In a prospective randomized clinical trial in elderly patients with metastatic breast cancer, pegylated liposomal doxorubicin and capecitabine demonstrated comparable efficacy and acceptable tolerance as first-line single-agent chemotherapy.
Many included patients were aged = 75 years (54%) and had ≥ 1 geriatric conditions (71%).
Patients aged ≥ 80 years were less likely to complete chemotherapy.
Keywords: metastatic breast cancer, capecitabine, pegylated liposomal doxorubicin, phase III, elderly, geriatric
Abstract
Background
Prospective data on chemotherapy for elderly patients with metastatic breast cancer (MBC) remain scarce. We compared the efficacy and safety of first-line chemotherapy with pegylated liposomal doxorubicin (PLD) versus capecitabine in MBC patients aged ≥65 years in a multicentre, phase III trial.
Patients and methods
Patients were randomized to six cycles of PLD (45 mg/m2 every 4 weeks) or eight cycles of capecitabine (1000 mg/m2 twice daily, day 1–14 every 3 weeks).
Results
The study enrolled 78 of the planned 154 patients and was closed prematurely due to slow accrual and supply problems of PLD. Many included patients were aged ≥75 years (54%) and vulnerable (≥1 geriatric condition: 71%). The median dose intensity was 85% for PLD and 84% for capecitabine, respectively. In both arms, the majority of patients completed at least 12 weeks of treatment (PLD 73%; capecitabine 74%). After a median follow-up of 39 months, 77 patients had progressed and 62 patients had died of MBC. Median progression-free survival was 5.6 versus 7.7 months (P = 0.11) for PLD and capecitabine, respectively. Median overall survival was 13.8 months for PLD and 16.8 months for capecitabine (P = 0.59). Both treatments were feasible, grade 3 toxicities consisting of fatigue (both arms: 13%), hand–foot syndrome (PLD: 10%; capecitabine: 16%), stomatitis (PLD: 10%; capecitabine: 3%), exanthema (PLD: 5%) and diarrhoea (PLD: 3%; capecitabine: 5%). Only 1 of 10 patients aged ≥80 years completed chemotherapy, while 3 and 6 patients discontinued treatment due to toxicity or progressive disease, respectively.
Conclusion
Both PLD and capecitabine demonstrated comparable efficacy and acceptable tolerance as first-line single-agent chemotherapy in elderly patients with MBC, even in vulnerable patients or patients aged ≥75 years. However, patients aged ≥80 years were unlikely to complete chemotherapy successfully.
Clinical Trial numbers
EudraCT 2006-002046-10; ISRCTN 11114726; CKTO 2006-09; BOOG 2006-02.
introduction
Data on chemotherapy in elderly patients with metastatic breast cancer (MBC) are limited [1, 2]. So far, ∼40 phase II trials and only 2 prospective randomized clinical trials have been reported, mainly in relatively fit patients aged 75 years or younger [2–4]. Results of studies on palliative chemotherapy in non-elderly patients, however, cannot be extrapolated to elderly patients because the latter are at an increased risk of toxicity due to altered pharmacokinetics related to impaired organ functions and potential drug interactions due to polypharmacy [5]. Moreover, quality of life and life expectancy may be hampered by comorbidities and older age itself.
Updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (Eusoma) recommend single-agent chemotherapy agents with favourable safety profiles for elderly patients having either ER-negative, hormone refractory or rapidly progressive disease (PD) [6]. Anthracyclines and taxanes are effective agents in breast cancer, at the cost of myelotoxicity, alopecia and potential cardiotoxicity for anthracyclines and neuropathy for taxanes, respectively. Pegylated liposomal doxorubicin (PLD; Caelyx®; Janssen Cilag) and capecitabine (Xeloda®; Roche) appear to be effective and well tolerated in elderly cancer patients even with compromised condition [7, 8].
