−) | NA |
| Barron et al. [37] | Tamoxifen (hormonal therapy) | Tamoxifen non-persistence was defined as 180 consecutive days of no tamoxifen supply after the index date (=first prescription) without alternative hormonal therapy during that time | Prescription refill data | Persistence rate: 77.9% at 1 year of treatment and 64.8% at 3.5 years of treatment | Cox proportional hazard regression analysis | Variables in univariate analysis with p< 0.1 were selected in the multivariable model and included age, types of prescription drug usage, number of having dementia/Parkinson disease, mean number of pharmacological agents per month | Age >75 compared with 45–54 years (−), using antidepressant medication at tamoxifen initiation (−), and having dementia/Parkinson disease (−), greater than one pharmacological agents per month a year before tamoxifen initiation (+) | |
| Demissie et al. [38] | Tamoxifen (hormonal therapy) | Women who were taking tamoxifen were classified at the second follow-up interview as either still taking tamoxifen (yes) or no longer taking tamoxifen (no) | Self-report during a telephone follow-up interview, questions not specified | Adherence: 85% at 21 months after surgery for breast cancer follow-up | Logistic regression analysis | All study variables were included in one model and then removed if not contributing. Two models were run: tamoxifen use at follow-up as outcome, and tamoxifen discontinuation at follow-up as outcome (n = 26, model underpowered) | No factor was associated with discontinuation of tamoxifen. Age (younger age +), stage 2 (+), ER positive status (+) and number of physicians (higher number +) and excellent ability (+) to communicate were all associated with tamoxifen use | |
| Dobie et al. [39] | Chemotherapy | Adherence: having received 5 months/cycles (one cycle a month) of chemotherapy within 9 months of diagnosis (liberal definition) and having received 6 months/cycles (one cycle a month) of chemotherapy within 9 months of diagnosis (conservative definition) | Claim codes in the administrative databases | Adherence rate using a conservative definition and full study sample: 78% | Logistic regression analysis | Race, age, sex, ethnicity, marital status, location of residence, and age-and race-specific household income and SEER registry were included in all models plus variables with P< 0.09 or those that significantly improved model fit | older age (−), female (−), readmission to hospital (−), recurrence of cancer (−) were associated with lower chemotherapy completion rates | |
| Fesinmeyer et al. [40] | Radiation therapy (RT) | Complete course of radiation: at least 30 treatments for those who did not have surgery before RT, at least 25 treatments for those who had prior surgery. An interruption or gap was defined as lapses of >4 but <31 days between RT treatments | Claim codes in the administrative databases | 70.4 of surgical patients and 52% of nonsurgical patients completed RT without interruptions/gaps | Logistic regression analysis, a separate model was calculated for each of the five tumour sites (larynx, nasal cavity, oral cavity, pharynx and salivary gland) | Each model included the receipt of surgery relative to radiation (yes/no and within 30 days), tumour stage, comorbidity, age, sex, race, urban versus rural residence | For oral cavity tumours: surgery within 30 days before RT (+), Charlson of 0(+), not chemo (+). For pharynx: surgery within 30 days of RT (+) and no chemo (+) and regional tumour (+). For laryngeal: surgery within 30 days of RT (+), no chemo (+), local tumours (+) and Charlson of 0 (+). For nasal cavity or salivary gland tumour: surgery within 30 days (+) | |
| Fink et al. [41]a | Tamoxifen (hormonal therapy) | Self-reported no longer taking tamoxifen, regardless of reason for stopping at 3, 6, 15 and 27 months after breast cancer surgery | Self-reported use during telephone interviews | Adherence: 83% at 1 year and 79% at 2 years of treatment | Logistic regression analysis | Predictors that were significant in univariate analyses were selected for inclusion as well as confounders not further specified | Decision balance scale score (having neutral or negative beliefs about the value of tamoxifen (−)) and number of positive nodes (−) | |
| Guth et al. [42]b | Hormonal therapy | Patients were divided into subgroups: those who did not initiate therapy (including those for whom therapy was not recommended/ was recommended but never began/refused) and those who initiated therapy (into discontinuation due to death/breast recurrence and/or distant metastasis, serious medical reasons other than breast cancer, therapy adverse effects, and other reasons) | Data were collected from the charts of follow-up consultations during which patients were asked about the treatments | Of the 325, 287 initiated endocrine therapy and One hundred and ninety-one of 287 (66.6%) completed 5-year therapy. Of the 96 who discontinued therapy, 31 were non-adherent (10.8%) | Logistic regression analysis | Only univariate analysis was conducted | Location of follow-up (GP follow-up (−)) | |
| Guth et al. [43]b | Hormonal therapy | Patients were divided in subgroups: those who did not initiate therapy (including those for whom therapy was not recommended/ was recommended but never began/refused) and those who initiated therapy (including those who completed 5 year therapy, those who completed >5 years, and those who discontinued due to drug-related side-effects and those who discontinued for other reasons such as death/recurrence/other serious medical reasons than breast cancer) | Data were collected from the charts of follow-up consultations during which patients were asked about the treatments | Non-persistence rate 37/400 (9.3%) | Descriptive analysis | NA | Of the 37 who were non-persistent, 24 discontinued because of side-effects, and 13 for other reasons including lack of motivation (5), lack of faith in therapy (2), misinformation by physician (2), errors regarding length of therapy (1), insurance reasons (1), denial of cancer diagnosis (1) and alcohol dependency/ psychiatric illness (2) | |
