Ki-67 is increasingly being used as a response biomarker in window of opportunity trials for breast cancer. We analyzed 274 patients who received no active treatment in pre-surgical trials and noticed a significant increase in Ki-67 from baseline biopsy to end point surgery in HER2-positive and triple-negative tumors. This association suggests a real increase in cancer proliferation and should be considered in the design and interpretation of pre-surgical studies.
Keywords: Ki 67 antigen, neoadjuvant treatment breast cancer, triple-negative breast cancer, molecular subtype breast cancer, window of opportunity
Abstract
Background
Ki-67 is increasingly being used as a response biomarker in window of opportunity, pre-surgical trials for breast cancer patients. Since Ki-67 is often higher at surgery than at baseline core biopsy in subjects allocated to placebo, we investigated which factors affected this change.
Patients and methods
We retrieved data from 274 patients who received no active treatment in three consecutive pre-surgical trials from a single institution. We assessed the association between changes in Ki-67 from diagnostic biopsy to surgical specimen and the following factors: age, body mass index, tumor prognostic and predictive factors, including immunohistochemical molecular subtype, number and size of biopsy specimens, time from biopsy to surgery, circulating insulin-like growth factor-I, sex hormone-binding globulin and hsCRP.
Results
A total of 269 patients with paired measures of Ki-67 at biopsy and surgery were analyzed. Overall, the mean (±SD) change was 2.2 ± 9.2% after a median interval of 41 days (inter-quartile range 33–48). Molecular subtype was the only factor associated with a significant change of Ki-67 (P = 0.004), with a mean absolute increase of 5.3% [95% confidence interval (CI): 2.3–8.3, P = 0.0005] in estrogen receptor-negative HER2-positive tumors (n = 36) and 5.4% (95% CI: 2.9–7.9, P < 0.0001) in triple-negative tumors (n = 78). No significant change in luminal-A (n = 46), luminal-B (n = 85) and luminal-B HER2-positive (n = 24) tumors was observed.
Conclusions
A significant increase in Ki-67 from baseline biopsy to end point surgery in untreated subjects was ascertained in HER2-positive and triple-negative tumors. This biological association suggests a real increase in cancer proliferation, possibly as a result of a biopsy-driven wound healing effect, and should be considered in the design and interpretation of pre-surgical studies.
Registered clinical trial numbers
ISRCTN86894592; ISRCTN16493703.
introduction
Although the sequential phase I–III model developed in advanced disease for cytotoxic chemotherapy remains a common strategy for drug development, for many new targeted therapies, there are limitations in assessing response by traditional methods, such as response rate defined by RECIST criteria, which may lead to erroneous conclusions about a drug's benefit [1]. A method to circumvent this issue is to assess the efficacy of novel agents, including first-in-humans, phase 0, pre-surgical (window of opportunity) trials during the interval between the diagnostic biopsy and planned surgical resection [2]. The goals of these trials include evaluation of target modulation after drug exposure and pharmacokinetic assessment of a potential anticancer agent. This is at variance with neo-adjuvant trials, in which an investigational agent is given preoperatively along with chemotherapy or hormonal therapy for a longer period of time and the primary end point is pathologic complete response. Both study types may expedite the drug development process by improving the understanding of an agent's biologic effect, validating markers that may predict which subsets of patients will benefit, and targeting patients in subsequent clinical trials.
Ki-67 labeling index modulation has been shown to be an appropriate end point for preoperative studies involving hormonal therapies [3], and a decrease in the pre-surgical levels of Ki-67 serves as an appropriate surrogate marker for outcome in patients who are administered antiestrogen therapy [4, 5] or chemotherapy [6]. However, variability in results can come from the lack of uniformity for measuring Ki-67, including the duration of tissue ischemia, formalin quality, length of fixation and measurement scoring (reviewed in [7]). Interestingly, we noted that Ki-67 is frequently higher in the surgical specimen than in the paired baseline core biopsy in subjects allocated to placebo within different trials [8–10]. This prompted us to investigate the association between several host and tumor factors and the change of Ki-67 in patients participating to three pre-surgical trials from a single institution.
