This study is the largest on treatment and prognosis of primary retroperitoneal sarcomas. One of the most important results is the impact of surgeon's specialization and piecemeal resection on patients’ outcome which strongly emphasizes the necessity of pretherapeutic referral to specialized centers. This study is also the first to consider prognostic factors in histological subtypes separately.
Keywords: retroperitoneal sarcoma, surgery, histology, prognosis
Abstract
Background
Retroperitoneal sarcomas (RPS) are heterogeneous. No previous study has investigated the impact of specialized surgery, evaluated locoregional relapse (LRR), abdominal sarcomatosis and distant metastatic relapse as separate events, or considered histological subtypes separately. This study addresses these specific points in a homogeneous cohort of patients with completely resected primary RPS.
Patients and methods
We conducted a retrospective analysis of adult patients diagnosed with a RPS between 1 January 1988 and 31 December 2008 and eventually referred to one of 12 centers of the French Sarcoma Group. All cases were centrally reviewed by an expert pathologist.
Results
Five hundred eighty-six patients were included. Median follow-up was 6.5 years [95% confidence interval (CI) 5.9–7.1]. Five hundred thirty-seven patients had localized disease and 389 patients (76%) had macroscopically complete resection of the tumor. In this latter group, the 5-year LRR-free survival rate was 46% [41–52] and the 5-year overall survival (OS) rate was 66% [61–71]. In multivariate analysis, gender, adjacent organ involvement, specialization of the surgeon, piecemeal resection and perioperative radiotherapy were independently associated with LRR. Specialization of the surgeon and piecemeal resection were independently associated with abdominal sarcomatosis whereas histology and adjacent organ involvement were independently associated with distant metastasis. Age, gender, grade, adjacent organ involvement and piecemeal resection were significantly associated with OS. Prognostic factors for LRR and OS were analyzed in well-differentiated and dedifferentiated liposarcomas and leiomyosarcomas. Grade 3 was an independent prognostic factor for OS of dedifferentiated liposarcomas.
Conclusion
This study underlines the crucial role of pretherapeutic assessment and meticulous histological examination of RPS as well as the need to consider histological subtypes separately. Surgery in a specialized center and avoidance of piecemeal resection stand out as the two most important prognostic factors for RPS and highlight the importance of treating these patients in specialized centers.
introduction
Retroperitoneal sarcomas (RPS) are a heterogeneous group mainly made up of three histological subtypes: well-differentiated liposarcomas (WDLPS), dedifferentiated liposarcomas (DDLPS) and leiomyosarcomas (LMS). Recent enhancements in molecular techniques have shown that a large proportion of tumors currently diagnosed as DDLPS were previously misdiagnosed as malignant fibrous histiocytoma (MFH) [1].
Surgery is the cornerstone of RPS treatment. Given their large anatomical relations with adjacent organs within the abdominal cavity, the relevance of negative microscopic margins is challenging in RPS [2]. Hence, most series evaluating prognostic factors for RPS define complete resection as removal of all macroscopic disease (R0/R1) in opposition to macroscopically incomplete resections (R2). Other than simple and large resection (including adjacent organ(s) involved by the tumor), compartmental resection is a more liberal, en bloc surgical procedure recently advocated by several experts [3–7] which has been suggested to decrease abdominal relapse risk by 3-fold [4]. However, its best indications remain to be clarified [8, 9], as is the role of adjuvant radiotherapy [6, 9–13].
Overall, prognosis of RPS is poor. LR relapse-free and overall survival (OS) rates are estimated around 50% at 5 years despite complete initial resection, and LR relapse is the main cause of disease-related death [2, 14]. Another specificity of RPS is the risk of developing abdominal sarcomatosis, which results in patient death even in the absence of systemic dissemination.
