In this study, we demonstrate that vemurafenib but not dabrafenib reduces peripheral lymphocyte counts in melanoma patients while both agents show similar clinical efficacy. Within the lymphocyte compartment, vemurafenib selectively decreases circulating CD4+ T cells and changes their phenotype and function. This indicates that selective BRAFi need to be assessed individually for immunomodulatory effects, especially, when planning combinations with immunotherapies.
Keywords: melanoma, vemurafenib, dabrafenib, lymphocytes, T cells, treatment
Abstract
Background
Since the majority of melanomas eventually become resistant and progress, combining selective BRAF inhibitors (BRAFi) with immunotherapies has been proposed to achieve more durable treatment responses. Here, we explored the impact of selective BRAFi on the hosts' immune system.
Patients and methods
Clinical data, whole blood counts (WBC) and serum lactate dehydrogenase (LDH) of 277 vemurafenib- and 65 dabrafenib-treated melanoma patients were evaluated. The frequency and phenotype of lymphocyte subpopulations were determined by flow cytometry while T cell cytokine secretion was measured by multiplex assays.
Results
Progression-free survival (PFS) as well as overall survival (OS) were similar in patients treated with either BRAFi. High pretreatment LDH was associated with shorter PFS and OS in both groups. During therapy, peripheral lymphocytes decreased by 24.3% (median, P < 0.0001) in vemurafenib-treated patients but remained unchanged in dabrafenib-treated patients (+1.2%, P = 0.717). Differentiation of peripheral lymphocytes of vemurafenib-treated patients showed a significant decrease in CD4+ T cells (P < 0.05). Within CD4+ T cells obtained during treatment, an increase in CCR7+CD45RA+ (naïve) and a decrease in CCR7+CD45RA− (central memory) populations were found (P < 0.01 for both). Furthermore, secretion of interferon-γ and interleukin-9 by CD4+ T cells was significantly lower in samples obtained during vemurafenib treatment compared with baseline samples.
Conclusion
While both compounds have comparable clinical efficacy, vemurafenib but not dabrafenib decreases patients peripheral lymphocyte counts and alters CD4+ T cell phenotype and function. Thus, selective BRAFi can significantly affect patients' peripheral lymphocyte populations. Fully understanding these effects could be critical for successfully implementing combinatorial therapies of BRAFi with immunomodulatory agents.
introduction
Despite the introduction of selective BRAF inhibitors (BRAFi) such as vemurafenib and dabrafenib, metastatic melanoma remains a disease with poor prognosis [1]. High rates of initial responses in melanoma patients treated with selective BRAFi have been observed, but tumors eventually become resistant and complete responses are rare [1]. In contrast, immunotherapies, e.g. with anti-CTLA4 and anti-PD-1 antibodies, can induce durable responses in a small subgroup of melanoma patients [2, 3]. Although a phase I trial employing vemurafenib in combination with ipilimumab, an anti-CTLA4 antibody, had to be discontinued due to severe liver toxicity [4], combining selective BRAFi and immunotherapies might prove beneficial in avoiding the shortcomings of the individual agents [1].
BRAFi impair cancer cell growth by decreasing MAPK pathway signaling. This pathway is relevant also in downstream T cell receptor signaling [4], implying that selective BRAFi could potentially alter immune responses and anti-tumor immunity in humans. Several in vitro studies have reported that analogs of vemurafenib do not inhibit human lymphocyte function [4, 5]. Comin-Anduix et al. [4] did not observe induction of apoptosis or inhibition of cytotoxicity in human T cells by vemurafenib in vitro. Similar results were obtained by Boni et al. [5] who found no impact of selective BRAFi on proliferation and viability of T cells. In this in vitro study, recognition and killing of tumor cells by T cells specific for melanoma differentiation antigens (MDA) was enhanced by selective BRAFi treatment, which up-regulated MDA expression [5]. Analysis of tumor biopsies obtained during treatment with dabrafenib or vemurafenib also showed an increase in infiltration of melanoma metastases by human CD4+ and CD8+ T cells and the presence of CD8+ T cells was found to be associated with the reduction in tumor mass [6]. For dabrafenib, Hong et al. [7] showed that composition and functionality of patients’ lymphocytes remained unaffected by treatment. In summary, lymphocyte function seems to be unaffected by selective BRAFi, while antigenicity of melanoma cells is increased.