patients and methods
patient selection
The cohort consisted of 181 breast cancer patients enrolled in the placebo arms of three randomized, placebo-controlled, phase II pre-surgical studies conducted at the European Institute of Oncology (EIO), Milan, between February 2004 and February 2011 [8, 9, 11, 12]. The main characteristics of the subjects included in the randomized trials have been published elsewhere [8, 9, 11, 12]. The participant flow diagram is shown in supplementary File S1 available at Annals of Oncology online. Briefly, in the S162 trial, pre-menopausal women with a histologically confirmed at tru-cut biopsy stage I–II ER+ breast cancer were randomized to either tamoxifen 10 mg/week (n = 50) or raloxifene 60 mg/day (n = 50) or placebo (n = 25) [12], whereas post-menopausal women were randomized to either exemestane 25 mg/day (n = 50), or celecoxib 800 mg/day (n = 50) or placebo (n = 25) [11] for 6 weeks before surgery. In the S291 trial, 60 pre- and post-menopausal women with a histologically confirmed stage I–II HER2-positive breast cancer were randomized to either lapatinib 1500 mg/day (n = 29) or placebo (n = 31) and treated for 3 weeks before surgery [9]. Finally, in the S425 trial, 200 women with stage I–IIa breast cancer were randomized to either metformin 850 mg/twice per day (n = 100) or placebo (n = 100) for 4 weeks before surgery [8]. A fourth cohort of 93 breast cancer patients who underwent a tru-cut biopsy and definitive surgery at the EIO but were not eligible for an endocrine treatment because they were triple-negative or pure HER2-positive, or because they refused to participate in these trials, was included. All participants signed an informed consent approved by the EIO Institutional Review Board.
pathology
Estrogen receptor (ER), progesterone receptor (PgR), Ki-67 labeling index and HER2 expression were evaluated by immunohistochemistry, as previously described [13, 14], whereas HER2 was measured according to the FDA recommendation. Specifically, Ki-67 was assessed by two expert pathologists (GP, GV) by IHC according to recent international recommendations [7] using the Mib-1 monoclonal antibody (1:50 dilution; Dako, Denmark), using an automated immunostainer (Dako). We evaluated all the cells in the diagnostic biopsies, and 2000 cells from three high power (×400) microscopic fields randomly selected at the periphery of the tumor in surgical samples, as previously reported [14]. The Ki-67 labeling index was calculated as the percentage of Ki-67 immunoreactive cells over the total number of counted cells. For all the remaining probes used, the immunohistochemical results were scored by recording the percentage of cells showing any definite nuclear (for ER and PgR) and membranous (for HER2) staining. HER2 immunoreactivity assessment was carried out according to the intensity and completeness of cell membrane staining, in a four-tier scale (0–3+), with 2+ cases reflex tested using fluorescence in situ hybridization (PathVysion; Abbott, Chicago, IL). HER2-positive cases were defined as immunohistochemically 3+ or amplified. A molecular subtype classification in five categories was adopted based upon the immunohistochemical assessment of ER, PgR, HER2 and Ki-67 according to the 2011 San Gallen consensus conference [15]: (i) luminal-A, when either one or both of ER and PgR were present, HER2 was not overexpressed/amplified and Ki-67 was <14% of the cells; (ii) luminal-B HER2-negative, when ER and/or PgR were present, Ki-67 was ≥14%, HER2 was not overexpressed/amplified; (iii) luminal-B HER2-positive, same as above but with HER2 amplified or overexpressed; (iv) pure HER2-positive, when ER and PgR were absent and HER2 was overexpressed/amplified, irrespective of the Ki-67 level; (v) triple-negative, when ER and PgR were absent and HER2 was not overexpressed/amplified.
