One of our major findings is that nonpegylated liposomal doxorubicin (NPLD; Myocet™), an anthracycline with an optimized cardiac safety profile, can be safely combined with trastuzumab: the frequency of cardiac events was extremely low and similar in both arms, with no cardiac deaths registered in the trial. Our study opens a potential new avenue for clinical research.
Keywords: breast cancer; anthracyclines, HER2, cardiac safety, Myocet, trastuzumab
Abstract
Background
Nonpegylated liposomal doxorubicin liposomal doxorubicin, (Myocet™; Sopherion Therapeutics, Inc Canada, and Cephalon, Europe) (NPLD; Myocet®) in combination with trastuzumabHerceptin® (Hoffmann-La Roche) has shown promising activity and cardiac safety. We conducted a randomized phase III trial of first-line NPLD plus trastuzumab and paclitaxel (Pharmachemie B.V.) (MTP) versus trastuzumab plus paclitaxel (TP) in patients with human epidermal growth factor 2 receptor (HER2)-positive metastatic breast cancer.
Patients and Methods
Patients were randomly assigned to NPLD (M, 50 mg/m2 every 3 weeks for six cycles), trastuzumab (T, 4 mg/kg loading dose followed by 2 mg/kg weekly), and paclitaxel (P, 80 mg/m2 weekly) or T + P at the same doses until progression or toxicity. The primary efficacy outcome was progression-free survival (PFS).
Results
One hundred and eighty-one patients were allocated to receive MTP, and 183 to TP. Median PFS was 16.1 and 14.5 months with MTP and TP, respectively [hazard ratio (HR) 0.84; two-sided P = 0.174]. In patients with estrogen receptor (ER)- and progesterone receptor (PR)-negative tumors, PFS was 20.7 and 14.0 months, respectively [HR 0.68; 95% confidence interval (CI) 0.47–0.99]. Median overall survival (OS) was 33.6 and 28.9 months with MTP and TP, respectively (HR 0.79; two-sided P = 0.083). In ER- and PR-negative tumors, OS was 38.2 and 27.9 months, respectively (HR 0.63; 95% CI 0.42–0.93). The frequency of adverse events was higher with MTP, but there was no significant difference in cardiac toxicity between treatment arms.
Conclusion(s)
The trial failed to demonstrate a significant clinical improvement with the addition of M to TP regimen. The clinical benefit observed in an exploratory analysis in the ER- and PR-negative population deserves consideration for further clinical trials.
Clinical trial number
introduction
The human epidermal growth factor 2 receptor (HER2) is a transmembrane tyrosine kinase that regulates cell growth, differentiation, and survival [1]. Overexpression of the HER2 protein or amplification of the HER2 gene occur in ∼20% of breast cancers (BCs) and are associated with poor prognosis [2, 3]. Trastuzumab, a humanized monoclonal antibody, targeting the extracellular domain of the HER2 protein, in combination with chemotherapy, improves disease-free and overall survival (OS) when compared with chemotherapy alone in patients with HER2-positive advanced BC [4]. Additional therapies directed at HER2 have since become available.
In its initial phase III randomized registrational trial, trastuzumab was combined with an anthracycline (doxorubicin or epirubicin) and cyclophosphamide (AC) in patients with no prior anthracycline therapy, or with paclitaxel (P) in patients who had received adjuvant anthracycline [4]. Addition of trastuzumab significantly prolonged time-to-disease progression and OS when compared with chemotherapy alone. Cardiotoxicity, however, was significant, with 16% New York Heart Association (NYHA) Class III–IV heart failure on the trastuzumab–AC arm compared with 3% with AC alone and similarly low rates in both paclitaxel-containing arms. Given the efficacy of anthracyclines in the management of BC, several strategies have been devised ranging from the sequential administration to careful cardiac monitoring [5–7]. Still, administration of trastuzumab with doxorubicin remains problematic and potentially underutilized.