| Guth in press et al. [44]b | Hormonal therapy | A patient was classified as compliant when she started with the treatment. A patient was classified as persistent when they took their medication for at least 36 months |
Data were collected from the charts of follow-up consultations during which patients were asked about the treatments | In the 80+ group, 87% were compliant and in the 60–79 group 95.5% were compliant. In the group 80+, 83% were persistence and in the group 60–79 88% were persistent. | Descriptive analysis | NA | Of those in the 80+ non-persistent, 13% were non- persistent due to side-effects and 4% for other reasons (2 lack of motivation). Of those aged 60–79, 7% were non-persistent due to side-effects and 5% due to other reasons (nine lack of motivation /resistance, one misinformation by physician, and two alcohol dependency/ psychiatric disease. In the older group, medications were more often discontinued by the physician for serious side-effects | |
| Hoskin et al. [45] | Radiation therapy | No definition provided | Not described | Non-adherence rate is 17/322 | Descriptive analysis | NA | Seventeen patients were non-adherent: three refused to wear the mask needed for the treatment, one was hospitalized for reasons not related to treatment, one was hospitalized for adverse effects of treatment and for ten no reason was defined | |
| Kimmick et al. [46] | Hormonal therapy | Prescription rate: at least one pharmacy filled prescription for a hormonal therapy agent within 1 year of diagnosis. Adherence: a Medication Possession Ratio (MPR) >80%. MPR is defined as the total days covered by the medication/total days needing the medication. Non-persistence = a gap of ≥90 days between medication refills |
Using prescription fill and refill data | Rate of prescription fill was 64% and 70% for those with hormone receptor-positive tumours. The mean MPR was 0.75. Adherence rate: 60% had a MPR of >80% during the first year after the initial prescription. The persistence rate was 80% | Logistic regression analysis | Age, race, comorbidity, number of prescription medications, stage, hormone receptor status, type of surgery, adjuvant chemo received, RT received, urban or rural residence, type of hospital. A separate model for adherence and persistence was calculated | Marital status (non-married (+). | Marital status (non-married +), Charlson comorbidity index of 3 compared with 0 (+), having a regional stage compared with local stage (+). |
| Lash et al. [47]a | Tamoxifen (hormonal therapy) | Self-reported discontinuation of tamoxifen, regardless of reason for stopping at 3, 6, 15, 27, 29, 51 and 63 months after breast cancer surgery | Self-reported use of tamoxifen during telephone interviews | After 5 years, 100 women (31%) had stopped taking Tamoxifen, 16 of those had restarted in the 5 year period | Cox proportional hazard regression analysis | Age, sex, estrogen receptor (ER) status, presence of tamoxifen side-effects, and number of prescription drugs | More prescription medications at baseline (−), new medication during follow-up (−). Severe side-effects at baseline and during follow-up (−). Positive views of tamoxifen (+) | |
| Lau et al. [48] | Chemotherapy and surgery | No definition was provided | Not reported | Twenty-five of 36 (69.4%) were adherent | Not reported, seems descriptive analysis only | NA | For two patients who received only one cycle the reasons are lack of immediate treatment effect, for nine patients who had completed chemo but refused the surgery they had a misconception that after chemo and they had been told their tumour had shrunk, because they were no longer in pain they could wait and perhaps avoid the surgery altogether. | |
| Neugut et al. [49] | Aromatase inhibitors (hormonal therapy) | Non-persistence: a supply gap of minimum 45 days and with no subsequent refills before the end of the study period. Non-adherence: a Medication Possession Ratio of less 80% | Using prescription refill data | Of those aged ≥65 , 24.7% were non-persistent and 8.9% were non-adherent over the 2-year study period | Logistic regression analysis | All study variables (out of pocket costs, number of other prescriptions, type of specialist, age, race, marital status, income, region of United States, and comorbidities) were included. Two models were calculated: one for adherence and one for persistence | A co-payment of $30–89.99 and $90 and more (−) | A co-payment of $30–89.99 and $90 and more (−). Age 84 and over (−), prescription of AI written by primary care specialist or different specialist (−), and increased number of prescriptions (−) |
| Owusu et al. [50] | Tamoxifen (hormonal therapy) | Tamoxifen discontinuation was operationalized as ever discontinuing tamoxifen for >60 days during the initial 5-year tamoxifen prescription | Prescription refill data | Forty-nine percent discontinued Tamoxifen before the 5 year completion | Cox proportional hazard regression analysis | All variables that were significant predictors of tamoxifen discontinuation at P< 0.10 were included in model which included age at diagnosis, race, lymph node involvement, estrogen and progesterone receptor status, and primary therapy received | Aged 75–80 or aged ≥80 compared to those <70 years, ER indeterminate status vs. ER+ (−), having received a breast-conserving surgery without radiation (−) | |
