Single patients have a worse overall survival when compared with married patients with extremity soft tissue sarcoma. Single patients do not undergo surgical resection or receive radiation therapy as frequently as their married counterparts. Social support systems and barriers to care should be evaluated at time of diagnosis and addressed in single patients to potentially improve survival outcomes.
Keywords: soft tissue sarcoma, discrepancies, survival, marital status
Abstract
Background
Spousal support has been hypothesized as providing important psychosocial support for patients and as such has been noted to provide a survival advantage in a number of chronic diseases and cancers. However, the specific effect of marital status on survival in soft tissue sarcomas (STSs) of the extremity has not been explored in detail.
Patients and methods
A total of 7384 patients were evaluated for this study using a Surveillance, Epidemiology, and End Results (SEER) registry query for patients over 20 years old with extremity STS diagnosed between 2004 and 2009. Survival outcomes were analyzed using Gray's test after patients were stratified by marital status. The Fine and Gray model, a multivariable regression model, was used to assess whether marital status was an independent predictor of sarcoma specific death. Statistical significance was maintained at P < 0.05.
Results
Analysis of the SEER database showed that single patients were more likely to die of their STS and at a faster rate than married patients. No differences were noted in tumor size and tumor site on presentation between married and single patients. However, single patients presented with higher grade tumors more frequently (P = 0.013), received less radiotherapy (P < 0.001), and had less surgery carried out (P < 0.001), compared with their married peers. Regression analysis showed that after accounting for tumor size, grade, site, histology, use of radiotherapy, age, gender, region where the patients were from, and income, being single continued to serve as an independent predictor of sarcoma-specific death; P < 0.0001.
Conclusion
Overall survival is worse for single patients, when compared with married patients, with STS. Single patients do not undergo surgical resection or receive radiation therapy as frequently as their married counterparts. Social support systems and barriers to care should be evaluated at time of diagnosis and addressed in single patients to potentially improve survival outcomes.
introduction
Being married has been shown to play an important role in promoting survival in many different disease processes including some cancers [1–3]. Patients who are married have been reported to have a greater degree of social support, and this has been subsequently implicated in affecting their overall health [4]. Studies have shown that spouses not only serve as pillars of support, in particular providing tremendous emotional support during some of the patient's most difficult times, but also in facilitating the patient's receipt of critical health care services [5, 6]. However, the role of marital status in affecting survival of patients with soft tissue sarcomas (STSs) has not yet been assessed.
STSs are rare tumors with an estimated annual incidence of 11 410 new cases each year [7]. As such, even specialized sarcoma centers only see a relatively small number of patients with STSs. Using data from individual centers usually present inherent disadvantages—the continued evolution of sarcoma care prevents grouping patients together over long periods of time (>10 years) as relevant trends and the ability to analyze details of patient care can be distorted [8]. Thus, in order to analyze pertinent trends with adequate powers, a large national database, Surveillance, Epidemiology, and End Results (SEER) database [9], was used to evaluate discrepancies in survival trends among single versus married patients.
Our primary objectives were to identify any survival discrepancies that might exist between single and married patients and to analyze if marital status served as an independent predictor of death after accounting for possible confounders in some tumor and sociodemographic-related factors. Secondary objectives included analyzing for discrepancies in tumor characteristics between single and married patients at disease presentation and identifying factors contributing to death due to sarcoma.
methods
data collection
The SEER database was queried for this study. SEER has been continuously collecting data since 1973 from 18 different registries that represent ∼28% of the United States population [10]. Inclusion criteria for our study included patients aged >20 years, diagnosis of a STS made between 2004 and 2009 and tumor site limited to the upper limb, pelvis, and lower limb. Following exclusion of patients with limited information, a total of 7384 patients were identified and analyzed in this study.
Demographic data collected included age at time of diagnosis, gender, marital status, and the median family income. Gender was reported as either male or female. Marital status was reported as either being married (married, domestic partner) or single (single, widowed, divorced, separated). The median family income was adjusted for the cost of living (COL) in the year 2000 and was listed by SEER for each patient.