A possible strategy to avoid the increased cardiac risk of the concomitant administration is the use of less cardiotoxic anthracyclines. Nonpegylated liposomal doxorubicin [NPLD, Myocet™, Sopherion (Canada) and Cephalon-Teva] is a nanotechnology product intended to passively accumulate into solid malignancies through gaps in the tumor microvasculature [8], while circumventing cardiac uptake. It has been shown to reduce the cardiac risk associated with conventional doxorubicin therapy in doxorubicin-naïve patients with metastatic BC (MBC), while preserving clinical activity, and is commercially available in Canada and in the EU [9, 10].
The three-drug combinations of NPLD, trastuzumab, and paclitaxel (MTP) was first investigated in a phase I–II trial, as first-line treatment of patients with HER2-overexpressing locally advanced BC or MBC and no prior exposure to anthracyclines, taxanes, or trastuzumab [11]. No patient developed treatment-related symptomatic congestive heart failure (CHF). Asymptomatic protocol-defined cardiac dysfunction was found in 11 (17%) of 54 patients at the recommended dose. Left ventricular ejection fraction (LVEF) recovered to ≥50% in eight patients and to >45% in the remaining three patients. Among 26 patients with MBC, 25 responded, median time to progression was 22.1 months and median OS was 40.4 months.
On the basis of the above results, a prospective, randomized phase III study (STM01-102) was designed in patients with HER2-overexpressing MBC and no prior chemotherapy for metastatic disease.
patients and methods
patients
Women with documented HER2-positive MBC who had received no prior chemotherapy for metastasic disease were eligible. Full eligibility criteria are published online.
study design and treatment
This prospective randomized phase III study aimed to compare the efficacy (progression-free survival, PFS) and safety of trastuzumab and paclitaxel with or without NLPD in patients with HER2-overexpressing MBC who had received no prior chemotherapy for their metastatic disease. Please refer to supplementary Material, available at Annals of Oncology online for further details.
study assessments
Tumor assessments were carried out using RECIST [12] at baseline, following every third cycle, and every 3 months post-treatment until disease progression or initiation of an alternate anticancer therapy. A computed tomography of the chest/abdomen/pelvis was required at baseline. Bone scans were to be done in the presence of symptoms or elevated alkaline phosphatase. Toxicities were graded according to NCI CTCAE, Version 3.0, except for modified lower limits of the normal range for leukocytes (4000 cells/µl), neutrophils (2000 cells/µl), and platelets (100 000 cells/µl). LVEF was measured at baseline and through the study by MUGA scan and echocardiogram. The same method of assessment was to be used in every patient throughout the study. Cardiac death was defined as death due to confirmed CHF, myocardial infarction (MI), documented primary arrhythmia, or sudden unexplained death. Additional secondary safety end points included laboratory abnormalities, worst-grade adverse events (AEs), serious AEs, and deaths.
Tumor response (based on RECIST) was evaluated by the investigators and retrospectively by the independent review committee (IRC) (Beacon Bioscience, Doylestown, PA, USA). The cardiac monitoring plan was developed based on the cardiac safety monitoring plan proposed by the National Surgical Adjuvant Breast and Bowel Project (NSABP) in protocol B31 [10]. LVEF measurements were also assessed blindly by the IRC. All cardiac events were adjudicated by the Cardiac Safety Monitoring Committee (CSMC). A data safety monitoring board periodically reviewed all available safety information.
statistical analysis
The sample size was determined based on a historical median PFS of <8 months with the combination of trastuzumab and paclitaxel, and based on the assumptions that the addition of NPLD would reduce the failure rate by ∼30%. Efficacy end points were analyzed on an intent-to-treat basis. The statistical analysis is published in supplementary Material, available at Annals of Oncology online .
results
patients and treatment exposure
Between July 2006 and March 2009, 363 patients from 83 centers in 12 countries were randomly assigned to receive NPLD plus trastuzumab and paclitaxel (MTP, n = 181) or trastuzumab and paclitaxel (TP, n = 182). Three patients were randomly assigned, but never received any study treatment (supplementary Figure S1, available at Annals of Oncology online). Two patients, randomly assigned to MTP, HER2, and FISH-negative, and one patient, randomly assigned to TP, both decided to receive standard treatment locally. Thus, the safety population consisted of 360 treated patients. As of the 30 June 2010 cut-off date for the primary analysis, the median follow-up time was 31 months. Forty-two (12%) patients were still receiving study treatment, and 318 (88%) had discontinued. The most frequent reasons for discontinuation were investigator-determined progressive disease, patient request, and toxicity. Eight patients, four in each arm, discontinued for a cardiac event. An updated survival analysis was conducted after a median follow-up time of 44 months.