| Partridge et al. [51]c | Chemotherapy/molecular-targeted therapy | Non-persistence: coming off therapy without completing the protocol specified treatment. Non-adherence, if fewer than 80% of doses expected were recorded by the MEMS. A missed dose of capecitabine was defined as no redosing within 20 h of the previous dose, when another dose was planned as per protocol. A dosing violation was defined as taking a dose <8 h or >16 h but <20 h from the previous dose. | MEMS | Eighty-three of patients were persistent. Average adherence across all cycles was 78% | Logistic regression analysis | Age, ethnicity, performance status, tumour size, hormone receptor status | Node-negative disease (−), received mastectomy (−) | The 26 (17%) who did not complete the protocol: 17 had toxicity/adverse effects or complications, 5 withdrew from the study and 2 had disease progression/ relapse and 2 died |
| Partridge et al. [52] | Tamoxifen (hormonal therapy) | Adherence: the proportion of eligible days during the 365 days following the first tamoxifen prescription. Patients with ≥80% days covered is adherent. | Prescription refill data | The overall adherence rate during the first year was 87%. Seventy-seven percent had filled prescriptions to cover ≥80% of the year and were classified as adherent | Logistic regression analysis | All variables were included in multivariable model which included age, race, surgery, visit to oncologist in past year, Charlson Comorbidity Score, other prescription drug use, number of outpatient services use and days hospitalized in the first year | Age 85 and older (−), non-White race (−), having had a mastectomy (−), having seen a seen a medical oncologist before starting tamoxifen (+), increasing Charlson scores (+). | |
| Regnier Denois et al. [53] | Chemotherapy/molecular-targeted therapy | Patient reported non-use of treatment | Focus group and individual interviews | Rate is NR, the majority of patients indicated that they never had forgotten their treatment | NA | NA | Patients reported that a change in their daily routine (such as outing in town, visiting friends or going on holiday) was associated with forgetting their medication (−), side-effects (−), not understanding the prescription (−). Timing of dosages was adjusted for convenience reasons | |
| Ruddy et al. [54]c | chemotherapy/molecular targeted therapy | Persistence with CMF: being prescribed six cycles of at least one of the three CMF drugs. Adherence to oral cyclophosphamide was calculated using the number of doses taken according to the medication calendars divided by the number of doses prescribed. Non-adherent was <80% of expected doses (11 or fewer of the 14 doses per cycle) |
Self-report using medication calendars and case-report forms filled out by study investigators | Sixty-five percent were persistent with CMF. Adherence with cyclophosphamide was 95% | Logistic regression analysis | The significant univariate variables were entered in a stepwise forward model predicting persistence which included only node status and hormone receptor status. A separate model was constructed to examine which grade 3 and 4 side-effects were associated with persistence, the final model included fatigue, vomiting and febrile neutropenia | Non-adherence was not modelled due to the small number of patients who were classified as non-adherent | Node negativity (−) and hormone receptor positive tumours (−), fatigue (−)and febrile neutropenia (−) |
| Shaheen et al. [55] | Luteinizing hormone-releasing hormone agonist (LHRH) | Non-compliance: missing 1 or more injections. Delay: more than 2 weeks after the scheduled time for the injection | Data collected from chart | Fifty-six perecnt were adherent, and 24% had a delay for one or more injections | Descriptive analysis | NA | Reasons for missing appointments were patients were confused about the treatment, long waiting times in clinics, having to travel a long distance to the clinic, clinic was closed due to holidays, and pain and bleeding at injection site | |
| Winterhalder et al. [56] | Chemotherapy/molecular-targeted therapy | Adherence: fully adherent to recommended dosage and intake interval for the duration of treatment | Self-reported intake of capecitabine using diaries which were completed daily | Ninety-one percent were fully adherent. The adherence rate among those with no adverse effects was 95%, and for those with three or more side-effects the adherence was 66.7%) | Not reported | Not reported. | The reasons for making mistakes included forgetting treatment (n = 9), side-effects (n = 4), and misunderstanding instructions (n = 3) There was a trend that those with less adverse events were more adherent (only P = 0.07 provided) |
|
| Ziller et al. [57] | Tamoxifen and anastrazole (hormonal therapy) | A patient was adherent when self-reported and if an MPR of ≥80% was achieved | Self-reported adherence measured using a questionnaire (questions not specified). Prescription checks were done using the charts. | Self-reported adherence 100%, MPR adherence 80% for tamoxifen and 69% for anastrazole using prescription information from charts | Logistic regression analysis | Only univariate models were calculated. Factors examined included age, job training, family risk, having children, tolerability to treatment, medication interruption, side-effects and quality of life | There was no significant predictor for adherence to tamoxifen or anastrazole. |
aThese two publications used data from the same prospective observational cohort study.
bIn these three publications part of the study sample selected from the clinical database is overlapping.
cThese two publications used data from the same companion study of a randomized clinical trial.