Most of studies that have investigated prognostic factors in RPS were limited by small sample size, bias resulting from single-center recruitment, heterogeneity caused by inclusion of both primary and recurrent diseases, or lack of histological review with modern molecular methods [10, 12, 15–20], and none considered histological subtypes separately. Several nomograms have been developed but none of them studied the impact of surgeon's specialization or piecemeal resection [21–23]. The aim of this study was to address these specific points in a large and homogeneous cohort of patients with completely resected primary RPS.
patients and methods
We retrospectively analyzed medical charts of patients older than 18 years, treated for a primary RPS between 1 January 1988 and 31 December 2008 and eventually referred to one of the 12 participating centers of the French Sarcoma Group (GSF-GETO). The list of patients originated from the GSF database (Conticabase). Inclusion criteria were (i) data available on initial treatment and follow-up and (ii) no concomitant uncontrolled other cancer. All histological diagnoses were systematically reviewed by an expert pathologist member of the GSF-GETO. Patients with fibrous solitary tumors and other sarcomas of uncertain malignancy were excluded from the analysis. In all cases, radiological, surgical and pathological reports were analyzed.
Patient characteristics at diagnosis included age, gender and performance status (PS). Tumor characteristics included tumor size, histology, grade [24], multifocality, adjacent organ involvement, vascular involvement, abdominal sarcomatosis [25] and distant metastasis.
Characteristics of initial treatment included diagnostic biopsy, pretreatment multidisciplinary team (MDT) assessment, type of surgery [4, 7], specialization of the surgeon, surgical margin status [2], tumor rupture because of piecemeal resection, number of resected organ(s) and tumor infiltration on histological report [26] and perioperative radiotherapy or chemotherapy. (Definitions are detailed in supplementary File, available at Annals of Oncology online.) Follow-up consisted in a CT scan within 3–6 months after surgery and usually every 6 months afterward. This retrospective study was approved by institutional ethics review boards.
statistical analysis
Median follow-up was calculated with reverse Kaplan–Meier method proposed by Shuster [27]. Differences between groups were evaluated with χ2 test or Fisher's exact test or Student's t-test.
Relapse-free survival for each end point and OS were, respectively, defined from diagnosis (biopsy or surgery) to event and death, or last follow-up. Survival rates were estimated with the Kaplan–Meier method [28]. Events competing with the event of interest [locoregional relapse (LRR), abdominal sarcomatosis, distant metastatic relapse] were ‘ignored' whereas patients who did not experience the event of interest were censored at their last follow-up.
Factors associated with complete resection were determined with odd ratios (OR) in the population of operated patients, excluding patients with unknown type of resection. Univariate and multivariate analyses were conducted with a binary logistic regression model using a backward stepwise analysis.
Prognostic factors for LR relapse, sarcomatosis, distant metastasis and OS were selected with the log-rank test in the population of R0/R1 patients [29]. Factors significantly associated with respective end points were included in a multivariate Cox regression analysis using the maximum likelihood method and a backward stepwise analysis [30] (Predefined variables are detailed in supplementary File, available at Annals of Oncology online).
All tests were two sided and P < 0.05 indicated statistical significance. In all cases, the proportional hazards assumption was assessed with Schoenfeld's test [31]. When it was not verified, interaction of covariates with a linear function of time was included in the model and hazard ratios (HRs) were given at 3 and 5 years. Analyses were conducted using commercially available software STATA V.11.
results
Between 1988 and 2008, 586 patients were treated for a primary RPS in 1 of the 12 participating centers. The median follow-up was 6.5 years [5.9–7.1] (Flowchart of patients' distribution in supplementary Figure S1, available at Annals of Oncology online).
characteristics at diagnosis and initial treatment modalities
Initial patient characteristics are given in Table 1. A diagnostic biopsy was done for 40% of the patients and 28% of the cases were discussed by an MDT before treatment. Five hundred thirty-seven patients (92%) had localized disease at diagnosis and 511 (87%) underwent resection. The 26 remaining patients were not operated mainly because of tumor nonresectability estimated by the surgeon (69%) or comorbidities (29%).
Table 1.