Whereas we reported a decrease in immunosuppressive myeloid cells in patients with advanced melanoma during vemurafenib therapy recently [8], no data following patients’ lymphocytes during vemurafenib treatment have been published yet. In this study, we explored the effects of selective BRAFi on the human immune system by analyzing T cells, B cells and natural killer (NK) cells as well as neutrophils. The retrospective analysis of clinical data from a large cohort of patients treated with selective BRAFi showed striking differences in the effects of vemurafenib and dabrafenib on patients’ peripheral lymphocytes.
materials and methods
clinical data and blood samples
Patients enrolled in this study started treatment with either vemurafenib or dabrafenib between May 2010 and March 2013 in 10 DeCOG (Dermatologic Cooperative Oncology Group) skin cancer units. After determining BRAF status, treatment was chosen based on availability. Whole blood counts (WBC) were carried out within 4 weeks before starting BRAFi treatment in 277 melanoma patients receiving vemurafenib and in 65 patients receiving dabrafenib and were repeated every 4–6 weeks during therapy. For our analyses, the nadir of lymphocytes within the first 12 weeks of treatment with either BRAFi was used. Peripheral blood mononuclear cells (PBMC) were obtained from 18 melanoma patients treated with vemurafenib (Stage IV, AJCC 2009 [9]) after written informed consent with local ethics approval. Clinicopathological characteristics are listed in Table 1. BRAF status in melanoma tissue was determined by Sanger sequencing or allele-specific PCR.
Table 1.
Clinicopathological characteristics of patients enrolled in this study
| Vemurafenib (n = 277) | Dabrafenib (n = 65) | P-value | |
|---|---|---|---|
| Age (years), median (range) | 56 (21–84) | 51 (20–79) | 0.069 |
| Sex (%) | 0.275 | ||
| Male | 164 (59.2) | 41 (63.1) | |
| Female | 113 (40.8) | 24 (36.9) | |
| Stage (AJCC 2009) (%) | 0.55 | ||
| Unknown | 1 (0.4) | 0 | |
| Stage III | 5 (1.8) | 0 | |
| Stage IV | 271 (97.8) | 65 (100) | |
| M stage (%) | 0.187 | ||
| Unknown | 1 (0.4) | 0 | |
| M1a | 21 (7.7) | 5 (7.7) | |
| M1b | 31 (11.4) | 2 (3.1) | |
| M1c | 218 (80.4) | 58 (89.2) | |
| LDH (U/l) before therapy, median (range) | 271 (137–9555) | 218 (135–2033) | 0.034 |
| Number of previous therapies, median (range) | 1 ( 0–6) | 1 (0–5) | 0.931 |
| Lymphocytes per nl before therapy, median (range) | 1.27 (0.16–4.81) | 1.4 (0.31–4.03) | 0.225 |
| PFS (weeks), median (range) | 21.3 (0.4–167) | 21.0 (3.6–142) | 0.98 |
| OS (weeks), median (range) | 44.1 (3–167) | 46.3 (11–142) | 0.84 |
antibodies
The following fluorochrome-labeled monoclonal antibodies (mAbs) purchased from Beckman Coulter (Brea, CA) were used: anti-CD8-APC-Alexa 700, anti-CD4-FITC and PECy7, anti-CD3-PECy5, anti-CD19-ECD, anti-CD45-FITC as well as anti-CD56-PE. Anti-CD45RO-Alexa Fluor 700®, CD69-PerCP/Cy5.5, CD8-APC, CD45RA-PECy7 and anti-CCR7-PE were purchased from Biolegend (San Diego, CA). Appropriate isotype controls were purchased from Beckman Coulter and BD Pharmigen (Heidelberg, Germany).
whole blood counts
WBC were carried out on clinical grade automated hematology analyzers, e.g. XE-5000 (Sysmex, Norderstedt, Germany).
isolation of PBMC
PBMC were isolated by density gradient centrifugation using Biocoll (Biochrom, Berlin, Germany) from heparinized venous blood and stored in liquid nitrogen until usage. In some samples, the number of PBMC recovered was too small to conduct all phenotypic and functional studies.
staining for flow cytometry
First, cells were incubated with Aqua Viability Dye (BD, Detected on FL-10) for 30 min at room temperature. After washing, samples were surface stained, acquired and analyzed as described [8].