Pathologic characteristics, treatment and outcome status collected include tumor site, tumor size, tumor grade, tumor histology, whether radiation was administered, surgery status, and survival status as of 31 December 2009. Tumor site was reported as upper limb and shoulder, lower limb and hip, or pelvis. Tumor grades were reported as either being: Grade 1, well-differentiated (low grade); Grade 2, moderately differentiated (intermediate grade); Grade 3, poorly differentiated (high grade); Grade 4, undifferentiated anaplastic. Due to the multiple histological subtypes of STS and to allow for statistical analysis with regression models, tumor histology was categorized as follows: liposarcoma, leiomyosarcoma, malignant fibrous histiocytoma/pleomorphic undifferentiated sarcoma, and others. Radiation received was reported as either yes or no. Patients were grouped according to their surgery status as follows: surgery was recommended and carried out, surgery was not recommended, surgery recommended but not carried out, and surgery could not be carried out. Survival status was current as of 31 December 2009 (when SEER ended the collection of data) and was reported as either alive, dead due to disease, or dead due to other causes.
To exclude patients who may have had disseminated disease on presentation, only patients who subsequently underwent a surgical resection after presentation (n = 6243) were included for survival curve and regression analyses. Multivariable regression analysis examined marital status as the main independent predictor of sarcoma-specific death after controlling for age, gender, region where the patients were from, tumor size, tumor grade, tumor site, histology subtype, radiation use, and income.
statistical methodologies
Demographic and clinical variables including risk factors were summarized and compared for the two groups. Descriptive summaries of continuous variables were presented in terms of median and interquartile range, whereas discrete variables were summarized in terms of frequencies and percentage. Wilcoxon rank-sum tests and χ2 test were used to do statistical comparisons. The end point of the study was designated as death due to sarcoma. Death was treated as a competing risk for patients who died from a cause not directly related to their STS. Gray's test was calculated and used to compare the disease-specific cumulative incidence of death [11, 12]. In addition to univariate comparisons, Fine and Gray's model, a multivariable regression analysis, was used to take into account potential confounders [13]. Statistical software R (version 1.11.1, www.r-project.org) was used for all data analysis. Reported P-values were two-sided and a P-value of < 0.05 was considered to indicate statistical significance.
results
The initial 7384 patients identified from the SEER database were grouped into either being married (married, domestic partner) or being single (single, widowed, divorced, separated). There were 4407 in the married group and 2977 in the single group (Table 1). No statistically significant differences were found in the tumor site or size. Significant differences were found in sex (a larger proportion of single patients were female; 57% of single patients were females, while only 39% of married patients were females; P< 0.001), tumor grade (a greater proportion of patients in the married group had Grade 1 tumors, whereas that of patients in the single group had Grade 4 tumors; P= 0.01), receipt of radiation (a greater proportion of married patients underwent radiation when compared with single patients—44% versus 39%; P < 0.001), surgery status (a higher proportion of patients in the married group had surgery carried out when compared with the single group—87% versus 82%; P< 0.001), and survival status (a larger proportion of patients in the single group died due to their sarcoma when compared with the married group—20% versus 15%; P< 0.001). Additionally, statistically significant differences were found in histological subtype of tumor, age at time of diagnosis, and COL-adjusted median family income; however, the differences were small and were deemed to not be clinically significant.
Table 1.
Demographics and tumor characteristics of patients classified by marital status
| Variable | Single (N = 2977) | Married (N = 4407) | P-value |
|---|---|---|---|
| Age, years, median (IQR) | 62 (45–78) | 61 (48–73) | 0.016 |
| Sex, n (%) | |||
| Male | 1274 (43) | 2693 (51) | <0.001 |
| Female | 1689 (57) | 1722 (39) | |
| COL-adjusted median family income | 51 950 (45 730, 59 410) | 51 580 (45 540, 57 040) | <0.001 |
| Site, n (%) | |||
| Lower limb and hip | 1697 (60) | 2550 (60) | 0.165 |
| Pelvis | 542 (19) | 818 (19) | |
| Upper limb and shoulder | 596 (21) | 875 (21) | |
| Size, cm, median (IQR) | 8.4 (4.5–13.5) | 8.0 (4.5–13.0) | 0.25 |
| Grade (%) | |||
| I | 385 (18) | 719 (21) | 0.013 |
| II | 375 (17) | 594 (17) | |
| III | 527 (24) | 799 (23) | |
| IV | 886 (41) | 1293 (38) | |
| Histology type, n (%) | |||
| Liposarcoma | 604 (23) | 1104 (28) | 0.001 |
| Leiomyosarcoma | 466 (18) | 700 (17) | |
| MFH/PUS | 624 (23) | 889 (22) | |
| Others | 965 (36) | 1313 (33) | |
| Radiation therapy, n (%) | |||
| Yes | 1147 (39) | 1937 (44) | <0.001 |
| No | 1794 (61) | 2465 (56) | |
| Surgery status, n (%) | |||
| carried out | 2436 (82) | 3807 (87) | <0.001 |
| Not recommended | 415 (42) | 490 (11) | |
| Could not be carried out | 46 (2) | 35 (1) | |
| Recommended but not carried out | 61 (2) | 58 (1) | |
| Survival status, n (%) | |||
| Alive | 1942 (65) | 3282 (74) | <0.001 |
| Died of disease | 605 (14) | 659 (15) | |
| Died of other causes | 430 (14) | 466 (11) | |
COL, cost of living; MFH, malignant fibrous histiocytoma; PUS, pleomorphic undifferentiated sarcoma.