The two treatment groups were well balanced for demographics, pretreatment characteristics, and extent of disease (supplementary Table S1, available at Annals of Oncology on line). In both groups, 62% of the patients had a performance status of 0. One-third of the patients had prior exposure to anthracyclines; 1% and 2% of the patients in the MTP and TP arms, respectively, had received trastuzumab previously.
A full treatment of NPLD therapy (six cycles) could be given to 72% of the patients. The median relative dose intensity of paclitaxel was reduced in the MPT arm when compared with the PT group (76% versus 95%). Primary prophylaxis with hematopoietic growth factors was administered to 124 patients in the MPT arm and 17 in the PT arm.
Post-treatment therapies were balanced between treatment arms. There was no cross-over therapy with NPLD.
efficacy
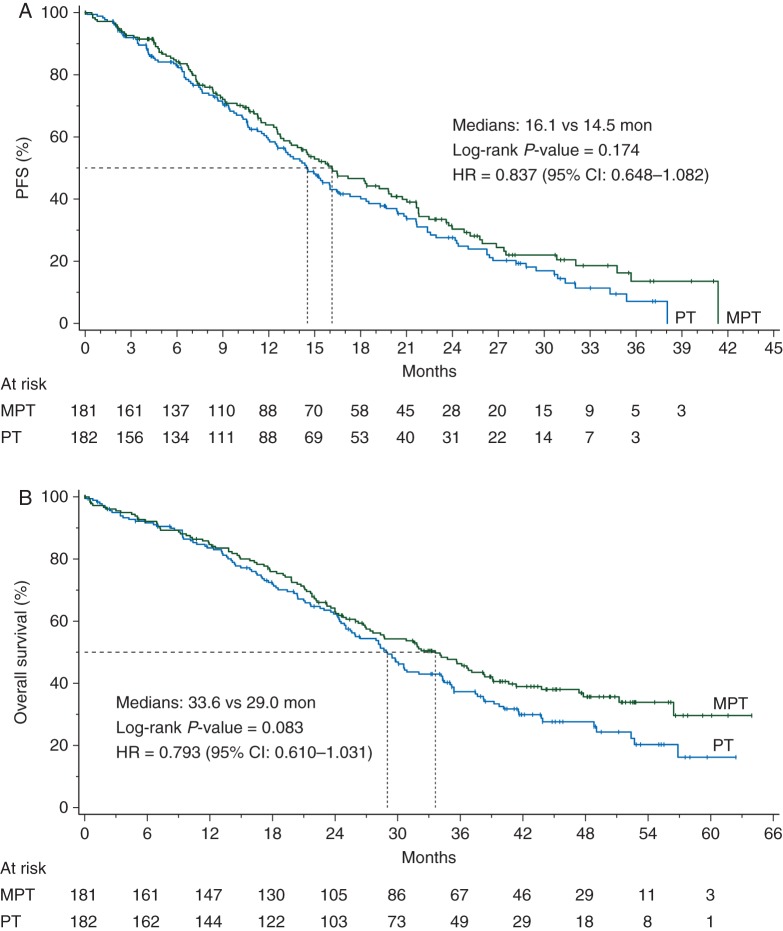
The trial failed to detect a statistically significant difference in the primary end point (Figure 1A and supplementary Table S2, available at Annals of Oncology online). The median PFS by the IRC (237 events) was 16.1 months in the MTP arm versus 14.5 months in the TP arm (P = 0.174).
Figure 1.