statistical methods
Ki-67 changes from baseline tru-cut biopsy to surgery were analyzed using random effects models, taking into account that data were collected from different studies, so each of the four cohorts was treated as a random factor. We evaluated the relationships between the changes in Ki-67 from core biopsy to surgery and the following host and tumor characteristics: age, body mass index, menopausal status, number and maximum size of biopsy specimens at baseline, histological type, tumor diameter, tumor grade, lymphnodal status, peritumoral vascular invasion, ER, PgR, HER2 IHC expression, molecular subtype, circulating testosterone, estradiol, insulin-like growth factor (IGF)-I, IGF-BP3, sex hormone-binding globulin and hsCRP. Molecular subtype was evaluated in the baseline trucut biopsy specimen to avoid category shifts due to the variations in Ki-67 level within each subject, but a sensitivity analysis was carried out also with the molecular subtype assessed at the time of surgery. All multivariate analyses were adjusted for Ki-67 value at baseline biopsy. We also evaluated the interactions between Ki-67 level at baseline and molecular subtype using F-tests based upon type 3 sums on squares. Subgroups and sensitivity analyses were also carried out to investigate the change in Ki-67 within each molecular subtype and the difference in molecular subtype between biopsy and surgery.
The above-described analyses relied on a normal distribution of the Ki-67 change. Such an assumption was graphically checked on residuals from saturated models, including all significant variables. Analyses with mixed effect models were carried out using PROC MIXED with the SAS Software® (SAS Institute Inc., Cary, NC). Two-sided P values below the 5% threshold were regarded as statistically significant.
Using as an example of window of opportunity trial, the study of lapatinib in HER2-positive breast cancer [9], sample size calculations for new trials based on different scenarios of Ki-67 changes were carried out using PASS 2008 (Hintze J, 2008. NCSS, LLC, Kaysville, UT).
results
The main subject and tumor characteristics are summarized in a supplementary File S2, available at Annals of Oncology online. The median and inter-quartile range (IQR) interval from biopsy to surgery was 41 days (33–48). Overall, the median patient's age was 51, half patients were premenopausal and over 55% had grade 3 tumors. The number and size of baseline biopsy specimens was available in over 50% of the samples.
Overall, the median change in Ki-67 was 0 (IQR, −2, 6) and the mean (±SD) change was 2.2 ± 9.2%. There was no influence of host and tumor characteristics on Ki-67 changes except for molecular subtype, which significantly correlated with the change of Ki-67 from baseline biopsy to end point surgery (P = 0.004, adjusted for baseline Ki-67 level). Table 1 illustrates the median and mean levels of Ki-67 at baseline biopsy and end point surgery and its changes by molecular subtype. As expected, Ki-67 levels were highest HER2-positive and triple-negative tumors. The median change was 0% for all molecular subtypes except for pure HER2-positive tumors, which showed a median absolute increase of 4% (IQR, 0–9%), whereas the mean change in Ki-67, after adjustment for the baseline value, was 5.3% [95% confidence interval (CI): 2.3, 8.3; P = 0.0005] for pure HER2-positive tumors and 5.4% (95% CI: 2.9, 7.9; P < 0.0001) for triple-negative tumors (Figure 1).
Table 1.