RT, radiation therapy; MEMS, microelectronic monitoring system; NA, not applicable; NR, not reported; CMF, cyclophosphamide methotrexate 5-fluorouracil.
patient-related factors
Patient-related factors associated with greater non-adherence and non-persistence were older than 75 years of age [36, 37, 39, 50], older than 84 [49, 52], black race [36], non-White race [52], being unmarried [36], having dementia/Parkinson disease [37], denial of cancer diagnosis/psychiatric illness/alcohol dependency [43, 44], change in normal daily routines [53], not understanding treatment (appointment) instructions [53, 55, 56] or forgetting the treatment [56]. Patient-related factors associated with greater adherence and persistence were younger age [38], being unmarried [46], excellent communication abilities [38], having no comorbidities [40] and having a Charlson Comorbidity Score of ≥3 / or increasing Charlson Comorbidity Scores (meaning adherence increased for each additional point on the Charlson Comorbidity Score) [46, 52].
therapy-related factors
Negative or neutral beliefs about the value of the treatment were associated with greater non-adherence and non-persistence [41, 43], as well as lack of immediate treatment effect and misconceptions about the treatment effect [48], therapy-related side-effects [43–45, 47, 51, 53–56], the treatment equipment itself (e.g. comfort of the mask needed for treatment radiation) [45], use of antidepressants at the time of cancer drug treatment initiation [37], higher number of drug prescriptions [47, 49], having received breast-conserving surgery without radiation [50] or mastectomy [51, 52]. Factors associated with greater adherence and persistence were having positive views about the treatment [47], not having chemo while receiving radiation [40], and having had surgery before radiation [40].
condition-related factors
Condition-related factors associated with greater non-adherence and non-persistence are (number of) hospitalizations [36, 39, 45], having positive lymph nodes [41], lymph node-negative disease [51, 54], Estrogen Receptor (ER) indeterminate status [50], hormone receptor-positive tumours [54] and cancer recurrence [39]. Factors associated with greater adherence were early-stage disease [38], ER+ status [38] and regional cancer stage [46].
health system factors
Factors associated with greater non-adherence included having follow-up appointments with a general practitioner instead of an oncologist [42], prescription for cancer treatment provided by a non-oncologist [49], receiving misinformation about the treatment from the physician [43, 44], long waiting times in the clinics and having to travel long distances to clinics [55]. Factors associated with greater adherence were a higher number of physicians involved in care [38] and having seen an oncologist before the start of treatment [52].
social economic factors
Factors associated with non-adherence and non-persistence included insurance reasons [43] and co-payments of ≥$30 USD [49].
discussion
To our knowledge, this is the first systematic review focusing on adherence to all active cancer treatments in older adults diagnosed with cancer. The reviewed studies represented a diverse range of cancer treatments; however, most focused on adjuvant hormonal therapy for women with early-stage breast cancer. Studies used very different definitions of both adherence and persistence which affected the adherence and persistence rates reported as well as factors associated with adherence and persistence. The WHO has described five groups of factors (patient-related factors, therapy-related factors, condition-related factors, health system factors and social economic factors) that influence treatment adherence and in our review we found evidence supporting each of these groups of factors affecting adherence in this population.
Factors associated with non-adherence were not all consistent across all studies conducted. For example, some studies reported that an age of ≥75 years were associated with non-adherence [36, 37, 39, 50], whereas many studies found no association between age and adherence [38, 42, 46, 47, 49, 51, 54, 57]. Similarly, some studies had conflicting findings about other factors, for example some reported that being unmarried, having several comorbidities or having lymph node-negative disease were associated with higher adherence and persistence [40, 46], while others reported the same factors being associated with greater non-adherence and non-persistence [36, 51,54]. These differences may be due to the different methods of data collection as some of these studies used administrative databases [36, 40, 46], while the other study used data collected within a clinical trial [51, 54]. Another possibility is that the study population within the clinical trial was more motivated to complete the treatment compared with the general older cancer population included in the administrative databases and therefore, factors influencing adherence rates are different. For several other factors, there were more consistent findings across studies. Specifically, hospitalizations, therapy-related side-effects and no visit to a medical oncologist before and during treatment were negatively associated with adherence and persistence [36, 39, 42–45, 47, 49, 51, 52, 56]. The latter finding may be particularly important since it has been reported that there is a referral bias of non-oncologist physicians not referring older adults with cancer to a medical oncologist [58].
What is surprising is that only a few studies examined factors that are known to affect cancer treatment decisions for patients, their families and their health care providers. This includes factors such as the number of hospital visits required for the treatment and travel time to the hospital, which was included in only one study [55]. Furthermore, no study examined classic geriatric factors—such as whether the ability to travel to the cancer treatment centre alone to receive the cancer treatment or the ability to fill the prescription at the pharmacy by themselves or having visual or hearing impairment—were associated with cancer treatment adherence. In addition, only one study examined the impact of economic factors such as co-payments on adherence and persistence [46]. In Canada, 5% of all seniors lived in poverty in 2010 [59]. In addition, the ‘out-of-pocket’ costs for cancer treatment in Ontario, Canada, are substantial, despite having a universal health care system with almost all medications covered by the public health plan. Longo et al. [60, 61] reported that ‘out-of-pocket’ costs for cancer treatment including transportation were on average $585 per month in 2003, and 20% of the studied sample reported that this financial burden was problematic. Although seniors in various jurisdictions might be eligible for publicly funded medication coverage, plans could require co-payments, payment of dispensing fees or only partial coverage of costs. In older adults with comorbidities, these additional costs could add to a significant financial burden that potentially impacts adherence to cancer treatment. Therefore, this needs to be examined in future studies.