Patient characteristics at diagnosis (n = 586)
| No. of patients | % | |
|---|---|---|
| Median age (min–max) | 57 years (18–89) | |
| Gender | ||
| Male | 273 | 47 |
| Female | 313 | 53 |
| PS | ||
| 0 | 378 | 65 |
| 1 | 105 | 18 |
| ≥2 | 31 | 5 |
| NS | 72 | 12 |
| Median tumor size (min–max) | 17 cm (4–85) | |
| Histology | ||
| DDLPS | 244 | 41.5 |
| WDLPS | 135 | 23 |
| LMS | 109 | 18.5 |
| US | 42 | 7 |
| Other | 56 | 10 |
| Grade | ||
| 1 | 152 | 26 |
| 2 | 244 | 41.5 |
| 3 | 179 | 30.5 |
| NS | 11 | 2 |
| Multifocality | ||
| No | 477 | 81.5 |
| Yes | 95 | 16 |
| NS | 14 | 2.5 |
| AO involvement | ||
| No | 314 | 54 |
| Yes | 248 | 42 |
| NS | 24 | 4 |
| Vascular involvement | ||
| No | 485 | 83 |
| Yes | 60 | 10 |
| NS | 41 | 7 |
| Abdominal sarcomatosis | ||
| No | 567 | 97 |
| Yes | 19 | 3 |
| Distant metastasis | ||
| No | 556 | 95 |
| Yes | 30 | 5 |
PS, performance status; NS, not specified; DDLPS, dedifferentiated liposarcoma; WDLPS, well-differentiated liposarcoma; LMS, leiomyosarcoma; US, unclassified sarcoma; AO, adjacent organ.
Type of surgery was a simple (37%), large (39%) or compartmental resection (24%). Two hundred twenty-two patients (43.5%) were operated on by a specialized surgeon, 389 patients (76%) had complete resection of the tumor and 332 patients (65%) had one or more adjacent organ(s) resected with the tumor. At least one of the resected organ(s) was infiltrated by the tumor on histological examination in 157 patients (47%) (Details of organ resections and infiltration patterns in supplementary Tables S1 and S2, available at Annals of Oncology online). Piecemeal resection occurred in 80 patients (15.5%).
Perioperative radiotherapy was decided for 146 patients (29%), mainly postoperatively (74%). The median dose was 50 Gray [0–76]. Eighty-nine patients (17%) received perioperative chemotherapy (median: 5 cycles [1–8]).
factors associated with macroscopically incomplete resection
Among the 511 patients who underwent surgery for a localized RPS, factors significantly associated with R2 resection in multivariate analyses were DDLPS and ‘Other’ histologies, multifocality, adjacent organ involvement, type of surgery (simple resection) and nonspecialization of the surgeon (supplementary Table S3, available at Annals of Oncology online).
survival
Survival and prognosis analyses focused on the homogeneous group of 389 patients who had R0/R1 resection at initial surgery.
LR relapse occurred in 211 patients (54%). The 5-year LR relapse-free survival rate was 46% [41–52].
Seventy-one patients (18%) developed abdominal sarcomatosis and 85 patients (22%) distant metastasis. Distant metastases occurred in the lung (57%), liver (33%) and other locations (35%). When considering the first event, median OS was 1.8 years [1.4–2.4] after abdominal sarcomatosis, and 2.2 years [1.3–2.9] after distant metastasis occurrence (P = 0.1).
At last update, 335 patients (57%) had died. The 5-year OS rate was 66% [61–71]. Median OS was 8 years [6.6–9]. Median OS of patients who did not undergo surgery was 16.2 months [10.2–26.7] and median OS of patients with incomplete initial resection who were not reoperated was 23 months [14–35] (P = 0.94).
prognostic analyses
LR relapse
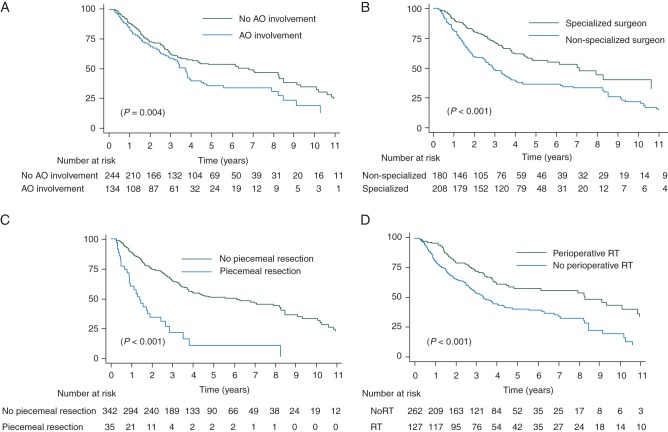
Factors significantly associated with LR relapse in univariate analysis were male gender, histology, grade, adjacent organ involvement, type of surgery, specialization of the surgeon, piecemeal resection and perioperative radiotherapy. In multivariate analysis, male gender, adjacent organ involvement, specialization of the surgeon and piecemeal resection as well as perioperative radiotherapy remained independent (Table 2, Figure 1A–D).