Kaplan–Meier estimates of progression-free survival based on (A) independent central review and (B) overall survival (intent-to-treat population). MTP, nonpegylated liposomal doxorubicin + trastuzumab + paclitaxel; TP, trastuzumab + paclitaxel; HR, hazard ratio.
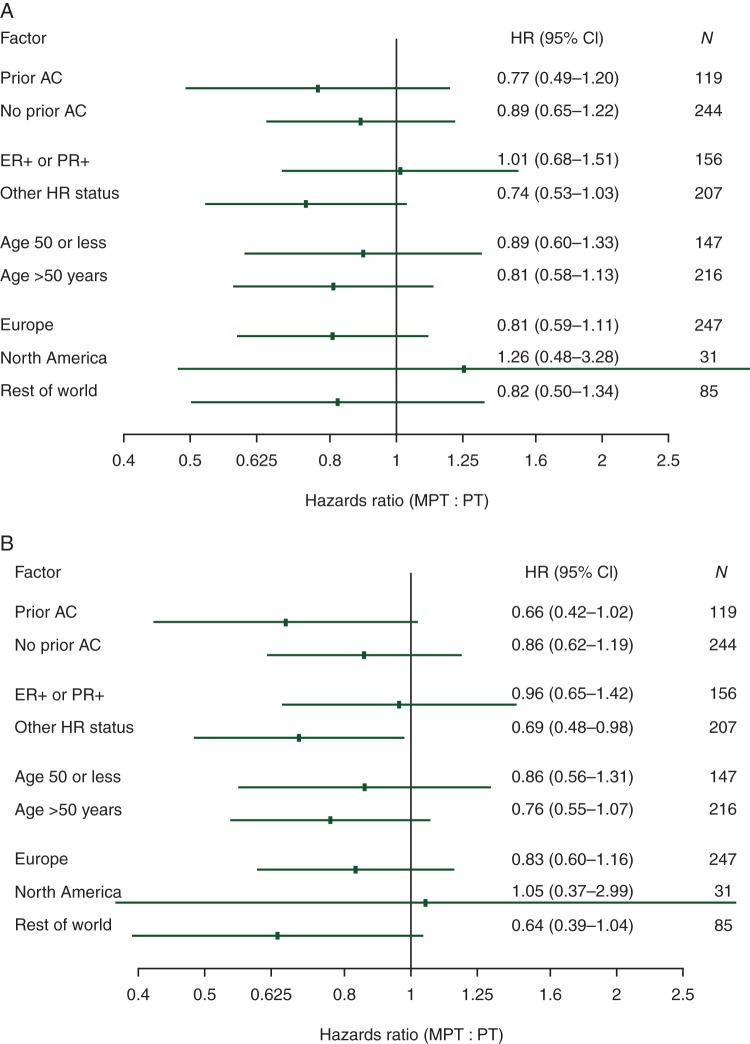
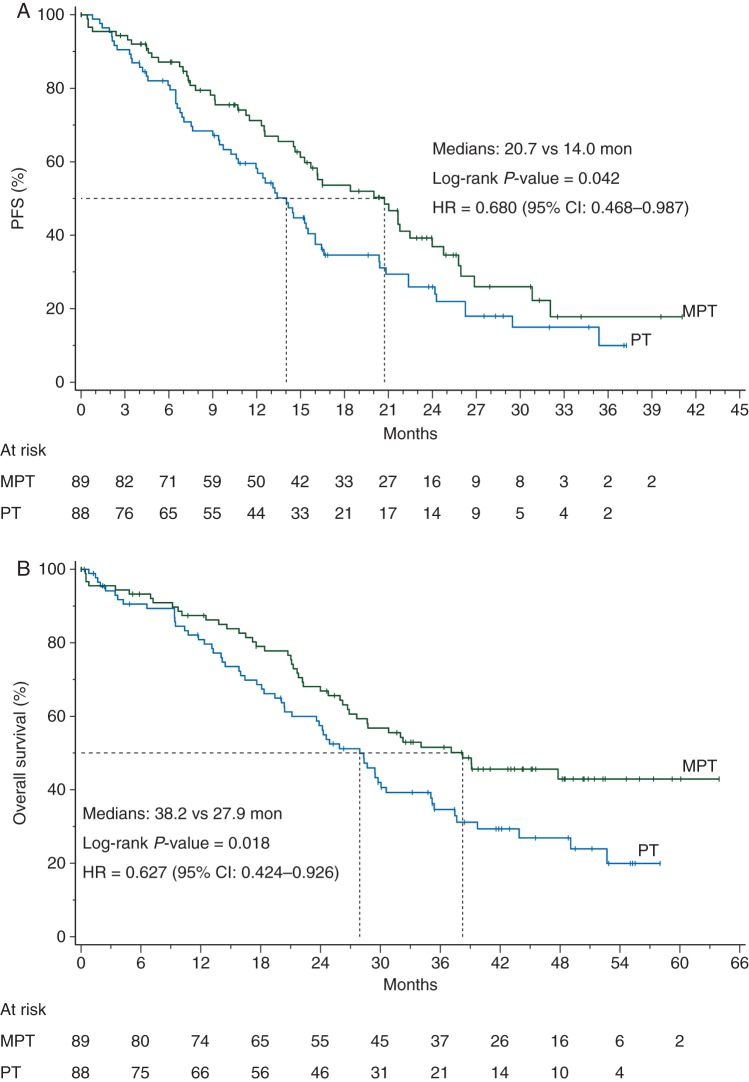
Hormone receptor status appeared to be of predictive significance. There were 177 patients with ER- and PR-negative tumors, 89 in the MTP arm and 88 in the PT, whereas 30 had unknown ER/PR status. Pretreatment characteristics in the subset of patients with ER- and PR-negative tumors were similar in both the MTP and TP arms (data not shown). The PFS analysis by prespecified strata revealed a trend favoring the MTP arm over the control arm in the ER−PR− stratum (also including unknown status), while no difference was detected among the ER+PR+ patients (Figure 2A). The exploratory analysis of PFS restricted to the 177 ER− and PR− patients (excluding unknown status) yielded a median PFS of 20.7 months in the MTP arm and 14.0 months in the TP arm (P = 0.042) (Figure 3A).
Figure 2.
Hazard ratios with 95% confidence intervals of progression-free survival (A) and survival (B) by prespecified stratification factors based on independent central review (intent-to-treat population). MTP, nonpegylated liposomal doxorubicin + trastuzumab + paclitaxel; TP, trastuzumab + paclitaxel; HR, hazard ratio.