At 5 years of follow-up, 432 of the 3807 (11%) married patients and 353 of the 2436 (14%) single patients had died due to their sarcoma. A comparison of the proportion who died from the disease between the two groups, as illustrated in Table 1, also shows the differences to be statistically significant (P< 0.001). After factoring in time, the results indicate that the time to death due to sarcoma is also different between single and married patients; P< 0.0001, Gray's test (Figure 1).
Figure 1.
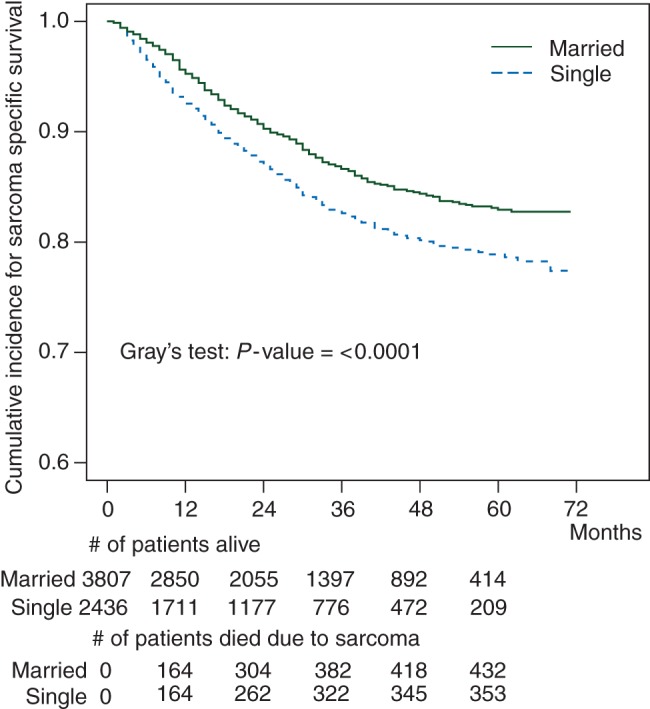
Sarcoma-specific survival for single versus married patients.
In addition to sarcoma-specific survival, overall survival curves were also plotted for both married and single patients. At 5 years of follow-up, 726 of the 3807 (19%) married patients had died and 635 of the 2436 single patients (26%) had died. This difference continues to remain statistically significant after factoring in time; P< 0.001 (Figure 2). Single patients encounter death at a larger proportion and faster rate than married patients.
Figure 2.
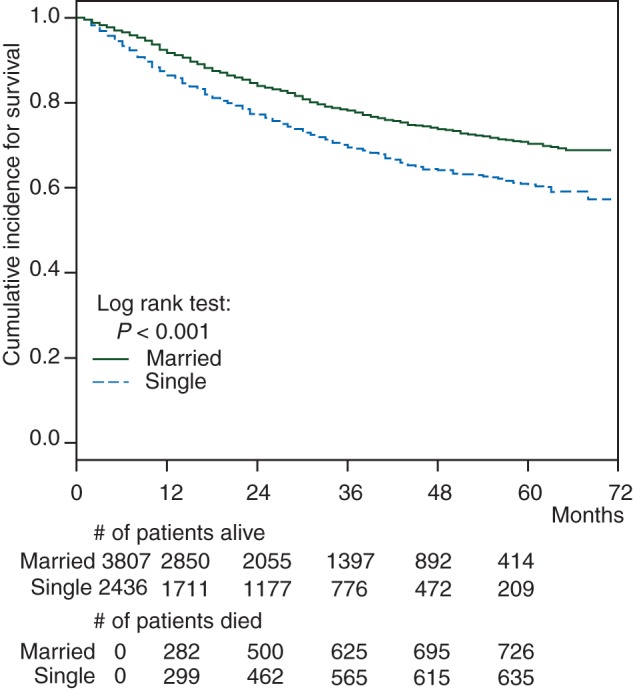
Overall survival for single versus married patients.