Table 2.
Multivariate analysis of factors significantly associated with risk of LR relapse, abdominal sarcomatosis (at 3 years), distant metastasis and overall survival in patients with initial complete resection of primary localized RPS (n = 389) (reference)
| LR relapse |
Abdominal sarcomatosis |
Distant metastasis |
Overall survival |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | [95% CI] | P | HRa | [95% CI] | P | HR | [95% CI] | P | HR | [95% CI] | P | ||||
| Male gender | 1.5 | [1.1–2.0] | 0.006 | Grade | not | Retained | 0.13 | Histology (WDLPS) | Age ≥ 56 years | 1.4 | [1.0–1.9] | 0.04 | |||
| DDLPS | 2.6 | [1.1–6.2] | <0.001 | ||||||||||||
| LMS | 11.9 | [5.3–27] | |||||||||||||
| AO involvement | 1.6 | [1.2–2.1] | 0.004 | AO involvement | 2 | [1.2–3.5] | 0.009 | US | 3.1 | [0.8–12] | Male gender | 1.7 | [1.3–2.3] | <0.001 | |
| Specialized surgeon | 0.5 | [0.4–0.7] | <0.001 | Specialized surgeon | 0.5 | [0.3–0.9] | 0.02 | Other | 9.6 | [3.8–24] | Grade (1) | ||||
| 2 | 2.1 | [1.4–3.2] | <0.001 | ||||||||||||
| Piecemeal resection | 2.9 | [1.9–4.5] | <0.001 | Piecemeal resection | 4.4 | [2.4–8.1] | <0.001 | AO involvement | 1.6 | [1–2.5] | 0.03 | 3 | 4.1 | [2.7–6.2] | |
| Perioperative RT | 0.5 | [0.4–0.7] | <0.001 | AO involvement | 1.6 | [1.2–2.2] | 0.002 | ||||||||
| Piecemeal resection | 2 | [1.3–3.0] | 0.002 | ||||||||||||
LR, locoregional; HR, hazard ratio; CI, confidence interval; AO, adjacent organ; RT, radiotherapy; WDLPS, well-differentiated liposarcoma; DDLPS, dedifferentiated liposarcoma; LMS, leiomyosarcoma; US, unclassified sarcoma.
aat 2 years.
Figure 1.
Kaplan–Meier locoregional relapse-free survival curves according to adjacent organ involvement (A), specialization of the surgeon (B), piecemeal resection (C) and perioperative radiotherapy (D). LR, locoregional; RT, radiotherapy.
abdominal sarcomatosis
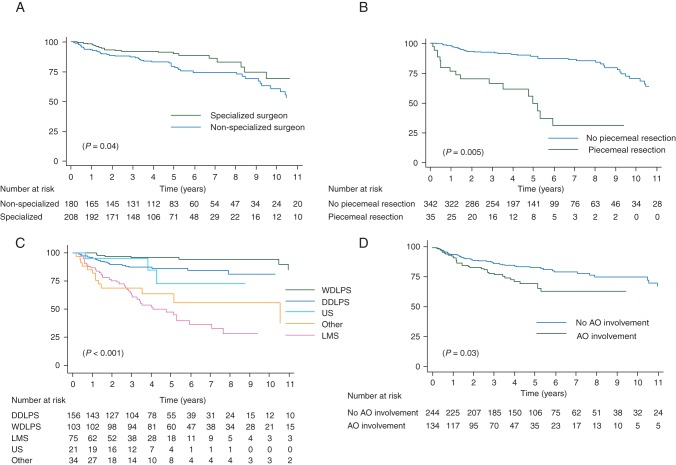
Factors significantly associated with development of abdominal sarcomatosis in univariate analysis were grade, adjacent organ involvement, specialization of the surgeon and piecemeal resection. Because nonproportionality was detected for grade, HRs were calculated at 3 and 5 years. At 3 years, the three last precited factors remained independent (Table 2). At 5 years, only specialization of the surgeon (HR = 0.5 [0.3–0.96], P = 0.04) and piecemeal resection (HR = 3.7 [1.5–9.2], P = 0.005) remained significant (Figure 2A and B).
