Despite an improved antitumor efficacy as noticed by an enhanced response rate and an improved progression-free survival, the hepatic intra-arterial fotemustine did not increase the overall survival of uveal melanoma patients with liver metastases only. We propose to consider intrahepatic treatment as an experimental approach.
Keywords: uveal melanoma, chemotherapy, intra-hepatic treatment, liver metastases
Abstract
Background
In uveal melanoma (UM) with metastatic disease limited to the liver, the effect of an intrahepatic treatment on survival is unknown. We investigated prospectively the efficacy and toxicity of hepatic intra-arterial (HIA) versus systemic (IV) fotemustine in patients with liver metastases from UM.
Patients and methods
Patients were randomly assigned to receive either IV or HIA fotemustine at 100 mg/m2 on days 1, 8, 15 (and 22 in HIA arm only) as induction, and after a 5-week rest period every 3 weeks as maintenance. Primary end point was overall survival (OS). Response rate (RR), progression-free survival (PFS) and safety were secondary end points.
Results
Accrual was stopped after randomization of 171 patients based on the results of a futility OS analysis. A total of 155 patients died and 16 were still alive [median follow-up 1.6 years (range 0.25–6 years)]. HIA did not improve OS (median 14.6 months) when compared with the IV arm (median 13.8 months), hazard ratio (HR) 1.09; 95% confidence interval (CI) 0.79–1.50, log-rank P = 0.59. However, there was a significant benefit on PFS for HIA compared with IV with a median of 4.5 versus 3.5 months, respectively (HR 0.62; 95% CI 0.45–0.84, log-rank P = 0.002). The 1-year PFS rate was 24% in the HIA arm versus 8% in the IV arm. An improved RR was seen in the HIA (10.5%) compared with IV treatment (2.4%). In the IV arm, the most frequent grade ≥3 toxicity was thrombocytopenia (42.1%) and neutropenia (62.6%), compared with 21.2% and 28.7% in the HIA arm. The main grade ≥3 toxicity related to HIA was catheter complications (12%) and liver toxicity (4.5%) apart from two toxic deaths.
Conclusion
HIA treatment with fotemustine did not translate into an improved OS compared with IV treatment, despite better RR and PFS. Intrahepatic treatment should still be considered as experimental.
EudraCT number and ClinicalTrials.gov identifier
2004-002245-12 and NCT00110123.
introduction
Uveal melanoma (UM) is a rare disease arising from the pigmented uveal tract of the eye. The incidence in Europe is 4.4 cases per million, varying between 2 in the south to 8 in the north of Europe [1]. Around 5% of patients with UM have distant metastases at diagnosis. Metastases appear usually within a median of 3 years after the treatment of the primary tumor with a range between 1 and 10 years. There is an unexplained hepato-tropism with the liver being the first site of metastases in up to 90% of patients and is the only site in 46% of them [2]. Some clinical and histological features may predict the development of metastases, tumor size being the most significant [3]. But chromosomal alterations like loss of chromosome 3 [4] or gene expression profiling have allowed to separate UM with a good or poor prognosis [5]. It has been postulated recently that, inactivating somatic mutation of BRCA-1 associate protein 1 (BAP1) on chromosome 3 might be one of the main genetic events for the acquisition of metastatic potential [6].
At present, there are no standard therapies for metastatic disease, and the treatments used for cutaneous melanoma have resulted in minimal efficacy [7]. The liver being the main site of metastasis and carrying a worse prognosis, efforts were made to develop locoregional intrahepatic strategies.