More than half of the included studies abstracted data from administrative and clinical databases and/or charts using claim codes and prescription refill data. Although this provides an estimate of when the prescriptions were filled, in most of these studies it was not examined or not possible to examine if the patient actually took the medication according to the treatment plan prescribed. Only the study by Regnier Denois et al. [53] explicitly asked older adults how they managed their capecitabine treatment. Using a qualitative study design, they reported that changes in regular routine (e.g. being out of town for family visits) are an important time when non-adherence to treatment occurs. Furthermore, they showed that that the treatment dosing schedules are being changed by older adults for convenience reasons (e.g. not before meals on an empty stomach but several hours later), which might impact treatment efficacy and safety. It is important that a patient understands the reasons for the treatment and the treatment itself. This should be addressed in patient education sessions by health care providers before and during cancer treatment. Several studies showed that patients’ beliefs about the value of treatment was an important factor influencing adherence and persistence [41, 43, 44, 48, 53] as well is the level of understanding of the treatment instructions [43, 44, 53, 55, 56]. However, only the study by Barron et al. [37] included a proxy measure for dementia/Parkinson disease (based on prescription information). No other study included a measure of cognitive functioning of the older adults or a measure of health literacy. Cognitive impairment and low health literacy are common in older adults in the oncology setting [62, 63], yet it is unclear whether this impacts cancer treatment adherence [64–67]. These are important factors and need to be included in future studies investigating adherence to active cancer treatments in older adults.
Another important issue is comorbidity. In half the included studies, it was not reported what type(s) or how many other chronic health conditions the older adults with cancer had [42–45, 47, 48, 51, 53–57], and only a few studies included the mean number of prescriptions taken [41, 46, 47, 52]. The three studies [38, 46, 52] that did find any association between the number of comorbid conditions and adherence/persistence showed conflicting findings. In these studies, adjuvant hormonal therapy in women with early-stage breast cancer was examined, and two of these studies used administrative databases [46, 52]. Further research is needed to examine how comorbidities and treatments for other chronic conditions affect adherence to active cancer treatment particularly for older adults with other cancers beyond early-stage breast cancer, and with other treatments than hormonal treatment in the adjuvant settings.
Although there have been previous reviews on adherence to some cancer treatments [18, 25–32], these have not focused on all forms of active cancer treatment adherence in the older population. Strengths of this review include the systematic methodology used to identify all relevant articles using two independent reviewers, inclusion of multiple databases and four languages, and not excluding studies based on the quality assessment criteria. This review also has several limitations. Of greatest importance is that the findings are limited by the scientific quality of the studies included. Additionally, we were unable to conduct a meta-analysis due to the heterogeneity of the studies included with regard to assessment methods used, study populations and outcomes.
In conclusion, non-adherence in older adults with cancer was common yet little is known about factors influencing non-adherence in this population, especially for cancer treatments than other hormonal therapy and among older men with cancer. Further studies exploring how older adults manage their cancer treatments are needed, including other forms of cancer treatment such as radiation therapy, chemotherapy and molecular-targeted therapy. Cancer treatment risks and benefits are not the same for the older and younger population [68–74] and this can affect adherence and persistence in older adults with cancer. With the expected increase of the older adult population around the world, and with the preference of both providers and patients for oral agents [26], it is important to understand how older adults manage their treatments at home as well as how cancer treatment adherence is influenced by the treatments for other chronic conditions and age-related changes in functioning for the older population.
funding
This work was supported by a University of Toronto Connaught New Researcher Award awarded to Dr M. Puts.
disclosure
The authors have declared no conflict of interest.