Figure 2.
Kaplan–Meier sarcomatosis-free survival curves according to specialization of the surgeon (A) and piecemeal resection (B); Kaplan–Meier distant metastasis-free survival curves according to histology (C) and adjacent organ involvement (D). WDLPS, well-differentiated liposarcoma; DDLPS, dedifferentiated liposarcoma; LMS, leiomyosarcoma; US, unclassified sarcoma; LR: locoregional.
distant metastasis
Factors significantly associated with development of distant metastasis in univariate analysis were tumor size ≤17 cm, histology, grade, adjacent organ involvement, vascular involvement and perioperative chemotherapy. In multivariate analysis, histology and adjacent organ involvement remained independent (Table 2, Figure 2C and D).
overall survival
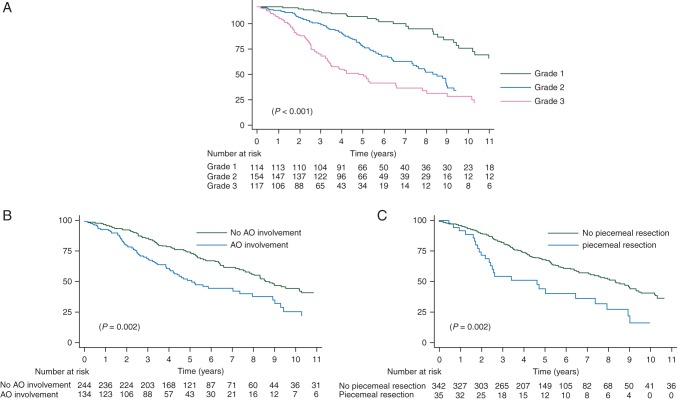
Factors significantly associated with OS in univariate analysis were age, male gender, histology, grade, adjacent organ involvement and piecemeal resection. In multivariate analysis, age, male gender, grade, adjacent organ involvement and piecemeal resection remained independent (Table 2, Figure 3A–C).
Figure 3.
Kaplan–Meier overall survival curves according to grade (A), adjacent organ involvement (B) and piecemeal resection (C). LR, locoregional.
subgroup analysis according to histology
Survival rates according to histology are given in supplementary Table S4, available at Annals of Oncology online. Results of prognostic analyses according to histology are presented in Table 3. Piecemeal resection was predictive of LR relapse for both WDLPS and LMS whereas surgeon specialization and perioperative RT were associated with a lower risk of LR relapse for DDLPS. Adjacent organ involvement was associated with worst OS for both WDLPS and LMS whereas grade 3 was associated with worst OS for DDLPS.
Table 3.
Factors significantly associated with LR relapse and overall survival in multivariate analysis, in patients with initial complete resection of primary localized WDLPS (n = 103), DDLPS (n = 156) and LMS (n = 75), respectively
| LR relapse |
Overall survival |
||||||
|---|---|---|---|---|---|---|---|
| HR | [95% CI] | P | HR | [95%CI] | P | ||
| WDLPS | |||||||
| Tumor size > 17 cm | 2.6 | [1.2–6.0] | 0.02 | Age ≥56 years | 3.3 | [1.5–7.5] | 0.004 |
| Piecemeal resection | 4 | [1.6–9.7] | 0.002 | Multifocality | 3.1 | [1.2–8.3] | 0.02 |
| AO involvement | 2.3 | [0.97–5.5] | 0.05 | ||||
| DDLPS | |||||||
| Specialized surgeon | 0.6 | [0.4–0.9] | 0.01 | Male gender | 1.8 | [1.1–2.8] | 0.014 |
| Perioperative RT | 0.6 | [0.4–0.9] | 0.028 | Grade 3 | 1.8 | [1.1–2.8] | 0.015 |
| Piecemeal resection | 2.3 | [1.2–4.2] | 0.008 | ||||
| LMS | |||||||
| Piecemeal resectiona | 10.1 | [3.3–30.5] | <0.001 | AO involvement | 2.4 | [1.1–5.6] | 0.035 |
LR, locoregional; HR, hazard ratio; CI, confidence interval; WDLPS, well-differentiated liposarcoma; DDLPS, dedifferentiated liposarcoma; AO, adjacent organ; RT, radiotherapy; LMS, leiomyosarcoma.
