Abstract
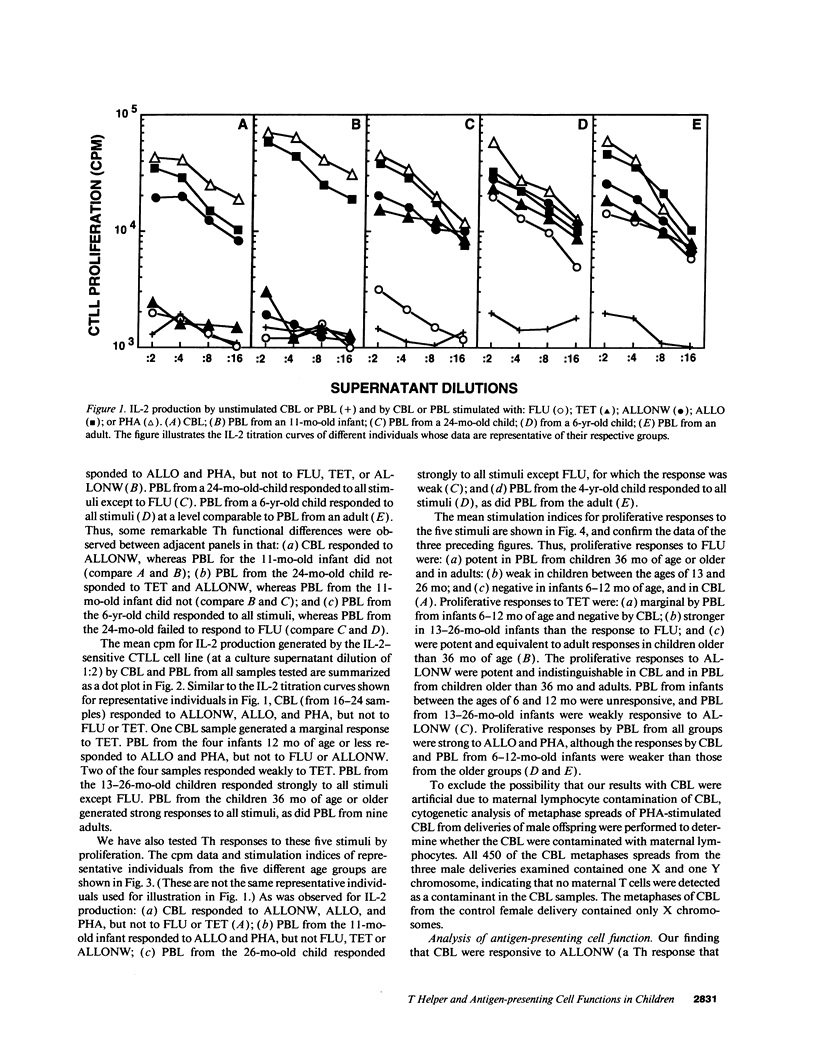
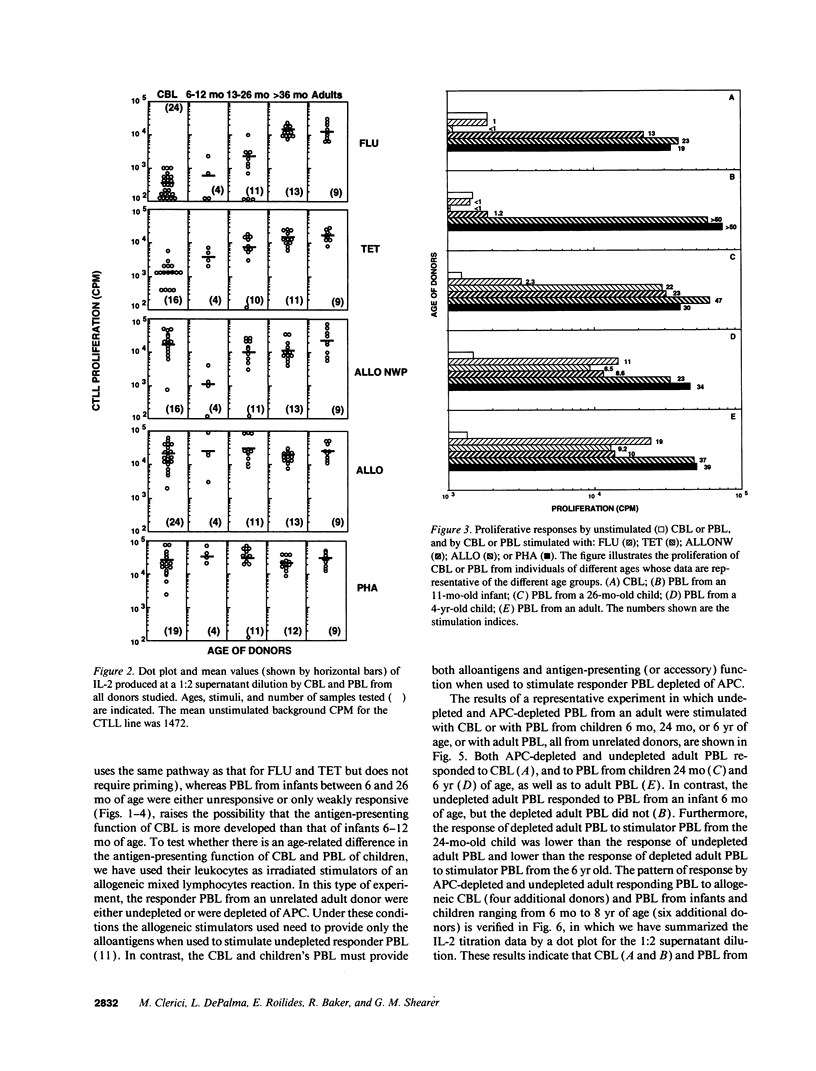
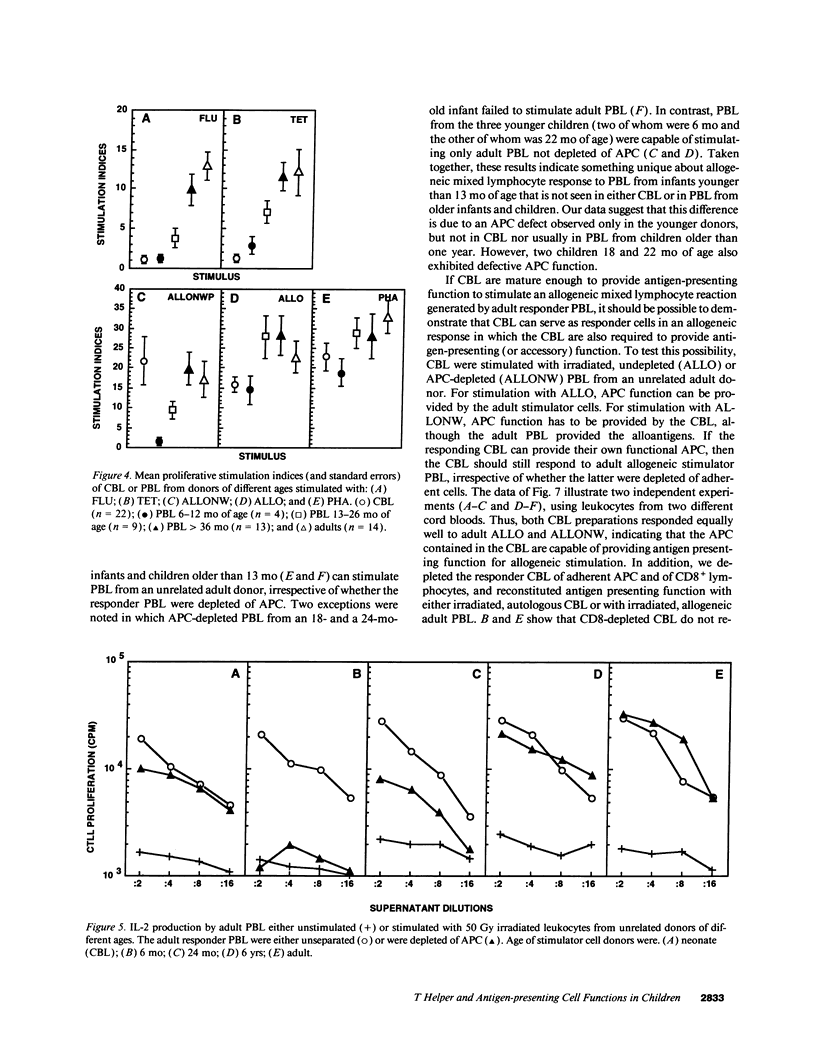
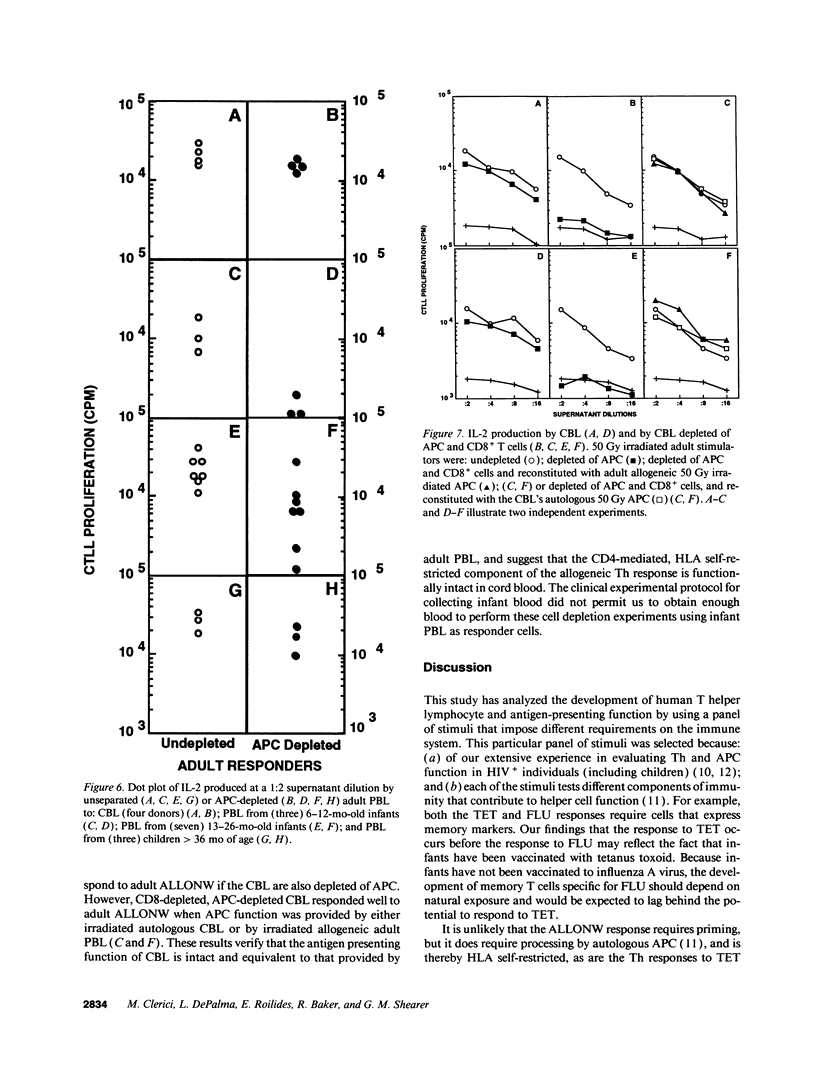
The development of antigen-specific functional T lymphocyte immunity in infants and children is an area of immunology that needs elucidation. Leukocytes from cord blood (CBL) and from PBL of children of different ages who were in the hospital for minor surgical procedures were compared with PBL from healthy adults for their ability to generate T helper cell (Th) responses assessed by in vitro proliferation and IL-2 production after stimulation with: influenza A virus (FLU); tetanus toxoid (TET); adult allogeneic PBL that were either undepleted (ALLO) or depleted of adherent antigen presenting cells (ALLONW); and PHA. CBL generated Th responses to ALLONW, ALLO, and PHA, but not to FLU or TET. PBL from infants between 6 and 13 mo of age responded to ALLO and PHA; none responded to FLU or ALLONW, and two of four responded weakly to TET. PBL from children between 13 and 26 mo of age responded to all stimuli except FLU, to which only one child responded marginally. PBL from children older than 36 mo responded to all stimuli at levels comparable to those of PBL from adults. The use of undepleted and adherent cell-depleted CBL and PBL from children of different ages as allogeneic stimulators of responses generated by PBL from adults indicated that the antigen presenting function of CBL and PBL from children 13 mo or older are sufficiently developed to present alloantigen, whereas PBL from children younger than 13 mo are not. Therefore, our results indicate that age-dependent differences