Supplementary Material
references
- 1.Canadian Cancer Society's Steering Committee on Cancer statistics. Canadian Cancer Statistics 2013. Canadian Cancer Society. Toronto, Canada: Canadian Cancer Society; 2013. [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. doi:10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.Sabata E on behalf of the WHO. Adherence to long-term therapies: Evidence for action. Geneva: World Health Organization; 2003. [Google Scholar]
- 4.Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol. 2011;86(6):471–474. doi: 10.1002/ajh.22019. doi:10.1002/ajh.22019. [DOI] [PubMed] [Google Scholar]
- 5.Mazzeo F, Duck L, Joosens E, et al. Nonadherence to imatinib treatment in patients with gastrointestinal stromal tumors: the ADAGIO study. Anticancer Res. 2011;31(4):1407–1409. [PubMed] [Google Scholar]
- 6.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4. doi:10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99(11):1763–1768. doi: 10.1038/sj.bjc.6604758. doi:10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srokowski TP, Fang S, Duan Z, et al. Completion of adjuvant radiation therapy among women with breast cancer. Cancer. 2008;113:22–29. doi: 10.1002/cncr.23513. doi:10.1002/cncr.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117(14):3733–3736. doi: 10.1182/blood-2010-10-309807. doi:10.1182/blood-2010-10-309807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allemani C, Storm H, Voogd AC, et al. Variation in ‘standard care’ for breast cancer across Europe: A EUROCARE-3 high resolution study. Eur J Cancer. 2010;46:1528–1536. doi: 10.1016/j.ejca.2010.02.016. doi:10.1016/j.ejca.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007;25:481–496. doi: 10.2165/00019053-200725060-00004. doi:10.2165/00019053-200725060-00004. [DOI] [PubMed] [Google Scholar]
- 12.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–2388. doi: 10.1200/JCO.2009.26.3087. doi:10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Chief Public Health Officer. The Chief Public Health Officer's annual report on the state of Public Health in Canada 2010: growing older-adding life to years. The Chief Public Health officer. Ottawa, Canada: Public Health Canada; 2010. [Google Scholar]
- 14.Canadian Institute for Health Information. Drug use among seniors on public drug programs in Canada 2002 to 2008. Ottawa, ON: CIHI. 1-7-2012; 2010. [Google Scholar]
- 15.Mangin D, Heath I, Jamoulle M. Beyond diagnosis: rising to the multimorbidity challenge. BMJ. 2012;344:e3526. doi: 10.1136/bmj.e3526. doi:10.1136/bmj.e3526. [DOI] [PubMed] [Google Scholar]
- 16.Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380:7–9. doi: 10.1016/S0140-6736(12)60482-6. doi:10.1016/S0140-6736(12)60482-6. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie CS, Kvale E, Fisch MJ. Multimorbidity: an issue of growing importance for oncologists. J Oncol Pract. 2011;7:371–374. doi: 10.1200/JOP.2011.000460. doi:10.1200/JOP.2011.000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banning M. Adherence to adjuvant therapy in post-menopausal breast cancer patients: a review. Eur J Cancer Care. 2012;21(1):10–19. doi: 10.1111/j.1365-2354.2011.01295.x. doi:10.1111/j.1365-2354.2011.01295.x. [DOI] [PubMed] [Google Scholar]
- 19.Banning M. Older people and adherence with medication: a review of the literature. Int J Nurs Stud. 2008;45:1550–1561. doi: 10.1016/j.ijnurstu.2008.02.009. doi:10.1016/j.ijnurstu.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Hughes CM. Medication non-adherence in the elderly: how big is the problem? Drugs Aging. 2004;21:793–811. doi: 10.2165/00002512-200421120-00004. doi:10.2165/00002512-200421120-00004. [DOI] [PubMed] [Google Scholar]
- 21.Schuz B, Marx C, Wurm S, et al. Medication beliefs predict medication adherence in older adults with multiple illnesses. J Psychosom Res. 2011;70:179–187. doi: 10.1016/j.jpsychores.2010.07.014. doi:10.1016/j.jpsychores.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Pound P, Britten N, Morgan M, et al. Resisting medicines: a synthesis of qualitative studies of medicine taking. Soc Sci Med. 2005;61:133–155. doi: 10.1016/j.socscimed.2004.11.063. doi:10.1016/j.socscimed.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 23.Mishra SI, Gioia D, Childress S, et al. Adherence to medication regimens among low-income patients with multiple comorbid chronic conditions. Health Soc Work. 2011;36:249–258. doi: 10.1093/hsw/36.4.249. doi:10.1093/hsw/36.4.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schlenk EA, Dunbar-Jacob J, Engberg S. Medication non-adherence among older adults: a review of strategies and interventions for improvement. J Gerontol Nurs. 2004;30:33–43. doi: 10.3928/0098-9134-20040701-08. [DOI] [PubMed] [Google Scholar]