Figure 3.
Kaplan–Meier estimates of progression-free survival (A) and survival (B) in patients with ER- and PR-negative tumors. Exploratory analysis excluding patients with undocumented ER and PR status from the prespecified stratum. MTP, nonpegylated liposomal doxorubicin + trastuzumab + paclitaxel; TP, trastuzumab + paclitaxel; HR, hazard ratio.
The interim OS analysis by the cut-off date of 30 June 2010 was based on 175 events, 82 (45%) in the MTP arm and 93 (51%) in the control arm (supplementary Table S2, available at Annals of Oncology online). A total of 30 patients were lost to follow-up for survival, 13 (7.2%) in the MTP group and 17 (9.3%) in the control group. The median survival was 32.3 months in the MTP group and 29.0 months in the TP group (P = 0.195). The final OS analysis, with 225 events, pointed to a trend in OS favoring the MTP arm over the TP arm (Figure 1B and supplementary Table S2, available at Annals of Oncology online), with corresponding medians of 33.6 versus 29.0 months, respectively (P = 0.083). The OS analysis by prespecified strata revealed that most of the apparent OS benefit was derived from the ER−PR− stratum (also including unknown status) (Figure 2B). The exploratory OS analysis restricted to the 177 patients with ER− and PR− patients negative status (excluding unknown status) yielded a median OS of 38.2 months for the MTP arm and 27.9 months for the TP arm (P = 0.018) (Figure 3B).