In this randomized phase III trial, we compared the efficacy and feasibility of PLD with capecitabine as single-agent first-line palliative chemotherapy in older metastatic breast cancer (MBC) patients (OMEGA trial).
methods
patients
Female patients aged ≥65 years with MBC and an indication for first-line chemotherapy were eligible for this trial. Additional inclusion criteria were as follows: ECOG performance status (PS) 0–2 (3 was allowed in case of pain or a pre-existing disabling disease); life expectancy of at least 3 months; adequate bone marrow function (white blood cells >3 × 109/l and platelets >100 × 109/l); acceptable renal function (creatinin clearance >40 ml/min); acceptable liver function (serum bilirubin <2 × upper normal limit (UNL), AST and ALT values <2 × UNL in the absence of liver metastases); normal baseline left ventricular ejection fraction by MUGA scan according to institutional limits. Previous adjuvant chemotherapy with anthracylines was allowed, considering a cumulative dose of <240 mg/m2 of doxorubicin or <450 mg/m2 of epirubicin and completion for at least 12 months. The study protocol was approved by the local ethics committees of all participating centres. All patients provided written informed consent before study entry.
treatment plan and evaluation
Patients were assigned to receive six cycles of PLD 45 mg/m2 given i.v. on day 1 every 4 weeks or eight cycles of capecitabine 1000 mg/m2 twice daily, taken orally on day 1–14 every 3 weeks. The dose of capacitabine was rounded to the nearest dose that could be administered using tablets of 500 mg. Stratification factors included ECOG PS (0–1 versus 2), HER2 status of the primary tumour (overexpression or not), site of metastatic disease (<or ≥3), previous adjuvant therapy (hormonal and/or chemotherapy, yes or no anthracyclines) and previous hormonal therapy for MBC (yes or no). Patient randomization was carried out at the datacentre of the Dutch Breast Cancer Research Group (BOOG). Study data were collected by the Integraal Kankercentrum Nederland.
Toxicity was graded according to the Common Toxicity Criteria (CTC) of the NCI, version 3. Patients were assessed clinically at least every 4 weeks and response evaluation was carried out after 12 weeks and at the end of study treatment, using RECIST criteria (version 1.0). Questionnaires on quality of life, using the EORTC Quality of Life Questionnaire C-30, and a geriatric assessment (GA) were completed at study entry, after 12 weeks and at the end of study treatment. GA examined the functional status, comorbidity, number of medications, nutritional status, cognition and mood. Patients were considered vulnerable if they had one or more geriatric conditions defined as full dependence of instrumental activities of daily living (IADL), comorbidity, polypharmacy (use of five or more types of medication), cognitive impairment, undernutrition and/or depressive syndromes. Methods and results of the GA in this study have been reported separately [9].
The primary objective of the study was to test the superiority of six cycles of PLD over eight cycles of capecitabine in regard to progression-free survival (PFS). Secondary objectives included the same comparisons regarding response rates (RRs), overall survival (OS) and the evaluation of toxicities and compliance.
statistical methods
Assuming a median PFS of 4 months in the standard treatment arm of capecitabine and a median PFS in the experimental arm (PLD) of 7 months [hazard ratio (HR) 0.57] at least 100 events needed to be observed to provide 80% power using a two-sided log-rank test with α = 0.05 and assuming exponential survival. Further, it was calculated that an accrual time of 15–18 months with a follow-up of 9 months would be sufficient to observe the required number of events in a total sample of 154 patients. All main analyses were done in accordance with the intention-to-treat (ITT) principle.
The formal comparison with respect to the PFS end point between the treatment arms was evaluated using a two-sided log-rank test with significance level of 0.05. PFS was defined as time from randomization to progression or death. Patients still alive without progression at the time of analysis were censored. All other analyses of the primary and secondary end points were of a non-inferential, i.e. of a hypotheses-generating, nature. These analyses included prognostic modelling with Cox proportional hazard regression.
An interim analysis was planned when 77 patients were assessable.