Frequencies of CD3+CD4+ T cells, CD3+CD8+ T cells, CD19+ B cells and CD3−CD56+ NK cells were determined after gating on lymphocytes defined by their forward and side scatter properties (FS/SS). Total numbers were then calculated using absolute lymphocyte counts measured in WBC.
magnetic cell sorting
From PBMC, CD4+ T cells and CD4− cells were isolated by magnetic bead-based separation (MACS technology, Miltenyi, Bergisch-Gladbach, Germany) according to the manufacturer's instructions. Purity, as determined by flow cytometry, was ≥97%.
cell culture
After thawing, PBMC were rested for 4 h in complete RPMI1640 medium (Life Technologies, Darmstadt, Germany) containing 10% FBS (PAA, Cölbe, Germany) in a humidified 5% CO2 atmosphere at 37°C. Afterwards, 106 PBMC were used for phenotypic studies. From the remaining cells, CD4+ and CD4− fractions were isolated as described. An aliquot of CD4+ cells was seeded in a 96-well round bottom plate (Corning, Kaiserslautern, Germany) and stimulated with anti-CD2/CD3/CD28-coated beads (Miltenyi) at a bead to cell ratio of 1:2 in complete RPMI1640 medium containing 10% FBS in humidified 5% CO2 atmosphere at 37°C. Supernatants (SN) were collected after 16 h of stimulation.
multiplex cytokine array
Cytokines in SN of CD4+ T cells were measured by flow cytometry using FlowCytomix (Human Th13-plex, eBioscience, San Diego, CA) according to the manufacturer's instructions.
statistical analysis
Data were analyzed with SPSS 20.0 (IBM Corp., Armonk, NY) using the Wilcoxon signed-rank and Mann–Whitney U-test for continuous variables. The χ2 tests and Fisher's exact tests were used to test for differences between categorical variables. Correlations were assessed by using the Spearman rank test. Survival was analyzed by the Kaplan–Meier method and tested for differences using the log-rank test.
results
study population
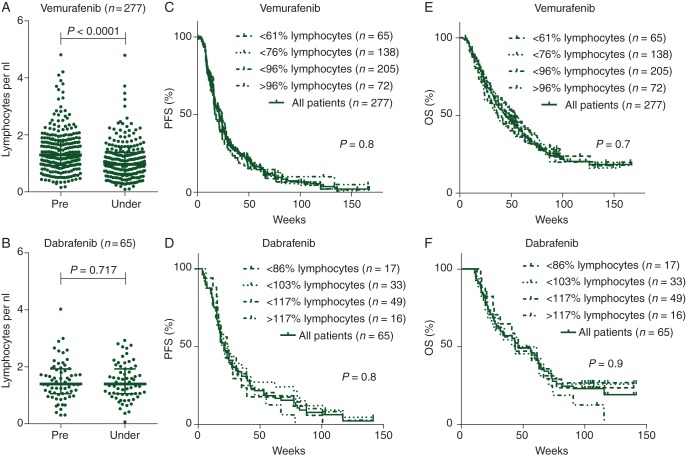
Clinicopathological characteristics of patients enrolled in this study and treated with either vemurafenib (n = 277) or dabrafenib (n = 65) are shown in Table 1 and supplementary Table S1, available at Annals of Oncology online. While age, sex and disease stage were similar between patients treated with either selective BRAFi, lactate dehydrogenase (LDH) levels before treatment were significantly higher in patients receiving vemurafenib than dabrafenib (P = 0.034). Pretreatment lymphocyte counts did not differ significantly between both groups. Both progression-free survival (PFS, 21.3 weeks for vemurafenib versus 21 weeks for dabrafenib, P = 0.98) and overall survival (OS) (vemurafenib: 44.1 versus dabrafenib: 46.3 weeks; P = 0.88) were similar in patients treated with either compound.