aSingle significant factor in univariate analysis.
discussion
This study is the largest study published to date on treatment and prognosis of primary RPS. Our results emphasize the crucial role of primary surgery. Indeed, prognosis of patients with R2 resection without subsequent re-excision was as poor as that of patients with localized disease who did not undergo initial surgery [15, 18]. Moreover, we showed no difference between median survival from the occurrence of abdominal sarcomatosis or distant metastasis, confirming the poor prognostic value of abdominal sarcomatosis.
The kidney and the colon were the most often resected organs but infiltration was less frequently observed in the kidney [4, 15, 17–20, 26]. This probably illustrates a more aggressive approach to uninvolved kidneys often encased by the tumor. Conversely, the rate of major vessel resection was only 10% but infiltration by the tumor was found in 67% of cases. These results illustrate the selective nature of adjacent organ(s) resection in surgery of RPS, a consequence of the balance between quality of margins and morbidity, ultimately assessed by the surgeon on an individual basis [7, 9].
The size of our series and the long median follow-up allowed us to identify strong prognostic factors. We confirmed well-recognized impact of tumor grade on OS [4, 12, 15, 17, 18, 20] and that histology is a stronger factor for risk of distant metastasis [12, 18, 20]. For the first time, we showed an impact of grade on survival of DDLPS.
For the first time, we also showed an impact of adjacent organ involvement on survival of WDLPS. WDLPS are usually seen by surgeons as compressing masses that do not infiltrate. Nevertheless, we showed that 33% of WDLPS resected with at least one organ were actually infiltrating. This is consistent with recent work by Mussi et al. [26]. Multifocality was also significantly associated with worse survival of WDLPS. Infiltrative patterns or multifocality can be difficult to identify in WDLPS where macroscopic aspects can be very close to normal fat. These results emphasize the importance of joint assessment of pretherapeutic imaging by trained radiologists and surgeons for optimal treatment of WDLPS.
Above all, our results clearly demonstrated the crucial importance of RPS patients' management in a sarcoma reference center, as suggested by a few previous reports [4, 16, 32]. We showed that piecemeal resection is a highly significant predictor of local relapse, sarcomatosis relapse and poorer OS. A strong correlation between this factor and the number of patients treated per center has been described [4]. Besides, we showed that surgical specialization was by itself a major prognostic factor for complete surgery, LR relapse and abdominal sarcomatosis, and specifically for LR relapse of DDLPS. Compartmental surgery also significantly decreased risk of LR relapse in univariate analysis. However, this variable was not retained in the multivariate model while surgeon specialization remained independent.
Perioperative radiotherapy was associated with a decrease in the risk of LR relapse after adjustment on the main potentially confounding factors. Our results are in agreement with several series [3, 10–12, 33] and reinforce the importance of current prospective trials assessing radiotherapy in RPS such as the ongoing EORTC/Eurosarc phase III randomized trial (NCT01344018) (supplementary Table S5, available at Annals of Oncology online). Moreover, this benefit seemed particularly important for DDLPS.
This retrospective study has several limits. It is associated with selection bias, as we only considered patients referred to a tertiary center at one point of their history. Further, the long inclusion period and its multicentric nature can be associated with heterogeneity, caused by changes in therapeutic approaches over time.