results
patient characteristics
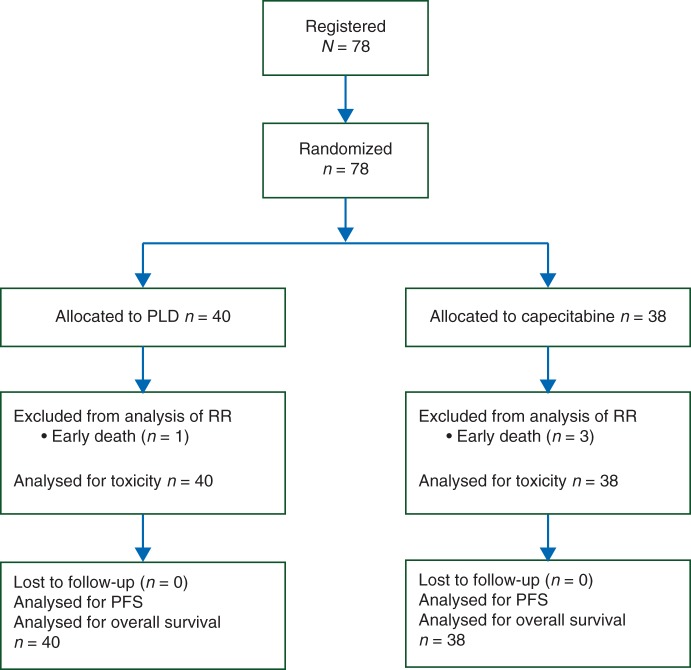
Between April 2007 and August 2011, 25 participating Dutch hospitals enrolled a total of 78 patients. The study was closed prematurely due to slow accrual, and eventually due to supply problems of PLD, before the interim analysis was carried out. The cut-off date for analysis was 10 September 2012, resulting in a median follow-up time of 39 months. All patients were eligible and none were lost to follow-up. Figure 1 depicts the CONSORT diagram of the study. Patient characteristics were well balanced between study arms (Table 1). The median age was 75 years (range 65–86 years) and 42 patients (54%) were ≥75 years. Seventeen patients (22%) had a PS of 2. Fifty-two of 73 patients assessable for a geriatric assessment (71%) had one or more geriatric conditions and were considered as being vulnerable. The most common geriatric conditions consisted of partial dependence in IADL, (89%), polypharmacy (51%) and depressive symptoms (33%). Undernutrition and cognitive impairment were less prevalent (5% and 7%, respectively).
Figure 1.
CONSORT diagram for the OMEGA study.
Table 1.
Patient pre-treatment characteristics by treatment arm
| PLD (N = 40) |
Capecitabine (N = 38) |
|||
|---|---|---|---|---|
| No. of patients | % | No. of patients | % | |
| Age, years | ||||
| <75 | 21 | 52 | 15 | 39 |
| 75–80 | 12 | 30 | 20 | 53 |
| ≥80 | 7 | 18 | 3 | 8 |
| ECOG performance score | ||||
| 0 | 12 | 30 | 11 | 29 |
| 1 | 19 | 48 | 18 | 47 |
| 2 | 8 | 20 | 9 | 24 |
| 3 | 1 | 2 | 0 | 0 |
| ER receptor status | ||||
| ER+ | 25 | 62 | 22 | 58 |
| ER− | 10 | 25 | 12 | 32 |
| Unknown | 5 | 12 | 4 | 11 |
| HER2 status | ||||
| HER2− | 25 | 62 | 23 | 61 |
| HER2 overexpression | 1 | 2 | 2 | 5 |
| Unknown | 14 | 35 | 13 | 34 |
| Metastatic sites | ||||
| Lung | 15 | 38 | 17 | 45 |
| Liver | 22 | 55 | 16 | 42 |
| Bone only | 3 | 8 | 4 | 11 |
| Visceral only | 12 | 30 | 12 | 32 |
| Prior systemic treatment | ||||
| Adjuvant chemotherapy | 5 | 12 | 5 | 13 |
| Adjuvant hormonal therapy | 19 | 48 | 18 | 47 |
| Palliative hormonal therapy | 24 | 60 | 21 | 55 |
treatment duration and compliance
The median number of cycles was five (range 1–6) in the PLD arm and seven (range 1–8) in the capecitabine arm. The median dose intensity was 85% for PLD and 84% for capecitabine, respectively, irrespective of age. Reason for treatment discontinuation was PD in 15 PLD patients and 10 patients on capecitabine, and toxicity in nine and eight patients, respectively.
Responses were evaluated after 12 weeks, i.e. three cycles of PLD or four cycles of capecitabine. In both arms, the majority of patients completed at least 12 weeks of treatment (PLD 73%; capecitabine 74%). Figure 2 depicts the number of delivered cycles of chemotherapy in relation to age. Eighteen of 21 PLD patients aged < 75 years (86%) completed three cycles, when compared with 11 of 19 patients aged ≥75 years (58%). Likewise, 13 of 15 patients aged <75 years (87%) completed four cycles of capecitabine, when compared with 16 of 23 patients aged ≥75 years (70%). Of 10 patients aged ≥80 years, 1 (10%) completed chemotherapy, while 3 and 6 patients discontinued treatment due to toxicity or PD, respectively. The percentage of patients who received a subsequent line of systemic therapy on disease progression was 60% in the PLD arm and 63% in the capecitabine arm.