The rationale for HIA treatment is based on the fact that metastases are fed primarily by the hepatic artery whereas the normal liver tissue is supplied by the portal vein. HIA chemotherapy using fotemustine has been shown to produce a high RR and encouraging median OS between 14 and 22 months were reported [8, 9]. The prospective randomized phase III trial (EORTC 18021) thus aimed to compare the efficacy and safety of HIA versus IV fotemustine in UM patients with metastases in liver only.
patients and methods
patients
Eligible patients were ≥18 years old, untreated, with histologically proven liver metastases from UM. Patients with extrahepatic metastases, severe cardiac disease or active duodenal ulceration were excluded. World Health Organization (WHO) performance status (PS) 0–2, absolute neutrophile count (ANC) ≥2.0 × 109/l, platelets count ≥100 × 109/l, hemoglobin ≥10 g/dl and ASAT/ALAT, alkaline phosphatase, γ-glutamyltransferase, LDH <5 × the upper normal limit (UNL), total bilirubin and serum creatinine <1.5 × UNL were required. Women of child bearing potential and men should be using an effective method of contraception.
The protocol was approved by the EORTC protocol review committee and local ethical committees of the participating institutions. A written informed consent was obtained from all patients before randomization in accordance with the declaration of Helsinki.
study design and treatment
The study is an open label randomized trial. Patients were centrally randomized to receive either IV or HIA fotemustine in a 1:1 ratio, using the minimization technique with stratification for PS (0 versus 1 versus 2), LDH (normal versus abnormal) and institution [10, 11]. The treatment schedule and dose modifications are reported in supplementary data, available at Annals of Oncology online.
treatment evaluation
The primary objective was to compare the overall survival (OS) defined as the time from date of randomization to the date of death from any cause. The secondary end points were progression-free survival (PFS), response rate (RR), pattern of progression, treatment-related toxicities and catheter-related complications. PFS was measured from the date of randomization to the date of progression or death. RR was based on measurable lesions according to RECIST criteria version 1.0. Tumor measurements were assessed radiologically by CT scan or MRI before start of treatment, then at week 7 from randomization before the maintenance treatment phase, then every 9 weeks, similarly in both arms, without central review. Stable disease was thus confirmed every 9 weeks. All responses had to be confirmed not <4 weeks after the first evaluation. Treatment-related toxicities were evaluated according to CTC version 2.0.
statistical analysis
The study aimed to detect a 50% increase in the median OS between the IV (8 months) and the HIA arms (12 months), corresponding to a hazard ratio (HR) of 0.67 (two-sided α = 0.05 and β = 0.15). A total of 262 patients were foreseen to be randomized over 3 years, of whom 220 had to be followed till death.
Due to a slow accrual, an unplanned interim futility analysis was recommended by the EORTC independent data monitoring committee (IDMC). At the time of the interim analysis, 134 deaths were recorded. Using a O'Brien-Fleming boundary, the observed treatment HR had to be >0.88 in order to reject the hypothesis of superiority in OS of HIA over IV, with a power of 80% [12]. This was the case, so the IDMC recommended closing the trial to patient's entry early for futility.
The Kaplan–Meier technique was used to estimate survival-type distributions, and standard errors (SE) of the estimates were obtained via Greenwood formula [13]. The log-rank two-sided test was used for the comparison of the treatment outcome. The 95% confidence interval (CI) of medians was obtained via the Brookmeyer and Crowley's nonparametric method. The Cox proportional hazards model was used to adjust the treatment comparison by factors used at randomization (LDH and performance status). This model provided an estimated treatment HR, its confidence interval with a boundary proper to each analysis (first and final), and a P-value (Wald test).
Main efficacy analyses were carried out in the intention-to-treat (ITT) population: all patients were considered in their initial randomized arm. Sensitivity analyses for OS and PFS were carried out in all patients who actually started the treatment allocated by randomization. A second sensitivity analysis was carried out for PFS analysis by censoring the follow-up at the time of cross-over to the other arm. SAS 9.2 software (SAS Institute, Inc., Cary, NC) was used for statistical analyses.
results
From February 2005 to February 2011, 171 patients were randomized. The primary OS analysis was based on a clinical cutoff date of 20 March 2012 by which time 155 patients had died and 16 were still alive. The median follow-up from randomization for the entire study was 5, 6 years (range 0.25–6 years) similar in the two treatment groups. For the 16 patients alive, it was 1.6 years (range 0.25–6 years). Overall, 30 patients were still alive 2 years after randomization. The efficacy population consisted in 86 in the HIA arm and 85 in the IV arm. Patient characteristics were well balanced between treatment arms (supplementary Table S1, available at Annals of Oncology online). However, in a small subgroup of patients with LDH > ULN, an unbalance favoring the IV arm was observed, with 2 (2.4%) >2 × UNL compared with 11 (12%) in the HIA arm.