There was no significant difference in PFS or OS by any of the other prespecified stratification factors, including the use of prior anthracyclines.
Patients who were nonassessable for tumor response (12 in the MTP arm and 13 in the TP arm) were considered nonresponders. Response rates were comparable (67% versus 62%), whereas response duration was numerically longer in the MTP arm than in the control arm (18.1 versus 15.3 months) (supplementary Table S2, available at Annals of Oncology online).
safety
The incidence of NYHA Class III–IV CHF or cardiac death per the blinded review of the CSMC, the primary cardiac toxicity end point, was 3% in the MTP arm and 1% in the control arm. There were no cardiac deaths in the MTP group per that blinded central assessment. The CSMC re-assessed LVEF changes in 297 of 360 (82.5%) patients. The proportion of patients meeting the criteria for treatment discontinuation was 6% in the MTP arm and 4% in the control arm. There were no reports of grade 3–4 myocardial ischemia, grade 3–4 MI, or grade 3–4 arrhythmia in either treatment arm.
Myelosuppression was the most frequent adverse reaction associated with the MTP arm (Table 1). It consisted of severe leucopenia and neutropenia as well as mild-to-moderate anemia and thrombocytopenia. The higher degree of myelosuppression in the MTP arm led to more transfusions, more febrile neutropenia, and more anti-infective therapy, although the incidence of infective episodes was similar in both arms. There was also a higher incidence of predominantly mild-to-moderate stomatitis and gastrointestinal intolerance in the MTP arm as well as more mild-to-moderate liver function tests alterations. Of note, hand–foot syndrome occurred in 8 (4.5%) patients in the MTP arm and 3 (1.7%) in the TP arm, with severe manifestations in a single patient in both arms (grade 3 in both cases). AEs, including treatment-related toxicities and deaths, led to more treatment discontinuations (23% versus 15%, supplementary Figure S1, available at Annals of Oncology online) and hospitalizations (23% versus 15%) in the MTP arm. The incidence of deaths within 30 days of last study therapy was similar in both treatment arms (5% versus 6%).
Table 1.
Most common all-causality adverse events
| Any grade (≥10% of patients) |
Grade 3–4 (≥2% of patients) |
|||||||
|---|---|---|---|---|---|---|---|---|
| MTP (N = 179) |
TP (N = 181) |
MTP (N = 179) |
TP (N = 181) |
|||||
| n | % | n | % | n | % | n | % | |
| Any adverse event | 178 | 99 | 177 | 98 | 159 | 89 | 101 | 56 |
| Leucocytes | 164 | 93 | 107 | 59 | 99 | 56 | 8 | 4 |
| Neutrophils | 163 | 92 | 100 | 56 | 138 | 78 | 15 | 8 |
| Hemoglobin | 153 | 86 | 124 | 69 | 16 | 9 | 8 | 4 |
| Alanine aminotransferase | 129 | 75 | 115 | 65 | 12 | 7 | 5 | 3 |
| Alkaline phosphatase | 127 | 73 | 109 | 61 | 14 | 8 | 10 | 6 |
| Aspartate aminotransferase | 123 | 71 | 99 | 56 | 4 | 2 | 7 | 4 |
| Alopecia | 121 | 68 | 114 | 63 | ||||
| Asthenia | 93 | 52 | 85 | 47 | 32 | 18 | 20 | 11 |
| Nausea | 73 | 41 | 42 | 23 | 4 | 2 | 1 | 1 |
| Diarrhea | 65 | 36 | 51 | 28 | 6 | 3 | 3 | 2 |
| Stomatitis | 59 | 33 | 23 | 13 | 16 | 9 | 2 | 1 |
| Vomiting | 52 | 29 | 23 | 13 | 5 | 3 | 0 | 0 |
| Peripheral edema | 47 | 26 | 47 | 26 | 2 | 1 | 5 | 3 |
| Peripheral neuropathy | 43 | 24 | 55 | 30 | 13 | 7 | 13 | 7 |