- 25.Verbrugghe M, Verhaeghe S, Lauwaert K, et al. Determinants and associated factors influencing medication adherence and persistence to oral anticancer drugs: A systematic review. Cancer Treat Rev. 2013;39:610–621. doi: 10.1016/j.ctrv.2012.12.014. doi:10.1016/j.ctrv.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Given BA, Spoelstra SL, Grant M. The challenges of oral agents as antineoplastic treatments. Semin Oncol Nurs. 2011;27:93–103. doi: 10.1016/j.soncn.2011.02.003. doi:10.1016/j.soncn.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Spoelstra SL, Given CW. Assessment and measurement of adherence to oral antineoplastic agents. Semin Oncol Nurs. 2011;27:116–132. doi: 10.1016/j.soncn.2011.02.004. doi:10.1016/j.soncn.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. doi:10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 29.Foulon V, Schoffski P, Wolter P. Patient adherence to oral anticancer drugs: an emerging issue in modern oncology. Acta Clin Belg. 2011;66:85–96. doi: 10.2143/ACB.66.2.2062525. [DOI] [PubMed] [Google Scholar]
- 30.Barton D. Oral agents in cancer treatment: the context for adherence. Semin Oncol Nurs. 2011;27:104–115. doi: 10.1016/j.soncn.2011.02.002. doi:10.1016/j.soncn.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Palmieri FM, Barton DL. Challenges of oral medications in patients with advanced breast cancer. Semin Oncol Nurs. 2007;23:S17–S22. doi: 10.1016/j.soncn.2007.10.004. doi:10.1016/j.soncn.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Hohneker J, Shah-Mehta S, Brandt PS. Perspectives on adherence and persistence with oral medications for cancer treatment. J Oncol Pract. 2011;7:65–67. doi: 10.1200/JOP.2010.000076. doi:10.1200/JOP.2010.000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. doi:10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. doi:10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 35.Pace R, Pluye P, Bartlett G, et al. Testing the reliability and efficiency of the pilot Mixed Methods Appraisal Tool (MMAT) for systematic mixed studies review. Int J Nurs Stud. 2012;49:47–53. doi: 10.1016/j.ijnurstu.2011.07.002. doi:10.1016/j.ijnurstu.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Barcenas CH, Zhang N, Zhao H, et al. Anthracycline regimen adherence in older patients with early breast cancer. Oncologist. 2012;17:303–311. doi: 10.1634/theoncologist.2011-0316. doi:10.1634/theoncologist.2011-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barron TI, Connolly RM, Bennett K, et al. Early discontinuation of tamoxifen—A lesson for oncologists. Cancer. 2007;109:832–839. doi: 10.1002/cncr.22485. doi:10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 38.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19:322–328. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 39.Dobie SA, Baldwin LM, Dominitz JA, et al. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98(9):610–619. doi: 10.1093/jnci/djj159. doi:10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fesinmeyer MD, Mehta V, Tock L, et al. Completion of radiotherapy for local and regional head and neck cancer in medicare. Arch Otolaryngol Head Neck Surg. 2009;135:860–867. doi: 10.1001/archoto.2009.108. doi:10.1001/archoto.2009.108. [DOI] [PubMed] [Google Scholar]
- 41.Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor–positive breast cancer. J Clin Oncol. 2004;22(16):3309–3315. doi: 10.1200/JCO.2004.11.064. doi:10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 42.Guth U, Huang DJ, Schotzau A, et al. Target and reality of adjuvant endocrine therapy in postmenopausal patients with invasive breast cancer. Br J Cancer. 2008;99(3):428–433. doi: 10.1038/sj.bjc.6604525. doi:10.1038/sj.bjc.6604525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guth U, Myrick ME, Schotzau A, et al. Drug switch because of treatment-related adverse side effects in endocrine adjuvant breast cancer therapy: How often and how often does it work? Breast Cancer Res Treat. 2011;129(3):799–807. doi: 10.1007/s10549-011-1668-y. doi:10.1007/s10549-011-1668-y. [DOI] [PubMed] [Google Scholar]
- 44.Guth U, Myrick ME, Kandler C, et al. The use of adjuvant endocrine breast cancer therapy in the oldest old. Breast. 2013;22(5):863–868. doi: 10.1016/j.breast.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Hoskin PJ, Rojas AM, Saunders MI, et al. Carbogen and nicotinamide in locally advanced bladder cancer: early results of a phase-III randomized trial. Radiother Oncol. 2009;91(1):120–125. doi: 10.1016/j.radonc.2008.10.001. doi:10.1016/j.radonc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Kimmick G, Anderson R, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27(21):3445–3451. doi: 10.1200/JCO.2008.19.2419. doi:10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99(2):215–220. doi: 10.1007/s10549-006-9193-0. doi:10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 48.Lau FW, Choi KT, Wei WI, et al. Induction chemotherapy for oral cancers in a non-compliant patient population. Asian J Surg. 1994;17(3):277–282. [Google Scholar]