Three patients were considered ineligible in the HIA arm: two with metastases outside the liver, one with LDH >5 × the UNL (supplementary Figure S1, available at Annals of Oncology online). Among the patients treated, the reason for stopping treatment was tumor progression or death in 37 patients (56.1%) in the HIA arm and 59 patients (71.1%) in the IV arm. The median (range) number of fotemustine cycles administered was 4 (1–17) and 3 (1–19) in the HIA and IV arms, respectively. Treatment discontinuation due to toxicity occurred in 14 (21.2%) (hematological n = 7, hepatic n = 3, septic shock n = 2, gastric ulcer n = 1, arterial thrombosis n = 1) and 22 (26.5%) patients (hematological n = 21, fatigue n = 1), respectively. In addition, in the HIA arm, 11 patients (16.7%) went off treatment due to catheter dysfunction. Since 20 patients in the HIA arm and 2 patients in the IV arm and did not start the protocol treatment, safety analysis was done on 66 and 83 patients, respectively.
efficacy
Based on the intention-to-treat analysis (ITT), the OS was similar in the HIA when compared with the IV group (HR, 1.09; 95% CI 0.79–1.50) (Figure 1). The median OS was 14.6 months (95% CI 10.2–15.4) and 13.8 months (95% CI 10.2–17.2) in the HIA and IV arms, respectively. At 2 years, 19.2% (SE 4.4%) survived in the HIA arm and 20.2% (SE 4.6%) in the IV arm. The treatment comparison did not change when adjusted by stratification factors or if only the patients who received the treatment according to protocol were considered.
Figure 1.
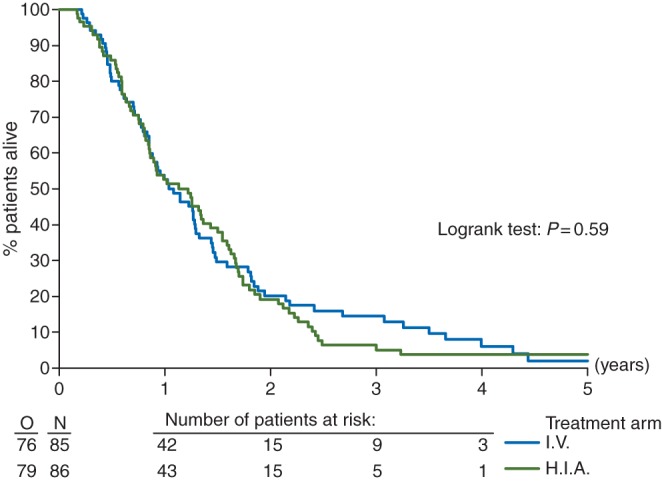
Overall survival by treatment group: ITT analysis in all randomized patients. O means observed events.
The initial stratification factors had an impact on OS. Patients with a PS 0 had a longer median OS than patients with a PS 1 (14.7 versus 9.5 months) (supplementary Figure S2, available at Annals of Oncology online). The median OS was 16.3 months when LDH ≤ UNL, 10.3 months when LDH between UNL and ≤2 × UNL, and 4.6 months when LDH >2 × UNL (supplementary Figure S3, available at Annals of Oncology online).
In the ITT analysis, there were 9 (10.5%) responses (1 complete, 8 partial) observed among the 86 patients in the HIA arm and 2 partial responses (2.4%) among 85 patients in the IV arm (P = 0.057). And considering the patients who started the protocol treatment (9/66 = 13.6% versus 2/83 = 2.4%), the difference was significant (P = 0.02). The median time to progression of the responding patients was 9.0 and 8.3 months in HIA and IV arms, respectively. Disease stabilization was reported 33 (38.4%) versus 44 (51.8%) in the HIA and IV arm, respectively.