exist in both T helper and antigen-presenting functions of CBL and PBL from children of different ages. Surprisingly, CBL appear to be more efficient in antigen-presenting function than PBL from children younger than 13 mo. These findings are important for establishing developmental parameters of T helper cell immunity relevant for pediatric infection and transplantation in infants and children.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bryson Y. J., Winter H. S., Gard S. E., Fischer T. J., Stiehm E. R. Deficiency of immune interferon production by leukocytes of normal newborns. Cell Immunol. 1980 Sep 15;55(1):191–200. doi: 10.1016/0008-8749(80)90150-1. [DOI] [PubMed] [Google Scholar]
- Bödeker B. G., Kortmann C., Peter H. H., Pichler W. J., Mühlradt P. F. Interleukin-2 in the ontogeny of human lymphoid tissues. Immunobiology. 1982;162(1):66–77. doi: 10.1016/S0171-2985(11)80018-5. [DOI] [PubMed] [Google Scholar]
- Calvelli T. A., Rubinstein A. Pediatric HIV infection: a review. Immunodefic Rev. 1990;2(2):83–127. [PubMed] [Google Scholar]
- Clerici M., Stocks N. I., Zajac R. A., Boswell R. N., Lucey D. R., Via C. S., Shearer G. M. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J Clin Invest. 1989 Dec;84(6):1892–1899. doi: 10.1172/JCI114376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerici M., Via C. S., Lucey D. R., Roilides E., Pizzo P. A., Shearer G. M. Functional dichotomy of CD4+ T helper lymphocytes in asymptomatic human immunodeficiency virus infection. Eur J Immunol. 1991 Mar;21(3):665–670. doi: 10.1002/eji.1830210319. [DOI] [PubMed] [Google Scholar]