This multicentric study gives an update on primary treatment modalities and patterns of relapse for patients with a primary RPS treated in France over the last 25 years. Our data indicate that diagnosis should include an accurate pretherapeutic assessment, even for WDLPS, and a meticulous histological examination and grading, especially for DDLPS. Systematic histological review appears crucial, as illustrated by the proportion of MFH which was only 7% in our study compared with rates of up to 30% reported in previous series [15]. However, accurate diagnosis and surgical procedure cannot always prevent disease-related death, particularly in sarcomas associated with a high risk of competitive systemic relapse, such as LMS.
By emphasizing the major prognostic role of surgical specialization and piecemeal resection, this study advocates for making RPS treatment in specialized centers mandatory. The first factor improving patients' prognosis is adequate initial treatment. Patients should therefore be referred to sarcoma centers from the initial diagnostic suspicion. Moreover, only grouping of these rare tumors in dedicated centers will allow prospective evaluation of the tailored treatments that we need to develop for each histological subtype of RPS.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors thank Pippa McKelvie-Sebileau for medical editorial assistance, Jean-Baptiste Courrèges, Myriam Jean-Denis and Frédéric Duee for data collection assistance. This study was carried out within the Netsarc (Reference Network for STS Clinical management) and RRePS (Reference Network for STS Pathology) Networks, supported by the French National Cancer Institute (INCa) and partly funded by Grant 'INCa-DGOS-Inserm 6046' from INCa.
references
- 1.Coindre JM, Mariani O, Chibon F, et al. Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: a review of 25 cases initially diagnosed as malignant fibrous histiocytoma. Mod Pathol. 2003;16:256–262. doi: 10.1097/01.MP.0000056983.78547.77. [DOI] [PubMed] [Google Scholar]
- 2.Anaya DA, Lev DC, Pollock RE. The role of surgical margin status in retroperitoneal sarcoma. J Surg Oncol. 2008;98:607–610. doi: 10.1002/jso.21031. [DOI] [PubMed] [Google Scholar]
- 3.Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24–30. doi: 10.1200/JCO.2008.17.8871. [DOI] [PubMed] [Google Scholar]
- 4.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–37. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 5.Bonvalot S, Miceli R, Berselli M, et al. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol. 2010;17:1507–1514. doi: 10.1245/s10434-010-1057-5. [DOI] [PubMed] [Google Scholar]
- 6.Gronchi A, Miceli R, Colombo C, et al. Frontline extended surgery is associated with improved survival in retroperitoneal low- to intermediate-grade soft tissue sarcomas. Ann Oncol. 2012;23:1067–1073. doi: 10.1093/annonc/mdr323. [DOI] [PubMed] [Google Scholar]
- 7.Bonvalot S, Raut CP, Pollock RE, et al. Technical considerations in surgery for retroperitoneal sarcomas: position paper from E-Surge, a master class in sarcoma surgery, and EORTC-STBSG. Ann Surg Oncol. 2012;19:2981–2991. doi: 10.1245/s10434-012-2342-2. [DOI] [PubMed] [Google Scholar]
- 8.Raut CP, Swallow CJ. Are radical compartmental resections for retroperitoneal sarcomas justified? Ann Surg Oncol. 2010;17:1481–1484. doi: 10.1245/s10434-010-1061-9. [DOI] [PubMed] [Google Scholar]
- 9.Pisters PW. Resection of some—but not all—clinically uninvolved adjacent viscera as part of surgery for retroperitoneal soft tissue sarcomas. J Clin Oncol. 2009;27:6–8. doi: 10.1200/JCO.2008.18.7138. [DOI] [PubMed] [Google Scholar]
- 10.Sampath S, Hitchcock YJ, Shrieve DC, et al. Radiotherapy and extent of surgical resection in retroperitoneal soft-tissue sarcoma: multi-institutional analysis of 261 patients. J Surg Oncol. 2010;101:345–350. doi: 10.1002/jso.21474. [DOI] [PubMed] [Google Scholar]
- 11.Heslin MJ, Lewis JJ, Nadler E, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15:2832–2839. doi: 10.1200/JCO.1997.15.8.2832. [DOI] [PubMed] [Google Scholar]