vemurafenib but not dabrafenib causes selective loss of peripheral lymphocytes
As shown in Figure 1A, patients treated with vemurafenib showed a significant decrease in peripheral lymphocytes within the first 12 weeks of treatment (median change: −24.3%, P < 0.0001) which was not observed in patients treated with dabrafenib (Figure 1B, median change: +1.2%, P = 0.72). The Kaplan–Meier analyses of both cohorts grouped by patients below the first quartile, the median, the third quartile, and patients above the third quartile showed no association of PFS or OS with the loss of peripheral lymphocytes (Figure 1C–F). As shown in supplementary Figure S1A–D, available at Annals of Oncology online, LDH serum levels negatively correlated with PFS and OS in patients treated with either selective BRAFi. However, loss of lymphocytes was not associated with a high level of LDH before therapy (supplementary Figure S1E and F, available at Annals of Oncology online) implying that the differing LDH levels in both groups before treatment did not contribute to the differential effects of dabrafenib and vemurafenib on peripheral lymphocytes. We further investigated the effect on leukocytes by analyzing neutrophil counts in the treated patients. Both selective BRAFi led to a significant decrease in peripheral neutrophils (vemurafenib: −23%, P < 0.0001; dabrafenib: −29.1%, P < 0.0001, data not shown). In contrast to lymphocytes, the change in peripheral neutrophil counts was similar in both treatment groups (P = 0.26, two-tailed Mann–Whitney test comparing the median change of neutrophil counts among both treatment groups). While dabrafenib and vemurafenib both decreased the number of circulating neutrophils, only vemurafenib-treated patients showed a loss of peripheral lymphocytes.
Figure 1.
Specific and common characteristics of selective BRAFi: vemurafenib (A), but not dabrafenib (B), causes a loss of absolute numbers of patients’ lymphocytes. Bars indicate medians, whiskers showing interquartile range. However, the Kaplan–Meier analyses of patients' OS and PFS grouped by patients below the first quartile, the median, the third quartile, and patients above the third quartile showed no association of changes in lymphocyte count and survival for both selective BRAFi (C–F). Absolute numbers of peripheral lymphocytes per nanoliter are shown (A and B). Each dot represents one individual patient. Cumulative survival expressed in weeks shown. Patients without an event were censored at last follow-up (C–F).
subset analyses of peripheral lymphocytes in patients treated with BRAFi
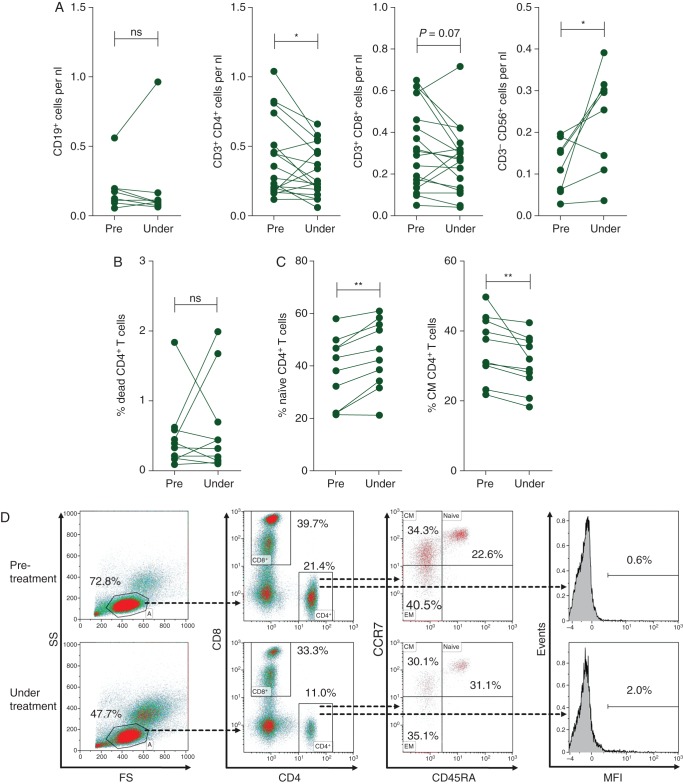
Human peripheral blood lymphocytes consist of three major subsets: NK cells (5–15%), B cells (5–15%) and T cells (70–90%). In vemurafenib-treated patients, the most profound loss of peripheral lymphocytes was in the CD4+ T cell compartment (Figure 2A). Absolute numbers of peripheral B cells remained unchanged. CD8+ T cells showed a trend toward decreased numbers. A significant increase in the absolute numbers of peripheral NK cells was noted (Figure 2A).