- 49.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–2542. doi: 10.1200/JCO.2010.33.3179. doi:10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Owusu C, Buist DS, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26(4):549–555. doi: 10.1200/JCO.2006.10.1022. doi:10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 51.Partridge AH, Archer L, Kornblith AB, et al. Adherence and persistence with oral adjuvant chemotherapy in older women with early-stage breast cancer in CALGB 49907: adherence companion study 60104. J Clin Oncol. 2010;28(14):2418–2422. doi: 10.1200/JCO.2009.26.4671. doi:10.1200/JCO.2009.26.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. doi:10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 53.Regnier DV, Poirson J, Nourissat A, et al. Adherence with oral chemotherapy: results from a qualitative study of the behaviour and representations of patients and oncologists. Eur J Cancer Care. 2011;20(4):520–527. doi: 10.1111/j.1365-2354.2010.01212.x. doi:10.1111/j.1365-2354.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 54.Ruddy KJ, Pitcher BN, Archer LE, et al. Persistence, adherence, and toxicity with oral CMF in older women with early-stage breast cancer (Adherence Companion Study 60104 for CALGB 49907) Ann Oncol. 2012;23(12):3075–3081. doi: 10.1093/annonc/mds133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaheen JA, Amin M, Harty JI. Patient compliance in treatment of prostate cancer with luteinizing hormone-releasing hormone (LHRH) agonist. Urology. 1993;42(5):533–535. doi: 10.1016/0090-4295(93)90265-c. doi:10.1016/0090-4295(93)90265-C. [DOI] [PubMed] [Google Scholar]
- 56.Winterhalder R, Hoesli P, Delmore G, et al. Self-reported compliance with capecitabine: findings from a prospective cohort analysis. Oncology. 2011;80(1–2):29–33. doi: 10.1159/000328317. doi:10.1159/000328317. [DOI] [PubMed] [Google Scholar]
- 57.Ziller V, Kalder M, Albert U-S, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20(3):431–436. doi: 10.1093/annonc/mdn646. doi:10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 58.Townsley C, Pond GR, Peloza B, et al. Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol. 2005;23:3802–3810. doi: 10.1200/JCO.2005.06.742. doi:10.1200/JCO.2005.06.742. [DOI] [PubMed] [Google Scholar]
- 59.Persons in low-income after tax 2006–2010. Ottawa, Canada: 2013. Statistics Canada. [Google Scholar]
- 60.Longo CJ, Deber R, Fitch M, et al. An examination of cancer patients’ monthly ‘out-of-pocket’ costs in Ontario, Canada. Eur J Cancer Care (Engl) 2007;16:500–507. doi: 10.1111/j.1365-2354.2007.00783.x. doi:10.1111/j.1365-2354.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 61.Longo CJ, Fitch M, Deber RB, et al. Financial and family burden associated with cancer treatment in Ontario, Canada. Support Care Cancer. 2006;14:1077–1085. doi: 10.1007/s00520-006-0088-8. doi:10.1007/s00520-006-0088-8. [DOI] [PubMed] [Google Scholar]
- 62.Retornaz F, Monette J, Batist G, et al. Usefulness of frailty markers in the assessment of the health and functional status of older cancer patients referred for chemotherapy: a pilot study. J Gerontol A Biol Sci Med Sci. 2008;63:518–522. doi: 10.1093/gerona/63.5.518. doi:10.1093/gerona/63.5.518. [DOI] [PubMed] [Google Scholar]
- 63.Puts MT, Monette J, Girre V, et al. Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol. 2011;78:138–149. doi: 10.1016/j.critrevonc.2010.04.003. doi:10.1016/j.critrevonc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Amalraj S, Starkweather C, Nguyen C, et al. Health literacy, communication, and treatment decision-making in older cancer patients. Oncology (Williston Park) 2009;23:369–375. [PubMed] [Google Scholar]
- 65.McCarthy DM, Waite KR, Curtis LM, et al. What did the doctor say? Health literacy and recall of medical instructions. Med Care. 2012;50:277–282. doi: 10.1097/MLR.0b013e318241e8e1. doi:10.1097/MLR.0b013e318241e8e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zamora H, Clingerman EM. Health literacy among older adults: a systematic literature review. J Gerontol Nurs. 2011;37:41–51. doi: 10.3928/00989134-20110503-02. doi:10.3928/00989134-20110106-06. [DOI] [PubMed] [Google Scholar]
- 67.Ngoh LN. Health literacy: a barrier to pharmacist-patient communication and medication adherence. J Am Pharm Assoc. 2009;49:e132–e146. doi: 10.1331/JAPhA.2009.07075. doi:10.1331/JAPhA.2009.07075. [DOI] [PubMed] [Google Scholar]
- 68.Papamichael D, Audisio R, Horiot JC, et al. Treatment of the elderly colorectal cancer patient: SIOG expert recommendations. Ann Oncol. 2009;20:5–16. doi: 10.1093/annonc/mdn532. doi:10.1093/annonc/mdn532. [DOI] [PubMed] [Google Scholar]
- 69.Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA) Lancet Oncol. 2012;13(4):e148–e160. doi: 10.1016/S1470-2045(11)70383-7. doi:10.1016/S1470-2045(11)70383-7. [DOI] [PubMed] [Google Scholar]
- 70.Aapro M, Bernard-Marty C, Brain EG, et al. Anthracycline cardiotoxicity in the elderly cancer patient: a SIOG expert position paper. Ann Oncol. 2011;22:257–267. doi: 10.1093/annonc/mdq609. doi:10.1093/annonc/mdq609. [DOI] [PubMed] [Google Scholar]
- 71.Droz J-P, Balducci L, Bolla M, et al. Background for the proposal of SIOG guidelines for the management of prostate cancer in senior adults. Critical Rev Oncol/Hematol. 2010;73(1):68–91. doi: 10.1016/j.critrevonc.2009.09.005. doi:10.1016/j.critrevonc.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Hurria A, Browner IS, Cohen HJ, et al. Senior adult oncology: Clinical practice guidelines in oncology. JNCCN. 2012;10(2):162–209. doi: 10.6004/jnccn.2012.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balducci L. Pharmacology of antineoplastic medications in older cancer patients. Oncology (Williston Park) 2009;23:78–85. [PubMed] [Google Scholar]
- 74.Extermann M. Geriatric oncology: an overview of progresses and challenges. Cancer Res Treat. 2010;42:61–68. doi: 10.4143/crt.2010.42.2.61. doi:10.4143/crt.2010.42.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.