Median (IQR) and mean (SD) Ki-67 level at baseline and surgery, change (Δ) and percentage change by molecular subtype
| n | Median Ki-67 (IQR) | Mean (SD) | |
|---|---|---|---|
| Overall | |||
| Baseline biopsy | 269 | 26 (15, 43) | 31.3 (21.3) |
| End point surgery | 28 (17, 42) | 33.4 (22.4) | |
| Change | 0 (−2, 6) | 2.2 (9.2) | |
| %change | 0 (−6.7, 28.6) | 13.58 (45.14) | |
| Luminal-A | |||
| Baseline biopsy | 46 | 10 (7, 11) | 8.8 (2.8) |
| End point surgery | 10 (8, 14) | 10.8 (5.2) | |
| Change | 0 (0, 4) | 1.9 (4.8) | |
| %change | 0 (0, 50) | 29.55 (70.02) | |
| Luminal-B | |||
| Baseline biopsy | 85 | 20 (17, 29) | 24.5 (11.4) |
| End point surgery | 22 (18, 30) | 25 (12.8) | |
| Change | 0 (−5, 4) | 0.5 (10.2) | |
| %change | 0 (−16.67, 22.22) | 6.79 (45.5) | |
| Luminal-B | |||
| HER2-positive | |||
| Baseline biopsy | 24 | 28 (21.5, 35) | 27.5 (9.9) |
| End point surgery | 28 (24, 35) | 29.8 (10) | |
| Change | 0 (−1.5, 7.5) | 2.3 (7) | |
| %change | 0 (−5.26, 32.78) | 15.6 (33.3) | |
| HER2-positive | |||
| Baseline biopsy | 36 | 30 (24.5, 45) | 33.3 (13.8) |
| End point surgery | 35.5 (29.5, 45) | 38.4 (15.8) | |
| Change | 4 (0, 9) | 5.1 (9.4) | |
| %change | 15.2 (0, 31.7) | 18.37 (27.88) | |
| Triple-negative | |||
| Baseline biopsy | 78 | 50 (30, 75) | 52 (22.9) |
| End point surgery | 53.5 (39, 80) | 54.9 (23.3) | |
| Change | 0 (−2, 10) | 2.8 (10.4) | |
| %change | 0 (−3.8, 23.1) | 8.74 (32.31) | |
Figure 1.
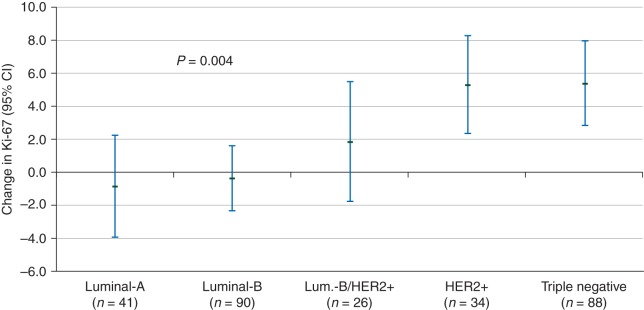
Means and 95% confidence interval of Ki-67 change (end point surgery minus baseline biopsy) by molecular subtype in untreated patients. Estimates of changes are adjusted for baseline Ki-67 value from a random effect model. P values are for molecular subtype. Means are least square means of Ki-67 change from a mixed effect model with each cohort treated as random factor and adjusted for Ki-67 at baseline.
Since the number of luminal-A and B tumors varied between baseline biopsy and surgery as a result of the variations in Ki-67 around the threshold level of 14%, we carried out a sensitivity analysis to look at the association between the molecular subtypes at the time of surgery and the Ki-67 change. The interaction between baseline Ki-67 level and any molecular subtype on Ki-67 change was significant (P = 0.004). Specifically, for luminal-B HER2-negative tumors, women with higher levels of Ki-67 at baseline exhibited a decrease, whereas those with lower baseline levels showed a slight increase in Ki-67 (P = 0.01; data not shown).
Subgroup analysis showed that the change in Ki-67 between triple-negative and HER2-positive tumors was not significantly different (P = 0.76). Likewise, the Ki-67 changes in luminal-A, luminal-B and luminal-B HER2-positive tumors were not significantly different among the three groups. As a result, a subgroup analysis was carried out by pooling subtypes into two classes, with a mean increase in Ki-67 of 5.2% (95% CI: 3.3, 7.1; P < 0.0001) in the triple-negative and pure HER2-positive group versus a mean change of −0.06% (95% CI: −1.6, 1.5; P = 0.9) in the other three molecular subtypes (P = 0.0002 between groups, adjusted for baseline Ki-67). The relationship between the interval from core biopsy to surgery and the change in Ki-67 within HER2-positive and triple-negative tumors is shown in a supplementary File S3, available at Annals of Oncology online. There was a non-significant trend to an increase in Ki-67 over time in both subtypes.