Figure 2.
Vemurafenib selectively diminishes circulating CD4+ T cells: (A) vemurafenib decreases the number of peripheral CD4+ T cells but increases NK cell numbers significantly while B cells and CD8+ T cells are unaffected. Absolute numbers of cells per nanoliter from 18 independent donors are shown. Each dot represents one individual patient. *P < 0.05. (B) The frequency of dead cells within the CD4+ gate is not affected by vemurafenib as determined in 10 independent donors before and during treatment. (C) However, an increase in the frequency of naïve CD4+ T cells and a decrease in CD4+ T cells with the phenotype of central memory (CM) T cells was observed. The frequency of CCR7+CD45RA+ (naïve) and CCR7+CD45RA− (CM) T cells within the CD4+ gate from 10 different donors is shown. **P < 0.01. (D) Representative density plots showing Th cell phenotype before therapy (top row) and during treatment (bottom row) of one melanoma patient. The frequency of lymphocytes as defined by their forward and side scatter (FS/SS) characteristics as well as the frequency of CD4+ cells within the lymphocyte gate decreases during treatment (left panel). In addition, the frequency of naïve CD4+ T cells increases while CM CD4+ T cells are reduced within peripheral lymphocytes during treatment (middle panel). However, no increase in the percentage of dead CD4+ T cells can be observed (right panel, MFI of aqua viability dye shown).
frequency and phenotype of peripheral CD4+ T cells under vemurafenib treatment
As peripheral CD4+ T cell numbers were most severely affected by vemurafenib, their phenotypical and functional properties were analyzed in more detail. As shown in Figure 2B, vemurafenib treatment did not increase the frequency of dead T cells among PBMC, but a significant decrease in CD4+ T cells with a central memory phenotype was observed, while the naїve cell population concurrently increased (Figure 2C and D).
CD4+ T cells can be subdivided in to several lineages and distinguished by their cytokine profile [10]. For functional analysis, we analyzed SN of polyclonal stimulated CD4+ T cells using multiplex cytokine arrays. As shown in supplementary Figure S2, available at Annals of Oncology online, a significant decrease in IFN-γ (P < 0.01) in SN from CD4+ T cells obtained during vemurafenib treatment compared with samples acquired before treatment was found, pointing to a preferential decrease in the numbers of type 1 helper T cells (Th1). A similar, also significant, change could be observed for interleukin (IL)-9 (P < 0.05). Levels of IL-2, IL-4, IL-10 and IL-17A remained unaffected. In summary, our ex vivo data indicate that vemurafenib alters the frequency, phenotype and function of human peripheral CD4+ T cells.
discussion
In this study, the impact of two selective BRAFi on the human peripheral immune system is investigated. While dabrafenib and vemurafenib showed similar clinical efficacy, only vemurafenib was found to cause a loss of peripheral lymphocytes. As previous studies relied on in vitro systems for functional analyses, our study also presents data on phenotype and function of human T cells obtained from patients treated with vemurafenib.