- Falloon J., Eddy J., Wiener L., Pizzo P. A. Human immunodeficiency virus infection in children. J Pediatr. 1989 Jan;114(1):1–30. doi: 10.1016/s0022-3476(89)80596-7. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Lee J., Beverley P. C. Ontogeny of expression of UCHL1 antigen on TcR-1+ (CD4/8) and TcR delta+ T cells. Eur J Immunol. 1989 Apr;19(4):771–773. doi: 10.1002/eji.1830190430. [DOI] [PubMed] [Google Scholar]
- Lewis D. B., Yu C. C., Meyer J., English B. K., Kahn S. J., Wilson C. B. Cellular and molecular mechanisms for reduced interleukin 4 and interferon-gamma production by neonatal T cells. J Clin Invest. 1991 Jan;87(1):194–202. doi: 10.1172/JCI114970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucey D. R., Melcher G. P., Hendrix C. W., Zajac R. A., Goetz D. W., Butzin C. A., Clerici M., Warner R. D., Abbadessa S., Hall K. Human immunodeficiency virus infection in the US Air Force: seroconversions, clinical staging, and assessment of a T helper cell functional assay to predict change in CD4+ T cell counts. J Infect Dis. 1991 Oct;164(4):631–637. doi: 10.1093/infdis/164.4.631. [DOI] [PubMed] [Google Scholar]
- Muluk S. C., Clerici M., Via C. S., Weir M. R., Kimmel P. L., Shearer G. M. Correlation of in vitro CD4+ T helper cell function with clinical graft status in immunosuppressed kidney transplant recipients. Transplantation. 1991 Aug;52(2):284–291. doi: 10.1097/00007890-199108000-00019. [DOI] [PubMed] [Google Scholar]
- Roilides E., Clerici M., DePalma L., Rubin M., Pizzo P. A., Shearer G. M. Helper T-cell responses in children infected with human immunodeficiency virus type 1. J Pediatr. 1991 May;118(5):724–730. doi: 10.1016/s0022-3476(05)80033-2. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Makgoba M. W., Sharrow S. O., Stephany D., Springer T. A., Young H. A., Shaw S. Human memory T lymphocytes express increased levels of three cell adhesion molecules (LFA-3, CD2, and LFA-1) and three other molecules (UCHL1, CDw29, and Pgp-1) and have enhanced IFN-gamma production. J Immunol. 1988 Mar 1;140(5):1401–1407. [PubMed] [Google Scholar]
- Schwartz R. H. Acquisition of immunologic self-tolerance. Cell. 1989 Jun 30;57(7):1073–1081. doi: 10.1016/0092-8674(89)90044-5. [DOI] [PubMed] [Google Scholar]
- Via C. S., Tsokos G. C., Stocks N. I., Clerici M., Shearer G. M. Human in vitro allogeneic responses. Demonstration of three pathways of T helper cell activation. J Immunol. 1990 Apr 1;144(7):2524–2528. [PubMed] [Google Scholar]
- Wakasugi N., Virelizier J. L. Defective IFN-gamma production in the human neonate. I. Dysregulation rather than intrinsic abnormality. J Immunol. 1985 Jan;134(1):167–171. [PubMed] [Google Scholar]
- Wilson C. B. The ontogeny of T lymphocyte maturation and function. J Pediatr. 1991 Mar;118(3):S4–S9. doi: 10.1016/s0022-3476(05)82182-1. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Westall J., Johnston L., Lewis D. B., Dower S. K., Alpert A. R. Decreased production of interferon-gamma by human neonatal cells. Intrinsic and regulatory deficiencies. J Clin Invest. 1986 Mar;77(3):860–867. doi: 10.1172/JCI112383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlabinger G. J., Mannhalter J. W., Eibl M. M. Cord blood macrophages present bacterial antigen (Escherichia coli) to paternal T cells. Clin Immunol Immunopathol. 1983 Sep;28(3):405–412. doi: 10.1016/0090-1229(83)90107-1. [DOI] [PubMed] [Google Scholar]