For the whole cohort of 171 patients, the median PFS was 4 months. On an ITT basis, there was a statistically significant (P = 0.002) increase in PFS in favor of HIA (HR, 0.62; 95% CI 0.45–0.84) (Figure 2). The median PFS was 4.5 months (95% CI 4.1–6.0) and 3.7 months (95% CI 2.0–4.1) for HIA and IV arms, respectively, and the 1-year PFS rate was 19% versus 8%. Adjusting by initial stratification factors (PS and LDH), treatment comparison provided similar results. Considering only the patients who started the treatment allocated by randomization, the HR was 0.53 (95% CI 0.38–0.75), and 0.49 when adjusted for PS and LDH (three-categorical variable). The 1-year PFS rate was 24% in the HIA arm versus 8% in the IV arm.
Figure 2.
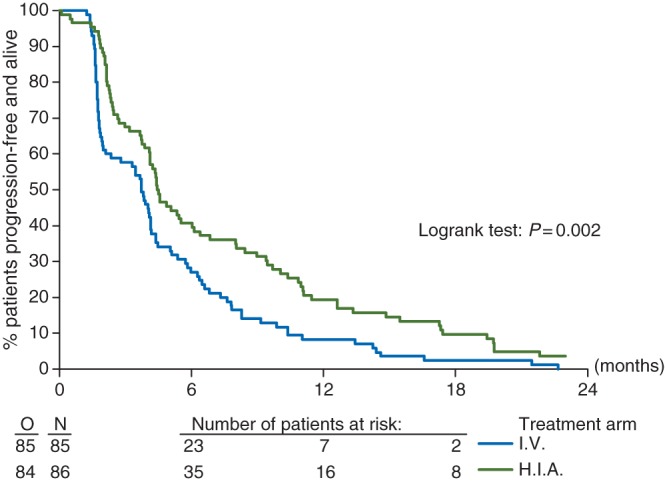
PFS by treatment group: ITT analysis in all patients randomized. O means observed events.
The initial PS (1 versus 0) had no impact on PFS (HR 0.84). Nevertheless, PFS was influenced by pretreatment LDH levels considered as either two or as three variables: the 6-month PFS rate was 40.4% (SE 4.9%), 28.8% (SE 5.9%) and 7.7% (SE 7.4%) when LDH was ≤UNL or UNL ≤ 2 × UNL or >2 × UNL, respectively (Wald test P = 0.04).
First progression in the liver was observed in 54.8% in the HIA arm compared with 89, 4% of the IV arm, whereas extrahepatic progression occurred in 26.2% in HIA arm and 5.0% in the IV arm. When off study treatment, 25 patients in the HIA arm received IV fotemustine, 3 had other intrahepatic therapies and all the remaining had systemic dacarbazine-based treatments. In the IV arm, one patient received HIA fotemustine and four had other intrahepatic treatments. Five additional patients with later progressions received intrahepatic treatment.
toxicity
The toxicity is summarized in Table 1. Hematological toxicity was more frequent in the IV than in the HIA arm. Thus, thrombocytopenia grade ≥3 was observed in 42.1% and in 21.2% in the IV and HIA arm, respectively. Neutropenia grade ≥3 was 62.6% in the IV arm and 28.7% in the HIA arm. But the incidence of infection or febrile neutropenia was not significantly different between the two arms. The nonhematological toxicity was mainly related to HIA therapy, with abdominal pain grade ≥3 in 12.1% of patients, and gastric ulcer in 3% of them. Moreover, 31.8% had catheter-induced complications (stenosis, thrombosis, dissection or misperfusion) and 4.5% had liver toxicity grade ≥3 (partial necrosis, impaired liver function tests). Two toxic deaths occurred in the HIA arm, one due to a septic shock and the other to a mesenteric artery thrombosis followed by a sepsis, and none in the IV arm.