Follow-up data from the BRIM-3 (vemurafenib [11]) and BREAK-3 trial (dabrafenib [12]) showed a median PFS of 6.9 months (∼30 weeks) for vemurafenib and dabrafenib. The median OS was 18.2 months (∼79.2 weeks) for dabrafenib and 13.6 months (∼59.2 weeks) for vemurafenib. The median PFS (vemurafenib: 21.3 weeks, dabrafenib: 21.0 weeks) and median OS (vemurafenib: 44.1 weeks, dabrafenib: 46.3 weeks) were lower in the patient cohort presented than in these prospective clinical trials. However, while both BRIM-3 trial and BREAK-3 trial solely enrolled previously untreated patients without active brain metastases, we included pre-treated patients as well as patients with active brain metastases into our analyses. Also, the percentage of patients classified M1c was lower in both prospective phase III trials (BRIM-3 and BREAK-3: 66%) when compared with the cohort analyzed in the present study (vemurafenib group: 80%, dabrafenib group: 89%). The differences in PFS and OS observed in our cohort compared with the prospective clinical trials BRIM-3 and BREAK-3 are thus most likely due to differences in the type of patients included in the respective studies.
Elevated serum LDH is known to be associated with a worse prognosis in patients with metastatic melanoma [9]. In the cohort presented, an association between serum LDH and PFS as well as OS was found for both selective BRAFi. To our knowledge, associations of pre-therapeutic serum LDH with survival rates in patients treated with selective BRAFi have not been reported previously. Clinical response to selective BRAFi seems to be limited in patients with higher pretreatment serum LDH. Although a difference in LDH levels could contribute to the lower PFS and OS in the patient cohort presented, an exact comparison was not possible as exact values of serum LDH were not published for BRIM-3 and BREAK-3 trial, respectively.
Analyzing WBC from a large cohort of patients treated with selective BRAFi, we found that vemurafenib, but not dabrafenib, reduces absolute numbers of peripheral lymphocytes. The loss of peripheral lymphocytes was previously related to disease progression rather than treatment [13]. In our Kaplan–Meier analyses, no association between loss of peripheral lymphocytes and PFS or OS was found. A decrease in peripheral lymphocytes is therefore associated with BRAFi therapy and depends on the specific BRAFi rather than disease progression. While only vemurafenib caused a loss of peripheral lymphocytes, both selective BRAFi led to a significant but similar decrease in peripheral neutrophils. Despite similar clinical efficacy as dabrafenib, vemurafenib shows a previously unrecognized specific and selective effect on human peripheral lymphocyte populations.
Selective BRAFi induce paradoxical activation of the MAPK pathway in BRAF wild-type cells which could contribute to the functional and phenotypic changes in CD4+ T cells observed during vemurafenib treatment [14]. In vitro, 50 μM vemurafenib was shown to lead to increased levels of pERK1/2 and pp38 in human T cells [4]. In contrast, Callahan et al. [15] reported increased activation of ERK1/2 in monocytes harboring an NRAS G12R mutation from a patient receiving vemurafenib, while no elevated levels of pERK1/2 were observed in the respective lymphocytes. So far, evidence of paradoxical activation of the MAPK pathway in human lymphocytes by selective BRAFi is limited to high doses used in in vitro studies. Paradoxical activation could however be one explanation for the changes in frequency, phenotype and function of human lymphocytes observed in our study.