Regression analysis (Table 2) found that age at time of diagnosis, gender, region where the patients were from, tumor site, and histology subtype did not serve as independent predictors of death due to sarcoma. However, being single independently increases the risk of sarcoma-specific death by 26% after accounting for some of the possible confounding variables. Similarly, for each 1 cm increase in tumor size, the risk of death increases by 5%; P < 0.0001. Increasing tumor grade also increases the risk of death (risk was increased by 20 times for a Grade 4 tumor when compared with a Grade 1 tumor); P < 0.0001. Receiving radiation therapy reduces the risk of sarcoma-specific death by 30% when compared with those who do not receive radiotherapy; P < 0.0001. In analyzing income, it was found that, for every $10 000 increase in income, the risk of sarcoma-specific death increases by 27%; P < 0.0001.
Table 2.
Multivariable regression analysis of sarcoma-specific death for selected variables
| Variable | Sarcoma-specific death |
|
|---|---|---|
| P-value | Hazard ratio (2.5%, 97.5%) | |
| Single (ref: married) | 0.0110 | 1.26 (1.05, 1.51) |
| Age | 0.1900 | 1.00 (0.99, 1.00) |
| Female gender (ref: male) | 0.9100 | 0.99 (0.82, 1.19) |
| Region | ||
| Pacific Coast (ref: Southwest) | 0.7400 | 0.93 (0.59, 1.45) |
| East (ref: Southwest) | 0.8200 | 1.05 (0.67, 1.66) |
| Northern Plains (ref: Southwest) | 0.3400 | 0.77 (0.45, 1.31) |
| Tumor size (per 1 cm increase) | <0.0001 | 1.05 (1.03, 1.06) |
| Tumor grade | ||
| Grade 4 (ref: Grade 1) | <0.0001 | 20.28 (10.98, 37.45) |
| Grade 3 (ref: Grade 1) | <0.0001 | 16.16 (8.68, 30.08) |
| Grade 2 (ref: Grade 1) | <0.0001 | 4.85 (2.49, 9.43) |
| Tumor site upper ext (ref: lower limb and hip) | 0.3600 | 0.89 (0.70, 1.14) |
| Histology subtype | ||
| Leiomyosarcoma (ref: MFH/PUS) | 0.5400 | 1.09 (0.83, 1.44) |
| Liposarcoma (ref: MFH/PUS) | 0.0550 | 0.75 (0.55, 1.01) |
| Others(ref: MFH/PUS) | 0.2700 | 1.14 (0.91, 1.43) |
| Radiation (ref: no radiation) | 0.0001 | 0.70 (0.58, 0.84) |
| Income (per $10 000 increase) | <0.0001 | 1.27 (1.14, 1.41) |
ref, reference.
discussion
This study showed that when compared with married patients, single patients encountered death due to their sarcoma in both larger proportions and increased rates—a statistically and clinically significant difference. Additionally, when confounding variables such as tumor size, grade, histological subtype, etc. were analyzed, it was found that being single continued to serve as an independent predictor of death secondary to sarcoma. In agreement with prior studies, it was also seen that increasing tumor size, grade, and failure to undergo radiotherapy all serve as independent predictors of sarcoma-specific death [14–18].
Being married has been shown in many studies to confer survival advantages for patients with chronic diseases and certain types of cancers including STS as evidenced in this study [2, 6, 19, 20]. Support systems seen in married patients are often lacking in single patients. Support can come in many forms ranging from financial to emotional. Medical expenses while receiving care can often put a huge strain on a patient's finances and having another source of income can help tremendously with this [21, 22]. This is particularly applicable in non-European countries where access to health care can be limited by the patient's financial means. Without adequate funding to cover the perceived costs of their treatment, some single patients might be reluctant to seek the care that they need in a timely manner. However, while spouses can often help lessen the financial burden involved in undergoing treatment of STS, it only serves as a partial explanation for the discrepancies seen as marital status continues to be an independent predictor of mortality even after accounting for family income. Other than financial support, spouses also serve in a variety of integral ways to help enable patients to receive the care that they need. For example, married patients can often rely on the instrumental role that their spouses play in managing home affairs and other commitments while they obtain care—a privilege single patients lack. Being single has also been associated with higher rates of depression among cancer patients [23]. Spousal support allows for patients to have an emotion pillar to lean on during some of the more difficult times of their lives.