Table 1.
Adverse events
| IV arm (N = 83) |
HIA arm (N = 66) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade |
Grade |
|||||||||
| 1 | 2 | 3 | 4 | Grade 3–4% | 1 | 2 | 3 | 4 | Grade 3–4% | |
| Hematological | ||||||||||
| Hemoglobin | 10 | 55 | 18 | 87.9 | 0 | 7 | 41 | 18 | 41.3 | |
| Leucocytes | 21 | 29 | 25 | 2 | 32.5 | 18 | 15 | 10 | 2 | 18.1 |
| Neutrophils | 12 | 7 | 23 | 29 | 62.6 | 11 | 8 | 11 | 8 | 28.8 |
| Thrombocytes | 7 | 15 | 20 | 15 | 42.1 | 6 | 8 | 10 | 4 | 21.2 |
| Nonhematological | ||||||||||
| Nausea-vomiting | 27 | 10 | 0 | 0 | 0 | 24 | 12 | 3 | 0 | 4.5 |
| Abdominal pain | 27 | 6 | 1 | 0 | 1.2 | 9 | 14 | 7 | 1 | 12.1 |
| Gastric ulcer | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 3.0 |
| Catheter complications | NA | 9 | 3 | 7 | 1 | 12.0 | ||||
| Infection | 0 | 7 | 3 | 0 | 3.6 | 0 | 8 | 1 | 2 | 4.5 |
| Febril neutropenia | 0 | 0 | 3 | 0 | 3.6 | 0 | 0 | 1 | 0 | 1.5 |
discussion
UM is an orphan disease for which large prospective studies do not exist and for which no efficient treatment is known in advanced stage. We present here the largest prospective randomized trial ever conducted that aimed to determine the actual outcome of patients with metastatic disease to the liver treated by an investigational approach through a locoregional treatment in the liver and compared with a similar cohort receiving intravenous chemotherapy.
Over the years, chemotherapy of metastatic UM has been tested mainly in multiple phase II trials of small and highly selected populations. It was based on regimens extrapolated from cutaneous melanoma. But these are two distinct diseases with a different pattern of metastases and different genetic profiles [2]. Activating V600E mutation of B-RAF has not been observed in UM [14]. On the contrary, 86% of UM exhibits GNAQ/11 mutations [15, 16].
Single-agent treatment with dacarbazine or temozolomide has been recognized as standard chemotherapy for metastatic cutaneous melanoma. Their activity in UM has been limited to a small phase II trials, where no responses were reported and the median survival was 6.7 months [17]. Fotemustine is a third-generation nitrosurea that was shown to be as active as dacarbazine in cutaneous melanoma [18]. In UM, its IV administration however has been tested in association with interferon α and IL-2 in 25 patients with an 8% RR and a 12.3-month median OS [19]. Interestingly, the same group treated another cohort of 23 patients with HIA Fotemustine and obtained a 22% RR and similar median survival [19]. Fotemustine has been accepted in some countries in Europe as a treatment option. Due to its high liver extraction rate at first pass, fotemustine has been also tested as a HIA administration. The results of a few phase II studies confirmed a RR ranging from 22% to 40%, and a median OS of 12–24 months [8, 9]. Multiple prognostic factors might have influenced these results when compared with the IV administration, and the strongest factor in multivariate analysis has been the LDH level [8].
We thus conducted a randomized trial balanced for the most important prognostic factors in order to compare the clinical outcome of patients with only liver metastases treated with the same chemotherapy administered either HIA or IV.
Our study confirmed that IV fotemustine led to a low RR (2.4%) and a high rate of progression (41.2%). These results are in line with any IV chemotherapy regimens [7]. The HIA fotemustine obtained a higher RR at 13.6% when considering patients that received the treatment as per protocol. It was however lower than the ones reported in the previous phase II trials.
In our study, PFS was influenced by the treatment type with a median PFS of 4.5 and 3.7 months for the HIA and IV, respectively. When considering only patients that received the treatment according to protocol, the 1-year PFS was 24% in HIA and 8% in IV arm. The most recent small phase II studies of IV therapies had median PFS ranging from 1.9 to 3 months [20].