Table 2 illustrates how the sample size in a window of opportunity trial is influenced by the change in the placebo arm. The treatment effect assumption is a 3% absolute decrease in the median Ki-67 between biopsy and surgery based on a prior trial of lapatinib in HER2-positive tumors [9]. If there is no change in Ki-67 in the placebo arm (conventional hypothesis), a sample size of 324 patients per arm is required to have 80% power and a two-sided 5% significance level to detect such an effect. If, however, Ki-67 increases by 3% in the placebo arm, the sample size is reduced to nearly one-quarter (n = 82 per arm) as a result of the increase in the placebo arm (new hypothesis). If the main end point is the percentage changes of Ki-67, the sample size is even smaller.
Table 2.
Sample size calculation for a window-of-opportunity randomized trial in HER2-positive breast cancer according to different Ki-67 changes in the placebo arm
| Main end point | Sample size per arm | Mean change (SD) on placebo | Mean change (SD) on active arm |
|---|---|---|---|
| Absolute change in Ki-67 from baseline | 324 | 0 (9) | −3 (17)a |
| 82 | 3 (9) | −3 (17) | |
| Percentage change in Ki-67 from baseline | 163 | 0 (30) | −10 (34) |
| 42 | 10 (3) | −10 (34) |
aEstimates for the decrease in the active arm are obtained from DeCensi et al. [14].
discussion
We report a significant association between selected breast cancer molecular subtypes and change in Ki-67 from baseline biopsy to end point surgery in subjects who received no treatment in window of opportunity trials. Specifically, Ki-67 significantly increased by an average of 5% in subjects with pure HER2-positive and in triple-negative tumors, whereas there was no change in luminal-A, luminal-B and luminal-B HER2-positive tumors. This specific association suggests a real biological phenomenon such as an increase in cancer proliferation in these cancer subtypes rather than an analytical artefact or a tissue sampling bias.
A previous clinical study in 32 breast cancer patients provides strong evidence for a real biopsy-driven wound healing stimulation of cancer proliferation in aggressive tumor subtypes. Tagliabue et al. [16] compared histological sections of primary breast cancers with residual tumors found in re-excision specimens and found that only the tumors overexpressing HER2 exhibited a 10% absolute Ki-67 increase after a mean interval of 5 weeks, whereas no change was noted in HER2-negative tumors. Moreover, drainage fluids collected from breast cancer patients shortly after surgery were particularly active in stimulating HER2-positive cell lines, whereas wound-induced in vitro proliferation was blunted when these cell lines were treated with trastuzumab before drainage fluid was added. Likewise, changes in gene expression of angiogenesis, proliferation and apoptosis and increased Ki-67 were noted in the control group in a pre-operative trial in ER-positive breast cancer [17], suggesting that local effects of wound healing may influence the interpretation of perioperative window of opportunity trials.
The strengths of our study are the large series of patients from a single institution and the strict compliance with the recommendations of the international working group for Ki-67 assessment in breast cancer, which included our laboratory as a member [7].
A study limitation is the pooling of patients from randomized trials with those who were not eligible or refused to participate in those trials to enrich our series with triple-negative and HER2-positive tumors. In addition, the Ki-67 differences between molecular subtypes may not be completely independent of study characteristics. Random effect models were used to take into account this heterogeneity. However, in order to avoid any selection bias, the results should be validated using independent data, also in light of the known variability of the Ki-67 antigen expression [7]. Moreover, given the different sources of our study populations, prospectively studies adequately designed and powered to investigate the change in Ki67 should confirm our finding.
Our data are at variance with those of Romero et al. [18], who showed a decrease in Ki-67 on surgical samples compared with core biopsy. Reasons for discrepancies with that study may depend on their smaller sample size (50 versus 270 cases), a different scoring system (assessment of hot spots in their study versus mean labeling index at the tumor periphery in our study), the lack of molecular subtype assessment and a crude analysis unadjusted for baseline values.