Figure 2.
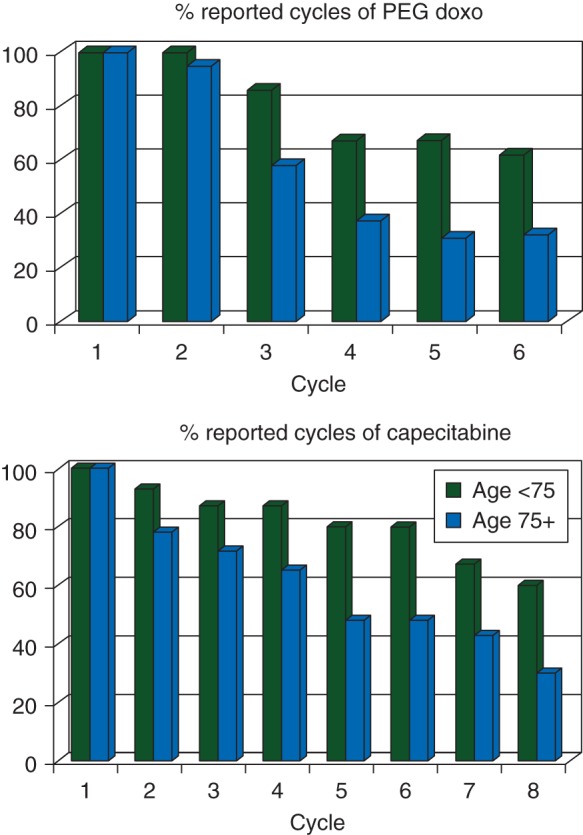
The number of reported cycles of chemotherapy in relation to age.
treatment response and outcome
Four patients (one on PLD, three on capecitabine) died within 1 month after randomization (3 due to early PD), and in accordance with protocol were excluded from response evaluation. However, all patients were included in the analysis of PFS, OS and toxicity. Sixty-five patients had assessable disease. In the ITT analysis, the overall RR for PLD and capecitabine was 18% and 17%, respectively. We did not observe a complete response. An additional 54% of patients treated with PLD and 45% of patients treated with capecitabine had stable disease (SD). Best response in nine assessable patients aged ≥80 years was SD in five patients and PD in four patients.
After a median follow-up of 39 months 77 patients (99%) had progressed and 62 patients (79%) had died. Causes of death were MBC (N = 56), cardiovascular disease (N = 1), early progression within 1 month after inclusion (N = 4) and toxicity (gastro-intestinal haemorrhage in a patient treated with capecitabine, N = 1).
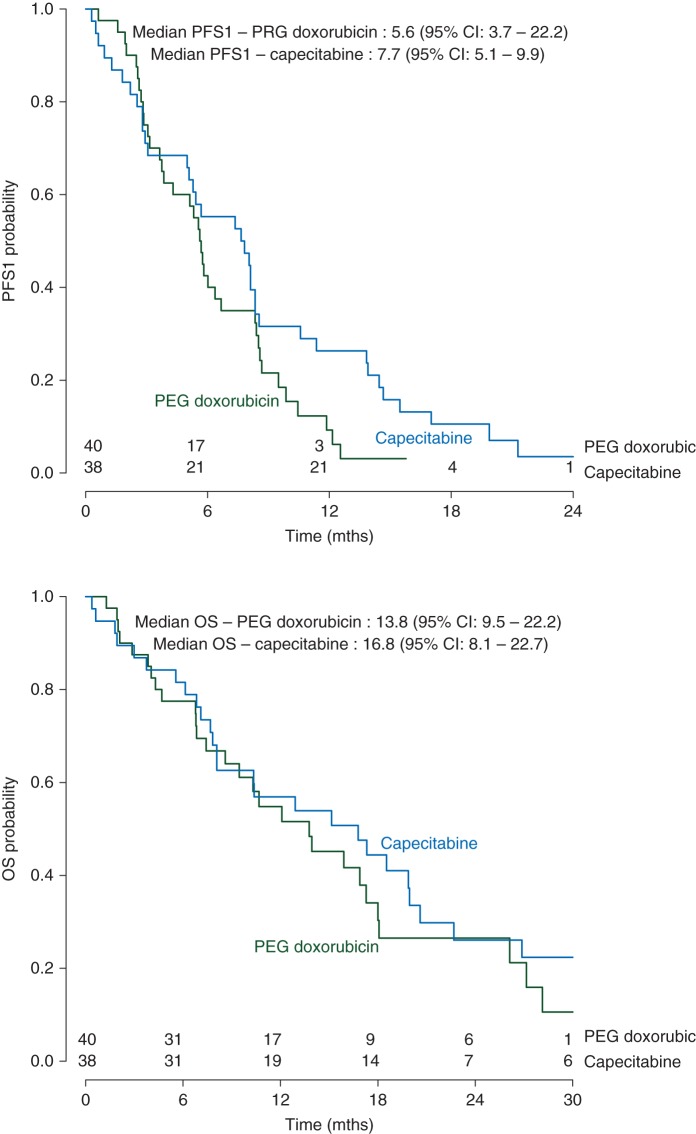
As depicted in Figure 3, median PFS was 5.6 months [95% confidence interval (CI) 3.7–8.4 months] for PLD and 7.7 months (95% CI 5.1–9.9) for capecitabine (HR 0.68; 95% CI 0.42–1.09; P = 0.11).
Figure 3.
Kaplan–Meier estimates of progression-free survival and overall survival comparing PLD versus capecitabine.
Likewise, the study did not observe a significant difference in OS between the chemotherapy regimens. Median OS was 13.8 months (95% CI 9.5–22.2 months) for PLD and 16.8 months (95% CI 8.1–22.7 months) for capecitabine (HR 0.87; 95% CI 0.53–1.44; P = 0.59).
In the multivariate Cox proportional hazards model, age was a significant predictor of OS (75–80 versus <75 years; HR 1.98; 95% CI 1.12–3.5; P = 0.02) (≥80 versus <75 years; HR 2.35; 95% CI 1.08–5.11; P = 0.03), but not of PFS (75–80 versus <75 years; HR 1.23; 95% CI 0.75–2.02; P = 0.41) (≥80 years versus <75 years; HR 1.63; 95% CI 0.80–3.34; P = 0.18).