The main end point of our study was however OS. The improved RR and PFS did not translate into a significant difference in median OS that was ∼14 months in both arms. Crossover or second-line treatments are unlikely to have had a major impact on OS. Similarly, a small randomized trial testing in 92 patients another locoregional strategy, the percutaneous hepatic perfusion with melphalan against systemic treatment, did not show any improvement in OS [21]. The known prognostic factors like PS or LDH level have had a major influence on OS in our study too, and were quite well balanced between both arms. The treatment comparisons did not change even when adjusted for an increase prevalence of LDH ≥ 2 × ULN in the HIA arm. Some biological characteristics, i.e. the presence of a GNA11 mutation [16], or the intratumoral immune status [22], might have influenced these results.
The treatment could be given on an outpatient basis in both arms reflecting the lack of major toxicity. In the IV arm, the toxicity was hematological without any increased risk of bleeding or infection compared with the HIA arm. In the latter, the morbidity was related mainly to catheter or hepatic arterial complications and liver toxicity that was the cause of treatment discontinuation in 11 patients (17%). This catheter complication rate correlated with the finding of similar multi-institutional studies in uveal or colorectal cancer [23]. In conclusion, despite an improved antitumor efficacy as noticed by an enhanced RR and an improved PFS, the HIA fotemustine did not increase the OS of UM patients with liver metastases only. We propose to consider intrahepatic treatment as an experimental approach that may not be appropriate as a single modality. But its combination with systemic targeted therapies, like MEK inhibitors that have recently demonstrated promising efficacy in UM [24], might be considered in future studies, similarly to what has been shown in other diseases [25–27].
funding
This work was supported by European Organisation for Research and Treatment of Cancer, and by Servier through an Educational Grant (IRIS trial number CL3-10036), and by a donation from the Schweitzerische Krebsliga from Switzerland through the EORTC Charitable Trust.
disclosure
AT disclosed potential conflict of interest being consultant for BMS, GSK, Amgen, and having received honoraria from these companies. SN disclosed potential conflict of interest being consultant for Pfizer, and Roche, GSK, Amgen, and having received research funding from these companies. TJ disclosed potential conflict of interest being consultant for BMS, and Roche and having received honoraria from these companies. All remaining authors have declared no conflict of interest.
Supplementary Material
acknowledgements
We warmly thank the EORTC Headquarters (data managers: S. Vanderschaeghe, I. Jagiello, L. Polders, C. Kluyskens; project manager: G. de Schaetzen; clinical research physicians: J. Flament, S. Margerit, K. Stoitchkov; pharmacovigilance managers: M.-P. Gauthier), and Mrs M. Gonin for typing the manuscript.