The implications of our study are threefold. First, interpretation of results of pre-surgical trials should consider the increase in Ki-67 in the placebo arm in HER2-positive and triple-negative tumors, and therefore lead to separated analyses (or trials) between highly proliferating and low proliferating molecular subtypes. In the former subtypes, where the use of a no treatment control arm is mandatory, a blunting of the expected increase in Ki-67 in the experimental arm may be regarded as an indication of activity. For instance, in a pre-surgical trial of metformin, the drug effect on Ki-67 change was evident only in certain subgroups, such as women with insulin resistance and women with HER2-positive tumors, and manifested itself as a blunting of Ki-67 in the metformin arm relative to the increase in the placebo arm [8]. Conversely, in the low proliferating tumor subtypes, the placebo arm might not be necessary given the stability of Ki-67 at least up to a 9-week interval.
A second implication regards the sample size calculation in the study design, which may greatly vary depending on the increase in Ki-67 in the placebo arm in highly proliferating tumors subtypes. Our simulation based on a prior study of lapatinib in HER2-positive tumors [9], where the mean (±SD) absolute pre-post treatment change of Ki-67 was −3 ± 17% in the lapatinib-treated patients, indicates that the sample size can be reduced by four times simply by assuming a 3 ± 9% absolute increase over time in the placebo arm rather than the conventional hypothesis of a 0 ± 9% change.
A third practical implication involves the possibility of tailoring the waiting list for surgery based on the molecular subtype as defined at the core biopsy, so that pure HER2-positive and triple-negative tumors may benefit from a shorter waiting due to their highly proliferating potential. Although there is no evidence that an increase in Ki-67 over a few weeks has a prognostic influence, prior data have suggested that these subtypes, especially triple-negative tumors, may benefit from an adjuvant treatment starting as soon as possible after surgery [19].
In conclusion, we have shown a significant increase in Ki-67 after a 6-week interval from baseline core biopsy to end point surgery in untreated subjects with pure HER2-positive and triple-negative tumors participating in pre-surgical trials. This selective association suggests a real increase in cancer proliferation, possibly as a result of the biopsy-driven wound healing effect, which should be taken into account in designing and interpreting window of opportunity trials.
funding
This work was supported by grants from the Italian Association for Cancer Research (AIRC), Italian League against Cancer (LILT), the Italian Ministry of Health (RF-09), and a contract from the Italian Foundation for Cancer Research (FIRC). AIRC: IG 12072; LILT: 14/08; MINISTRY OF HEALTH: RF-2009-153226.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Schramm N, Englhart E, Schlemmer M, et al. Tumor response and clinical outcome in metastatic gastrointestinal stromal tumors under sunitinib therapy: comparison of RECIST, Choi and volumetric criteria. Eur J Radiol. 2013;82:951–958. doi: 10.1016/j.ejrad.2013.02.034. doi:10.1016/j.ejrad.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 2.Kalinsky K, Hershman DL. Cracking open window of opportunity trials. J Clin Oncol. 2012;30:2573–2575. doi: 10.1200/JCO.2012.42.3293. doi:10.1200/JCO.2012.42.3293. [DOI] [PubMed] [Google Scholar]
- 3.Decensi A, Robertson C, Viale G, et al. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–790. doi: 10.1093/jnci/95.11.779. doi:10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 4.Decensi A, Guerrieri-Gonzaga A, Gandini S, et al. Prognostic significance of Ki-67 labeling index after short-term presurgical tamoxifen in women with ER-positive breast cancer. Ann Oncol. 2010;22:582–587. doi: 10.1093/annonc/mdq427. doi:10.1093/annonc/mdq427. [DOI] [PubMed] [Google Scholar]