In this study, being single served as an independent predictor of death due to sarcoma when certain tumor and social factors were accounted for. However, being single can also entail additional confounding variables that were not accounted for in the study. For example, single patients may have barriers that may prevent them from obtaining timely care following diagnosis—i.e. living far away from the treatment center might also involve dependence on others for transportation. Increased distance has been well established to have a negative impact on a patient's care and without having support personnel to facilitate hospital trips will delay timely and appropriate care [24–26]. Additionally, poor adherence to clinic appointments has also been reported for those who are not married [27].
In comparing the two groups of patients, it is seen that both single and married patients present with comparable disease progression at time of diagnosis—i.e. no significant differences in tumor size. However, when analyzing rates of surgical treatment and adjuvant radiotherapy, it is seen that single patients do not undergo such treatment modalities as often as their married counterparts. This, subsequently, translates into poor survival in single patients. Thus, while single and married patients may present at a similar time point in their disease course, they subsequently diverge with married patients obtaining more aggressive treatment.
With an increasing shift toward health care being provided through a more psychosocial framework, marital statuses of patients should also be taken into account in order to determine those at risk of decreased survival and provide optimal care for them. For example, the use of counselors could allow single patients the ability to discuss their sarcoma diagnosis and any possible barriers in seeking care [28]. Additionally, social workers can also assist patients who are single or those without adequate familial support during their initial visit to facilitate visits to the treatment facility and work with them in overcoming such barriers [29, 30].
Marital status has a significant effect on survival in patients with STS. Single patients encounter death due to their sarcomas at a much larger proportion and faster rate when compared with their married counterparts. While both groups of patients have comparable progression of their sarcoma at diagnosis, their survival outcomes are significantly different. Social support systems and barriers to treatment options should be investigated in single patients and care tailored to their needs, with the aid of other members of an interdisciplinary health care team, such that the above shown discrepancies can be minimized.
study limitations
Marital status of patients is a dynamic factor that has the potential to change during a patient's disease course. However, SEER only collects marital status at time of diagnosis. Owing to this restriction, marital status cannot be analyzed as a time dependent variable in affecting survival. An additional limitation of the SEER database is that since it supplies data at a population level, the specific details of the resection, such as margin status, are not provided. Despite these limitations, the SEER database provides large, robust data on a heterogeneous group of patients from which conclusions such as the one shown in this study can be presented with validity and applied to a large group of patients.
disclosure
The authors have declared no conflicts of interest.
references
- 1.Vercelli M, Lillini R, Capocaccia R, et al. Cancer survival in the elderly: effects of socio-economic factors and health care system features (ELDCARE project) Eur J Cancer. 2006;42:234–242. doi: 10.1016/j.ejca.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 2.Lai H, Lai S, Krongrad A, et al. The effect of marital status on survival in late-stage cancer patients: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, in the United States. Int J Behav Med. 1999;6:150–176. doi: 10.1207/s15327558ijbm0602_4. [DOI] [PubMed] [Google Scholar]
- 3.de Graeff A, de Leeuw JR, Ros WJ, et al. Sociodemographic factors and quality of life as prognostic indicators in head and neck cancer. Eur J Cancer. 2001;37:332–339. doi: 10.1016/s0959-8049(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 4.Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29:377–387. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- 5.Molloy GJ, Stamatakis E, Randall G, et al. Marital status, gender and cardiovascular mortality: behavioural, psychological distress and metabolic explanations. Soc Sci Med. 2009;69:223–228. doi: 10.1016/j.socscimed.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schone BS, Weinick RM. Health-related behaviors and the benefits of marriage for elderly persons. Gerontologist. 1998;38:618–627. doi: 10.1093/geront/38.5.618. [DOI] [PubMed] [Google Scholar]
- 7.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 8.Manfrini M, Tiwari A, Ham J, et al. Evolution of surgical treatment for sarcomas of proximal humerus in children: retrospective review at a single institute over 30 years. J Pediatr Orthop. 2011;31:56–64. doi: 10.1097/BPO.0b013e318202c223. [DOI] [PubMed] [Google Scholar]
- 9.SEER. Surveillance, Epidemiology, and End Results (SEER) Program (http://www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2011 Sub (1973–2009) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2010 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012, based on the November 2011 submission.