references
- 1.Virgili G, Gatta G, Ciccolallo L, et al. Incidence of uveal melanoma in Europe. Ophthalmology. 2007;114(12):2309–2315. doi: 10.1016/j.ophtha.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 2.Diener-West M, Reynolds SM, Agugliaro DJ, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123(12):1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 3.Shields CL, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127(8):989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 4.Prescher G, Bornfeld N, Hirche H, et al. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1996;347(9010):1222–1225. doi: 10.1016/s0140-6736(96)90736-9. [DOI] [PubMed] [Google Scholar]
- 5.Onken MD, Ehlers JP, Worley LA, et al. Functional gene expression analysis uncovers phenotypic switch in aggressive uveal melanomas. Cancer Res. 2006;66(9):4602–4609. doi: 10.1158/0008-5472.CAN-05-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harbour JW, Onken MD, Roberson ED, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(6009):1410–1413. doi: 10.1126/science.1194472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedikian AY, Legha SS, Mavligit G, et al. Treatment of uveal melanoma metastatic to the liver: a review of the M. D. Anderson Cancer Center experience and prognostic factors. Cancer. 1995;76(9):1665–1670. doi: 10.1002/1097-0142(19951101)76:9<1665::aid-cncr2820760925>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Leyvraz S, Spataro V, Bauer J, et al. Treatment of ocular melanoma metastatic to the liver by hepatic arterial chemotherapy. J Clin Oncol. 1997;15(7):2589–2595. doi: 10.1200/JCO.1997.15.7.2589. [DOI] [PubMed] [Google Scholar]
- 9.Siegel R, Hauschild A, Kettelhack C, et al. Hepatic arterial Fotemustine chemotherapy in patients with liver metastases from cutaneous melanoma is as effective as in ocular melanoma. Eur J Surg Oncol. 2007;33(5):627–632. doi: 10.1016/j.ejso.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Freedman LS, White SJ. On the use of Pocock and Simon's method for balancing treatment numbers over prognostic factors in the controlled clinical trial. Biometrics. 1976;32(3):691–694. [PubMed] [Google Scholar]
- 11.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–115. [PubMed] [Google Scholar]
- 12.Fleming TR, Harrington DP, O'Brien PC. Designs for group sequential tests. Control Clin Trials. 1984;5(4):348–361. doi: 10.1016/s0197-2456(84)80014-8. [DOI] [PubMed] [Google Scholar]
- 13.Kalbfleisch JD, Prentice RL. Hokboken, NJ: John Wiley & Sons, Inc; 2002. The Survival Analysis of Failure Time Data. 2nd edition. [Google Scholar]
- 14.Greaves WO, Verma S, Patel KP, et al. Frequency and spectrum of BRAF mutations in a retrospective, single-institution study of 1112 cases of melanoma. J Mol Diagn. 2013;15:220–226. doi: 10.1016/j.jmoldx.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457(7229):599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bedikian AY, Papadopoulos N, Plager C, et al. Phase II evaluation of temozolomide in metastatic choroidal melanoma. Melanoma Res. 2003;13(3):303–306. doi: 10.1097/00008390-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22(6):1118–1125. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 19.Becker JC, Terheyden P, Kampgen E, et al. Treatment of disseminated ocular melanoma with sequential fotemustine, interferon alpha, and interleukin 2. Br J Cancer. 2002;87(8):840–845. doi: 10.1038/sj.bjc.6600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmittel A, Schmidt-Hieber M, Martus P, et al. A randomized phase II trial of gemcitabine plus treosulfan versus treosulfan alone in patients with metastatic uveal melanoma. Ann Oncol. 2006;17(12):1826–1829. doi: 10.1093/annonc/mdl309. [DOI] [PubMed] [Google Scholar]
- 21.Pingpank JF, Hughes MS, Faries MB, et al. A phase III random assignment trial comparing percutaneous hepatic perfusion with melphalan (PHP-mel) to standard of care for patients with hepatic metastases from metastatic ocular or cutaneous melanoma. J Clin Oncol. 2010;28(suppl; abstr LBA8512) 18s. [Google Scholar]
- 22.Toivonen P, Mäkitie T, Kujala E, et al. Microcirculation and tumor-infiltrating macrophages in choroidal and ciliary body melanoma and corresponding metastases. Invest Ophthalmol Vis Sci. 2004;45(1):1–6. doi: 10.1167/iovs.03-0622. [DOI] [PubMed] [Google Scholar]
- 23.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341(27):2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 24.Carvajal RD, Sosman JA, Quevedo F, et al. Phase II study of selumetinib versus temozolomide in gnaq/Gna11 mutant uveal melanoma. J Clin Oncol. 2013;31 [Google Scholar]
- 25.Kemeny NE, Melendez FDH, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2009;27(21):3465–3471. doi: 10.1200/JCO.2008.20.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sansonno D, Lauletta G, Russi S, et al. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist. 2012;17(3):359–366. doi: 10.1634/theoncologist.2011-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leyvraz S, Keilholz U. Ocular melanoma: what's new? Curr Opin Oncol. 2012;24(2):162–169. doi: 10.1097/CCO.0b013e32834ff069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