- 12.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–368. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Choi AH, Barnholtz-Sloan JS, Kim JA. Effect of radiation therapy on survival in surgically resected retroperitoneal sarcoma: a propensity score-adjusted SEER analysis. Ann Oncol. 2012;23:2449–2457. doi: 10.1093/annonc/mdr616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stojadinovic A, Yeh A, Brennan MF. Completely resected recurrent soft tissue sarcoma: primary anatomic site governs outcomes. J Am Coll Surg. 2002;194:436–447. doi: 10.1016/s1072-7515(02)01120-1. [DOI] [PubMed] [Google Scholar]
- 15.Kilkenny JW, III, Bland KI, Copeland EM., III Retroperitoneal sarcoma: the University of Florida experience. J Am Coll Surg. 1996;182:329–339. [PubMed] [Google Scholar]
- 16.van Dalen T, Hennipman A, Van Coevorden F, et al. Evaluation of a clinically applicable post-surgical classification system for primary retroperitoneal soft-tissue sarcoma. Ann Surg Oncol. 2004;11:483–490. doi: 10.1245/ASO.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Strauss DC, Hayes AJ, Thway K, et al. Surgical management of primary retroperitoneal sarcoma. Br J Surg. 2010;97:698–706. doi: 10.1002/bjs.6994. [DOI] [PubMed] [Google Scholar]
- 18.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan I, Park SZ, Donohue JH, et al. Operative management of primary retroperitoneal sarcomas: a reappraisal of an institutional experience. Ann Surg. 2004;239:244–250. doi: 10.1097/01.sla.0000108670.31446.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gronchi A, Casali PG, Fiore M, et al. Retroperitoneal soft tissue sarcomas: patterns of recurrence in 167 patients treated at a single institution. Cancer. 2004;100:2448–2455. doi: 10.1002/cncr.20269. [DOI] [PubMed] [Google Scholar]
- 21.Anaya DA, Lahat G, Wang X, et al. Postoperative nomogram for survival of patients with retroperitoneal sarcoma treated with curative intent. Ann Oncol. 2010;21:397–402. doi: 10.1093/annonc/mdp298. [DOI] [PubMed] [Google Scholar]
- 22.Ardoino I, Miceli R, Berselli M, et al. Histology-specific nomogram for primary retroperitoneal soft tissue sarcoma. Cancer. 2010;116:2429–2436. doi: 10.1002/cncr.25057. [DOI] [PubMed] [Google Scholar]
- 23.Gronchi A, Miceli R, Shurell E, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31:1649–1655. doi: 10.1200/JCO.2012.44.3747. [DOI] [PubMed] [Google Scholar]
- 24.Trojani M, Contesso G, Coindre JM, et al. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer. 1984;33:37–42. doi: 10.1002/ijc.2910330108. [DOI] [PubMed] [Google Scholar]
- 25.Rossi CR, Casali P, Kusamura S, et al. The consensus statement on the locoregional treatment of abdominal sarcomatosis. J Surg Oncol. 2008;98:291–294. doi: 10.1002/jso.21067. [DOI] [PubMed] [Google Scholar]
- 26.Mussi C, Colombo P, Bertuzzi A, et al. Retroperitoneal sarcoma: is it time to change the surgical policy? Ann Surg Oncol. 2011;18:2136–2142. doi: 10.1245/s10434-011-1742-z. [DOI] [PubMed] [Google Scholar]
- 27.Shuster JJ. Median follow-up in clinical trials. J Clin Oncol. 1991;9:191–192. doi: 10.1200/JCO.1991.9.1.191. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 29.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 30.Cox DR. Regression modelas and life-tables. J Stat Soc (Series B) 1972;34:187–202. [Google Scholar]
- 31.Schoenfeld DA. Chi-squared goodness of fit tests for the proportional hazards regression model. Biometrika. 1980;67:145–153. [Google Scholar]
- 32.Merchant S, Cheifetz R, Knowling M, et al. Practice referral patterns and outcomes in patients with primary retroperitoneal sarcoma in British Columbia. Am J Surg. 2012;203:632–638. doi: 10.1016/j.amjsurg.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Mussi C, Collini P, Miceli R, et al. The prognostic impact of dedifferentiation in retroperitoneal liposarcoma: a series of surgically treated patients at a single institution. Cancer. 2008;113:1657–1665. doi: 10.1002/cncr.23774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.