- 10.SEER. SEER Registries: About SEER.
- 11.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 13.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 14.Fiore M, Casali PG, Miceli R, et al. Prognostic effect of re-excision in adult soft tissue sarcoma of the extremity. Ann Surg Oncol. 2006;13:110–117. doi: 10.1245/ASO.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Grimer RJ. Size matters for sarcomas! Ann R Coll Surg Engl. 2006;88:519–524. doi: 10.1308/003588406X130651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alamanda VK, Crosby SN, Archer KR, et al. Primary excision compared with re-excision of extremity soft tissue sarcomas—is anything new? J Surg Oncol. 2012;105:662–667. doi: 10.1002/jso.23021. [DOI] [PubMed] [Google Scholar]
- 17.Atalar H, Basarir K, Yildiz Y, et al. Prognostic factors in patients with malignant fibrous histiocytoma of the extremities. Acta Orthop Traumatol Turc. 2007;41:271–276. [PubMed] [Google Scholar]
- 18.Chandrasekar CR, Wafa H, Grimer RJ, et al. The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br. 2008;90:203–208. doi: 10.1302/0301-620X.90B2.19760. [DOI] [PubMed] [Google Scholar]
- 19.Holm KE, Plaufcan MR, Ford DW, et al. The impact of age on outcomes in chronic obstructive pulmonary disease differs by relationship status. J Behav Med. 2013 doi: 10.1007/s10865-013-9516-7. May 4 [epub ahead of print], doi: 10.1007/s10865-013-9516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelles JL, Joseph SA, Konety BR. The impact of marriage on bladder cancer mortality. Urol Oncol. 2009;27:263–267. doi: 10.1016/j.urolonc.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 21.O'Neill CB, Atoria CL, O'Reilly EM, et al. Costs and trends in pancreatic cancer treatment. Cancer. 2012;118:5132–5139. doi: 10.1002/cncr.27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker MS, Kessler LG, Urban N, et al. Estimating the treatment costs of breast and lung cancer. Med Care. 1991;29:40–49. doi: 10.1097/00005650-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Schlegel RJ, Manning MA, Molix LA, et al. Predictors of depressive symptoms among breast cancer patients during the first year post diagnosis. Psychol Health. 2012;27:277–293. doi: 10.1080/08870446.2011.559232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strauss K, MacLean C, Troy A, et al. Driving distance as a barrier to glycemic control in diabetes. J Gen Intern Med. 2006;21:378–380. doi: 10.1111/j.1525-1497.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peipins LA, Graham S, Young R, et al. Time and distance barriers to mammography facilities in the Atlanta metropolitan area. J Community Health. 2011;36:675–683. doi: 10.1007/s10900-011-9359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph AE, Hallman BC. Over the hill and far away: distance as a barrier to the provision of assistance to elderly relatives. Soc Sci Med. 1998;46:631–639. doi: 10.1016/s0277-9536(97)00181-0. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler E, Prettyman A, Lenhard MJ, et al. Adherence to outpatient program postoperative appointments after bariatric surgery. Surg Obes Relat Dis. 2008;4:515–520. doi: 10.1016/j.soard.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Marcus AC, Garrett KM, Cella D, et al. Can telephone counseling post-treatment improve psychosocial outcomes among early stage breast cancer survivors? Psychooncology. 2010;19:923–932. doi: 10.1002/pon.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cwikel JG, Behar LC. Organizing social work services with adult cancer patients: integrating empirical research. Soc Work Health Care. 1999;28:55–76. doi: 10.1300/J010v28n03_04. [DOI] [PubMed] [Google Scholar]
- 30.Sharp JW, Blum D, Aviv L. Elderly men with cancer: social work interventions in prostate cancer. Soc Work Health Care. 1993;19:91–107. doi: 10.1300/J010v19n01_06. [DOI] [PubMed] [Google Scholar]