- 5.Dowsett M, Smith IE, Ebbs SR, et al. Prognostic value of Ki67 expression after short-term presurgical endocrine therapy for primary breast cancer. J Natl Cancer Inst. 2007;99:167–170. doi: 10.1093/jnci/djk020. doi:10.1093/jnci/djk020. [DOI] [PubMed] [Google Scholar]
- 6.Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. doi:10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 7.Dowsett M, Nielsen TO, A'Hern R, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. doi:10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonanni B, Puntoni M, Cazzaniga M, et al. Dual effect of metformin on breast cancer proliferation in a randomized presurgical trial. J Clin Oncol. 2012;30:2593–2600. doi: 10.1200/JCO.2011.39.3769. doi:10.1200/JCO.2011.39.3769. [DOI] [PubMed] [Google Scholar]
- 9.Decensi A, Puntoni M, Pruneri G, et al. Lapatinib activity in premalignant lesions and HER-2-positive cancer of the breast in a randomized, placebo-controlled presurgical trial. Cancer Prev Res (Phila) 2011;4:1181–1189. doi: 10.1158/1940-6207.CAPR-10-0337. doi:10.1158/1940-6207.CAPR-10-0337. [DOI] [PubMed] [Google Scholar]
- 10.Dowsett M, Bundred NJ, Decensi A, et al. Effect of raloxifene on breast cancer cell Ki67 and apoptosis: a double-blind, placebo-controlled, randomized clinical trial in postmenopausal patients. Cancer Epidemiol Biomarkers Prev. 2001;10:961–966. [PubMed] [Google Scholar]
- 11.Aristarco V, Gandini S, Johansson H, et al. Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research. Philadelphia, PA: AACR; 2011. A randomized, placebo-controlled, phase II, presurgical biomarker trial of celecoxib or exemestane in postmenopausal breast cancer patients. , Orlando, FL, 2–6 April 2011. [DOI] [PubMed] [Google Scholar]
- 12.Serrano D, Lazzeroni M, Gandini S, et al. A randomized phase II pre-surgical trial of weekly low-dose tamoxifen versus raloxifene versus placebo in premenopausal women with estrogen receptor positive breast cancer. Breast Cancer Res. 2013;15:R47. doi: 10.1186/bcr3439. doi:10.1186/bcr3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viale G, Regan MM, Maiorano E, et al. Prognostic and predictive value of centrally reviewed expression of estrogen and progesterone receptors in a randomized trial comparing letrozole and tamoxifen adjuvant therapy for postmenopausal early breast cancer: BIG 1-98. J Clin Oncol. 2007;25:3846–3852. doi: 10.1200/JCO.2007.11.9453. doi:10.1200/JCO.2007.11.9453. [DOI] [PubMed] [Google Scholar]
- 14.Viale G, Giobbie-Hurder A, Regan MM, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: results from Breast International Group Trial 1-98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. doi:10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. doi:10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tagliabue E, Agresti R, Carcangiu ML, et al. Role of HER2 in wound-induced breast carcinoma proliferation. Lancet. 2003;362:527–533. doi: 10.1016/S0140-6736(03)14112-8. doi:10.1016/S0140-6736(03)14112-8. [DOI] [PubMed] [Google Scholar]
- 17.Morrogh M, Andrade VP, Patil AJ, et al. Differentially expressed genes in window trials are influenced by the wound-healing process: lessons learned from a pilot study with anastrozole. J Surg Res. 2012;176:121–132. doi: 10.1016/j.jss.2011.05.058. doi:10.1016/j.jss.2011.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero Q, Bendahl PO, Klintman M, et al. Ki67 proliferation in core biopsies versus surgical samples—a model for neo-adjuvant breast cancer studies. BMC Cancer. 2011;11:341. doi: 10.1186/1471-2407-11-341. doi:10.1186/1471-2407-11-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colleoni M, Bonetti M, Coates AS, et al. Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. The International Breast Cancer Study Group. J Clin Oncol. 2000;18:584–590. doi: 10.1200/JCO.2000.18.3.584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