toxicity
Toxicity was assessed in all patients and in all chemotherapy cycles (Table 2). Generally, treatment was well tolerated. The most common grade 3 toxicities associated with PLD were fatigue (13%), hand–foot syndrome (HFS) (10%), stomatitis (10%) and exanthema (5%), without any grade 4 toxicity. In patients who received capecitabine, grade 3 toxicities consisted of fatigue (13%), HFS (16%), diarrhoea (5%) and pulmonary embolism (3%). One patient on capecitabine died due to a gastrointestinal haemorrhage. Alopecia was more often observed in patients treated with PLD (grade 1: 35%; grade 2: 3%) than in patients on capecitabine (grade 1: 11%).
Table 2.
Grade 3 and 4 related adverse events by treatment arm
| PLD (N = 40) |
Capecitabine (N = 38) |
|||
|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Fatigue | 5 (13%) | – | 5 (13%) | – |
| Exanthema | 2 (5%) | – | – | – |
| Hand–foot syndrome | 4 (10%) | – | 6 (16%) | – |
| Stomatitis | 4 (10%) | – | 1 (3%) | – |
| Nausea | – | – | 1 (3%) | – |
| Diarrhoea | 1 (3%) | – | 2 (5%) | – |
| Neutropenic fever | 1 (3%) | – | – | – |
| Cardiac | 1 (3%) | – | – | – |
| Pulmonary embolism | 1 (3%) | – | – | – |
Three of 10 patients aged ≥80 years stopped treatment due to toxicity grade 3 (1 patient on PLD with HFS, 1 patient on PLD with hypertension, 1 patient on capecitabine with HFS).
discussion
In this multicentre, randomized, phase III study in MBC patients aged ≥65 years, first-line chemotherapy with either PLD or capecitabine appeared feasible, even in vulnerable patients or patients aged ≥75+. Efficacy regarding RR, PFS and OS was similar in both treatment arms. However, as this study was closed prematurely due to slow accrual and supply problems of PLD and enrolled only 78 of the planned 154 patients, it may have failed to meet its primary end point to show a difference in PFS between the two arms. Of notice, the vast majority of patients in our study died of breast cancer and not due to comorbidity or toxicity, implying that results on OS in our study resemble disease-specific survival.