| Pyrexia | 39 | 22 | 29 | 16 | 5 | 3 | 1 | 1 |
| Fatigue | 39 | 22 | 28 | 15 | 3 | 2 | 4 | 2 |
| Bilirubin | 38 | 22 | 30 | 17 | ||||
| Decreased appetite | 35 | 20 | 22 | 12 | 6 | 3 | 1 | 1 |
| Peripheral sensory neuropathy | 34 | 19 | 37 | 20 | 15 | 8 | 13 | 7 |
| Headache | 33 | 18 | 28 | 15 | ||||
| Hypertension | 29 | 16 | 19 | 11 | 9 | 5 | 7 | 4 |
| Nail disorders | 29 | 16 | 40 | 22 | 2 | 1 | 8 | 4 |
| Cough | 27 | 15 | 24 | 13 | ||||
| Dyspnea | 25 | 14 | 25 | 14 | 4 | 2 | 4 | 2 |
| Influenza-like illness | 25 | 14 | 16 | 9 | ||||
| Left ventricular dysfunction | 24 | 13 | 17 | 9 | ||||
| Nasopharyngitis | 22 | 12 | 15 | 8 | ||||
| Erythema | 22 | 12 | 18 | 10 | 4 | 2 | 3 | 2 |
| Constipation | 21 | 12 | 15 | 8 | ||||
| Paresthesia | 21 | 12 | 29 | 16 | 2 | 1 | 5 | 3 |
| Epistaxis | 21 | 12 | 26 | 14 | ||||
| Febrile neutropenia | 20 | 11 | 2 | 1 | 19 | 11 | 2 | 1 |
| Pain in extremity | 20 | 11 | 23 | 13 | ||||
| Platelets | 19 | 11 | 9 | 5 | 7 | 4 | 3 | 2 |
| Respiratory tract infection | 19 | 11 | 20 | 11 | ||||
| Rhinorrea | 19 | 11 | 7 | 4 | ||||
| Dyspepsia | 18 | 10 | 11 | 6 | ||||
| Arthralgia | 18 | 10 | 18 | 10 | ||||
| Dry skin | 17 | 9 | 6 | 3 | 5 | 3 | 2 | 1 |
| Onycholysis | 7 | 4 | 3 | 2 | 6 | 3 | 3 | 2 |
discussion
We did not observe a significant improvement in PFS with the addition of NPLD to standard paclitaxel and trastuzumab as first-line therapy for HER2-positive MBC. The hazard ratio (HR) was 0.84 [95% confidence interval (CI) 0.65–1.08; P = 0.174]. Numerically, the median duration of PFS favored the MTP arm (16.1 versus 14.5 months), while PFS in the control arm largely exceeded expectations based on prior experience with trastuzumab and paclitaxel in first-line metastatic HER2-overexpressing MBC [13, 14]. A trend favoring the three-drug combination arm in terms of OS was apparent, with a HR of 0.79 (95% CI 0.61–1.03) and a P-value of 0.083 (supplementary Table S2, available at Annals of Oncology online). The suggested clinical benefit of the MPT regimen appeared to be mostly associated with ER- and PR-negative tumors (Figure 2B), which prompted an unplanned analysis of the data according to these biomarkers. Clinical trials have consistently shown that hormone receptor-negative and hormone receptor-positive BCs are distinct disease entities with different chemotherapy sensitivity [15]. Greater efficacy of anthracycline-based regimens among patients with hormone receptor-negative tumors has been reported by others [16, 17]. Overall, the safety profile of NPLD was consistent with previous clinical experience, and safety continues to appear manageable and acceptable. The most important adverse reactions associated with the MPT arm were myelosuppression and myelosuppression-related complications. One of the main objectives of the trial was to provide conclusive evidence that NPLD could be safely administered concomitantly with trastuzumab. Previous trials had already shown greater cardiac safety with NPLD when compared with doxorubicin [9, 10] as well as its safety in combination with trastuzumab [11]. This trial extends these observations and supports its cardiac safety when administered concomitantly with trastuzumab and paclitaxel. Of note, the overall incidence of hand–foot syndrome in the MPT arm was <5%, and the incidence of severe hand–foot syndrome was 1% in each arm.