Recently, Hong et al. [7] carried out an extensive analysis of PBMC obtained from patients treated with dabrafenib and found no changes in the absolute numbers of lymphocyte subsets (T, B and NK cells) or ex vivo functionality of T cells. We observed a decline in peripheral CD4+ T cells and an increase in circulating NK cell numbers in patients treated with vemurafenib. Decreased survival as well as changes in the compartmental distribution of CD4+ T cell subsets are possible explanations. Survival of CD4+ T cells seems to be unaffected by vemurafenib treatment as no increased frequency of apoptotic CD4+ was observed, a finding consistent with previous in vitro studies [4]. An intriguing possible explanation would be that peripheral lymphocytes progressively infiltrate tumor tissue in response to vemurafenib. Increased infiltration of melanoma metastases by both CD8+ and CD4+ T cells has been reported in patients receiving vemurafenib but also for patients treated with dabrafenib [6]. As dabrafenib does not change the number of CD4+ and CD8+ T cells in the blood of treated patients [7], we believe a shift of cells from blood into tumor tissue is an unlikely mechanism for the loss of peripheral CD4+ cells observed in our study. Migration into secondary lymphoid tissue could be another explanation for the loss of peripheral CD4+ cells observed in our study. CCL21 is a major chemoattractant for CD4+ T cells [16]. Since activation of the MAPK pathway results in increased secretion of CCL21 by lymphatic endothelial cells [16], vemurafenib could induce migration into secondary lymphoid organs, but the effect of vemurafenib on endothelial cells remains to be determined. The decline in circulating CD4+ T cells appears not to be due to decreased survival but to a change in the compartmental distribution of CD4+ T cells. However, the reason for the decrease in circulating CD4+ T cells under therapy with vemurafenib remains unclear and has to be explored in future studies.
Despite a decrease in absolute numbers, we found phenotypic and functional changes in circulating CD4+ T cells in patients treated with vemurafenib. While the proportion of peripheral naїve CD4+CCR7+CD45RA+ T cells increased significantly, a concomitant decrease in the frequency of central memory (CM, CD4+CCR7+CD45RA−) T cells was noted [17]. Expansion of naїve CD4+ T cells was noted in patients with systemic lupus erythematosus receiving an IL-6 receptor-blocking antibody [18]. Since silencing mutated BRAF also leads to decreased secretion of IL-6 by melanoma cells [19], vemurafenib might cause an increase in circulating naїve CD4+ cells by blocking IL-6 production from melanoma cells. Compared with memory cells, naїve CD4+ T cells only produce low levels of effector cytokines [17]. Thus, the decrease in interferon-γ (IFN-γ) and IL-9 production by bulk CD4+ T cells from patients under vemurafenib treatment could be explained by the increased proportion of naїve CD4+ T cells. Since IFN-γ and IL-9 have been reported to play an important role in cancer immunosurveillance [20, 21], vemurafenib treatment of melanoma patients might have a dampening effect on anti-tumor immunity.
In summary, our data show unexpected differences between vemurafenib- and dabrafenib-treated patients. Both agents showed similar clinical efficacy with comparable PFS and OS which inversely correlated with pre-therapeutic serum LDH levels. The influence on patients' lymphocyte populations however differed significantly, with vemurafenib but not dabrafenib leading to a loss of peripheral lymphocytes. Vemurafenib further altered the frequency, function and phenotype of CD4+ T cells in patients with advanced melanoma. We believe our findings highlight that selective BRAFi need to be assessed individually for immunomodulatory effects, in particular, when planning combination therapies with other agents, such as immunotherapeutic substances.
funding
This work was supported by MERCUR Foundation (AN-2012-0030).
disclosure
BS has received honoraria from Roche and travel support from Bristol-Myers Squibb. WS has received honoraria from Roche and travel support from Bristol-Myers Squibb. EL has received honoraria from Roche, Bristol-Myers Squibb, Amgen, Boehringer Ingelheim, Merck Sharp & Dohme, and Merck, and travel support from Bristol-Myers Squibb. BW has received honoraria, travel and research support from Bristol-Myers Squibb. UT has been on the advisory board or has received honoraria from Roche, GlaxoSmithKline, Bristol-Myers Squibb and Merck. CB has been on the advisory board or has received honoraria from Bristol-Myers Squibb, GlaxoSmithKline, Merck Sharp & Dohme, Novartis and Roche. CL has received honoria from Roche, Bristol-Myers Squibb, Celgene and Johnson & Johnson and travel support from Roche, Bristol-Myers Squibb and Teva. JU is on the advisory board or has received honoraria and travel support from Roche, GlaxoSmithKline, Bristol-Myers Squibb, LEO Pharma and Merck. RG is on the advisory board or has received honoraria from Roche, Novartis, Amgen, GlaxoSmithKline, Bristol-Myers Squibb, Merck Sharp & Dohme, MerckSerono, Janssen and AlmirallHermal. LZ has received honoraria from Roche, Bristol-Myers Squibb, Boehringer Ingelheim and Amgen, and travel support from Merck Sharp & Dohme and Bristol-Myers Squibb. UH has received honoraria from Roche. DS is on the advisory board or has received honoraria from Roche, Genentech, Novartis, Amgen, GlaxoSmithKline, Bristol-Myers Squibb, Boehringer Ingelheim and Merck Sharp & Dohme. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The authors would like to thank I. Moll and B. Gutt for their technical support and N. Rompoti, B. Reising, I. Hermes and E. Muehlenstaedt for their clinical assistance.