To our knowledge, the current trial is only the third randomized study on chemotherapy in elderly MBC patients. O'Shaughnessy et al. compared capecitabine (daily dose of 2500 mg/m2) with i.v. CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line chemotherapy in 95 women aged 55 years or older [3]. Patients had a median age of 69 years and a median Karnofsky PS of 90%. Median time to progression (TTP) for capecitabine was 4.1 months and for CMF 3.0 months and median OS was 19.6 and 17.2 months in both arms, respectively. Capecitabine was stopped due to toxicity in 16% of patients and reduced in dose in 34% of patients. In the second randomized study, 410 patients aged 60 years or older were randomized for first-line chemotherapy with either gemcitabine or weekly epirubicin (at a dose of 35 mg/m2) [4]. Median age of patients was 68 years and 20% of patients had a Karnofsky PS of 60%–70%. Weekly epirubicin was superior to gemcitabine with a median TTP of 6.1 versus 3.4 months (P = 0.0001) and a median OS of 19.1 versus 11.8 months (P = 0.0004). Weekly epirubicin was well tolerated with grade 3–4 neutropenia and mucositis in 19% and 8% of patients, respectively. Results of our study regarding efficacy and tolerability of monotherapy with PLD or capecitabine as palliative chemotherapy in MBC are in line with these studies, which included relatively fit and young patients, while our study adds data on the feasibility of these regimens in vulnerable elderly patients and in patients aged ≥75 years.
Results of the German PELICAN study, a randomized phase III study evaluating PLD (50 mg/m2) versus capecitabine (2500 mg/m2) as first-line chemotherapy in 210 MBC patients (range 22–85 year) have not yet been presented in full paper [10]. However, this study will not entirely be comparable with our study as the inclusion criteria regarding age and doses of chemotherapy differ.
Although the paucity of randomized clinical trials in elderly MBC patients is compensated by prospective phase II trials in this patient group, even these trials suffer from inclusion bias by tending to include mainly relatively young and fit patients. Phase II studies on capecitabine [8, 11] and PLD [7, 12] in the elderly included mainly patients with an ECOG PS of 0–1 and a median age <75 year and do not report any results on efficacy or safety in vulnerable patients or patients aged 75 years or older.
To improve knowledge on chemotherapy in the elderly, the lower age limit of 65 years in our study may have been too low. Considering the overall improvement of health during the past decades and the improved life expectancy, Debled et al. already stated that a cut-off point of 75 years might be more appropriate for future trials focusing on palliative chemotherapy in the elderly [2]. A French observational study in elderly women with MBC, using a discriminating function analysis, found the age of 76 years to be the age above which patients were treated in a different way when compared with younger patients [13]. Although one-third of women who die of breast cancer is ≥75 years, only a small number of patients older than 75 years has been included in chemotherapy studies. Data on efficacy and feasibility of chemotherapy for these patients therefore remain scarce. We observed comparable efficacy and acceptable tolerance of both PLD and capecitabine in patients aged ≥75 years but a poor outcome in patients aged 80 years or older. Due to the low number of 10 patients aged ≥80 years, we can only speculate on reasons for their poor outcome in our study. A study on docetaxel chemotherapy in patients with castration-resistant prostate cancer reported a worse OS in 18 patients aged ≥80 years with poor treatment compliance due to more toxicity [14]. Other data on palliative chemotherapy in very elderly patients are extremely rare.
Regarding the accrual problems in our study, we have previously reported on barriers to accrual such as the patient's refusal of either chemotherapy or randomization, or a medical condition considered as being too fit or too frail for inclusion [15]. Prior studies focussing on adjuvant chemotherapy in elderly breast cancer patients (CASA and ACTION) have also been closed prematurely due to poor recruitment [16, 17]. Numerous reviews and retrospective studies have already emphasized the under-representation and under-treatment of elderly cancer patients and the lack of evidence-based treatment guidelines for these patients. The EORTC and SIOG recently reported various recommendations for better clinical trial design in the elderly such as using composite end points and obligatory integration of some form of GA [18].