Doxorubicin is one of the most active chemotherapeutic agents against BC. Its concomitant use with trastuzumab is limited by confounding cardiac toxicity, warranting the development of anthracyclines with reduced cardiac toxicity for patients with HER2-positive BC. Trial STM01-102 may not have been adequately powered to demonstrate a significant improvement in clinical outcomes with the addition of M to the TP regimen when given as first-line treatment to an unselected patient population with HER2-overexpressing MBC. However, the encouraging PFS and OS results observed in patients with ER- and PR-negative tumors, as well as the cardiac safety of the MTP combination, suggest that NPLD deserves consideration for further clinical trials in HER2-positive BC in order to further define the benefits of this agent versus the potential added costs and associated health risks.
funding
This work was supported by Sopherion Therapeutics.
disclosure
S.F., R.H.G., J.M., and M.R. are employees of Sopherion Therapeutics and have declared stock ownership. N.A. has declared compensated consultancy to Sopherion Therapeutics. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The free supply of trastuzumab by Roche Holding, Switzerland, for part of the trial is kindly acknowledged. This work is dedicated to the memory of Sal Forenza.
references
- 1.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Hudis CA Trastuzumab. Mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. New Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 6.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi G, Albanell J, Eiermann W, et al. Pilot trial of trastuzumab starting with or after the doxorubicin component of a doxorubicin plus paclitaxel regimen for women with HER-2 positive advanced breast cancer. Clin Cancer Res. 2003;9:5944–5951. [PubMed] [Google Scholar]
- 8.Mayer LD, Bally MB, Cullis PR, et al. Comparison of free and liposome encapsulated doxorubicin tumor drug uptake and antitumor efficacy in the SC115 murine mammary tumor. Cancer Lett. 1990;53:183–190. doi: 10.1016/0304-3835(90)90212-g. [DOI] [PubMed] [Google Scholar]
- 9.Harris L, Batist G, Belt R, et al. Liposome-encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first-line therapy of metastatic breast carcinoma. Cancer. 2002;94:25–36. doi: 10.1002/cncr.10201. [DOI] [PubMed] [Google Scholar]
- 10.Batist G, Ramakrishnan G, Rao C, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized, multicenter trial of metastatic breast cancer. J Clin Oncol. 2001;19:1444–1454. doi: 10.1200/JCO.2001.19.5.1444. [DOI] [PubMed] [Google Scholar]
- 11.Cortés J, Di Cosimo S, Climent M, et al. Nonpegylated liposomal doxorubicin (TLC-D99), paclitaxel, and trastuzumab in HER-2-overexpressing breast cancer: a multicenter phase I/II study. Clin Cancer Res. 2009;15:307–314. doi: 10.1158/1078-0432.CCR-08-1113. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Bullock K, Blackwell K. Clinical efficacy of taxane-trastuzumab combination regimens for HER-2-positive metastatic breast cancer. Oncologist. 2008;13:515–525. doi: 10.1634/theoncologist.2007-0204. [DOI] [PubMed] [Google Scholar]
- 14.Gasparini G, Gion M, Mariani L, et al. Randomized phase II trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Res Treat. 2007;101:355–365. doi: 10.1007/s10549-006-9306-9. [DOI] [PubMed] [Google Scholar]
- 15.Andre F, Pusztai L. Molecular classification of breast cancer: implications for selection of adjuvant chemotherapy. Nat Clin Pract Oncol. 2006;3:621–632. doi: 10.1038/ncponc0636. [DOI] [PubMed] [Google Scholar]
- 16.Conforti R, Boulet T, Tomasic G, et al. Breast cancer molecular subclassification and estrogen receptor expression to predict efficacy of adjuvant anthracyclines-based chemotherapy: a biomarker study from two randomized trials. Ann Oncol. 2007;18:1477–1483. doi: 10.1093/annonc/mdm209. [DOI] [PubMed] [Google Scholar]
- 17.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.