references
- 1.Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14:e60–e69. doi: 10.1016/S1470-2045(12)70539-9. [DOI] [PubMed] [Google Scholar]
- 2.Wolchok JD, Weber JS, Maio M, et al. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol. 2013;24:2174–2180. doi: 10.1093/annonc/mdt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comin-Anduix B, Chodon T, Sazegar H, et al. The oncogenic BRAF kinase inhibitor PLX4032/RG7204 does not affect the viability or function of human lymphocytes across a wide range of concentrations. Clin Cancer Res. 2010;16:6040–6048. doi: 10.1158/1078-0432.CCR-10-1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 6.Wilmott JS, Long GV, Howle JR, et al. Selective BRAF inhibitors induce marked T cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 7.Hong DS, Vence L, Falchook G, et al. BRAF(V600) inhibitor GSK2118436 targeted inhibition of mutant BRAF in cancer patients does not impair overall immune competency. Clin Cancer Res. 2012;18:2326–2335. doi: 10.1158/1078-0432.CCR-11-2515. [DOI] [PubMed] [Google Scholar]
- 8.Schilling B, Sucker A, Griewank K, et al. Vemurafenib reverses immunosuppression by myeloid derived suppressor cells. Int J Cancer. 2013;133:1653–1663. doi: 10.1002/ijc.28168. [DOI] [PubMed] [Google Scholar]
- 9.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2009;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman PB, Robert C, Larkin JMG, et al. Updated overall survival (OS) results for BRIM-3, a phase III randomized, open-label, multicenter trial comparing BRAF inhibitor vemurafenib (vem) with dacarbazine (DTIC) in previously untreated patients with BRAFV600E-mutated melanoma. J Clin Oncol. 2012;30(suppl) abstract 8502. [Google Scholar]
- 12.Hauschild A, Demidov LV, Jouary T, et al. An update on BREAK-3, a phase III, randomized trial: Dabrafenib (DAB) versus dacarbazine (DTIC) in patients with BRAF V600E-positive mutation metastatic melanoma (MM) J Clin Oncol. 2013;31(suppl) abstract 9013. [Google Scholar]
- 13.Bouwhuis MG, ten Hagen TL, Eggermont AM. Immunologic functions as prognostic indicators in melanoma. Mol Oncol. 2011;5:183–189. doi: 10.1016/j.molonc.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010;464:427–430. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Callahan MK, Rampal R, Harding JJ, et al. Progression of RAS-mutant leukemia during RAF inhibitor treatment. N Engl J Med. 2012;367:2316–2321. doi: 10.1056/NEJMoa1208958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyagaki T, Sugaya M, Okochi H, et al. Blocking MAPK signaling downregulates CCL21 in lymphatic endothelial cells and impairs contact hypersensitivity responses. J Invest Dermatol. 2011;131:1927–1935. doi: 10.1038/jid.2011.135. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Forster R, et al. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 18.Shirota Y, Yarboro C, Fischer R, et al. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann Rheum Dis. 2013;72:118–128. doi: 10.1136/annrheumdis-2012-201310. [DOI] [PubMed] [Google Scholar]
- 19.Sumimoto H, Imabayashi F, Iwata T, et al. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203:1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purwar R, Schlapbach C, Xiao S, et al. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012 doi: 10.1038/nm.2856. doi:10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.