In conclusion, in this randomized study on first-line single-agent chemotherapy in elderly MBC patients comparable efficacy and acceptable tolerance of both PLD and capecitabine were observed, even in vulnerable patients or patients ≥75 years. However, patients aged ≥80 years were less likely to complete chemotherapy successfully.
funding
This work was supported by unrestricted grants from Amgen BV the Netherlands (166214), Schering Plough the Netherlands (BCA 3001) and Janssen Cilag BV the Netherlands, MSD (p05028).
disclosure
The authors have declared no conflict of interest.
acknowledgements
The authors thank the patients and the other investigators participating in this study.
references
- 1.Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer. Breast. 2012;21:242–252. doi: 10.1016/j.breast.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Debled M, Bellera C, Donamaria C, et al. Chemotherapy treatment for older women with metastatic breast cancer: what is the evidence? Cancer Treat Rev. 2011;37:590–598. doi: 10.1016/j.ctrv.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 3.O'Shaughnessy JA, Blum J, Moiseyenko J, et al. Randomized, open label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate, 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol. 2001;12:1247–1254. doi: 10.1023/a:1012281104865. [DOI] [PubMed] [Google Scholar]
- 4.Feher O, Vodvarka P, Jassem J, et al. First-line gemcitabine versus epirubicin in postmenopausal women aged 60 or older with metastatic breast cancer: a multicenter, randomized, phase III study. Ann Oncol. 2005;6:899–908. doi: 10.1093/annonc/mdi181. [DOI] [PubMed] [Google Scholar]
- 5.Hamberg P, Verweij J, Seyanaeve C. Cytotoxic therapy for the elderly with metastatic breast cancer: a review on safety, pharmacokinetics and efficacy. Eur J Cancer. 2007;43:1514–1528. doi: 10.1016/j.ejca.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Biganzoli L, Wildiers H, Oakman C, et al. Management of elderly patients with breast cancer: updated recommendations of the International Society of Geriatric Oncology (SIOG) and European Society of Breast Cancer Specialists (EUSOMA) Lancet Oncol. 2012;13:e148–e160. doi: 10.1016/S1470-2045(11)70383-7. [DOI] [PubMed] [Google Scholar]
- 7.Green H, Stal O, Bachmeier K, et al. Pegylated liposomal doxorubicin as first-line monotherapy in elderly women with locally advanced or metastatic breast cancer: novel treatment predictive factors identified. Cancer Lett. 2011;27:145–153. doi: 10.1016/j.canlet.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Bajetta E, Procopio G, Celio L, et al. Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J Clin Oncol. 2005;23:2155–2161. doi: 10.1200/JCO.2005.02.167. [DOI] [PubMed] [Google Scholar]
- 9.Hamaker ME, Seynaeve C, Wymenga ANM, et al. Baseline comprehensive geriatric assessment is associated with toxicity ad survival in elderly metastatic breast cancer patients receiving single-agent chemotherapy: results from the OMEGA study of the Dutch Breast Cancer Trialists’ Group. 2014;23:81–87. doi: 10.1016/j.breast.2013.11.004. Breast. [DOI] [PubMed] [Google Scholar]
- 10.Jager E, Al-Batran S, Saupe S, et al. A randomized phase III study evaluating pegylated liposomal doxorubicin versus capecitabine for metastatic breast cancer. J Clin Oncol. 2010;28:15s. doi: 10.1007/s10549-016-4033-3. (supll; abstr 1022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotsori AA, Noble JL, Ashley S, et al. Moderate dose capecitabine in older patients with metastatic breast cancer: a standard option for first line treatment? Breast. 2010;19:377–381. doi: 10.1016/j.breast.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Biganzoli L, Coleman R, Minisini A, et al. A joined analysis of two European Organisation for the Research and Treatment of Cancer (EORTC) studies to evaluate the role of pegylated liposomal doxorubicin (Caelyx) in the treatment of elderly patients with metastatic breast cancer. Crit Rec Oncol Hematol. 2007;61:84–89. doi: 10.1016/j.critrevonc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Freyer G, Braud A-C, Chaibi P, et al. Dealing with metastatic breast cancer in elderly women: results from a French study on a large cohort carried out by the ‘Observatory on Elderly Patients. Ann Oncol. 2006;17:211–216. doi: 10.1093/annonc/mdj043. [DOI] [PubMed] [Google Scholar]
- 14.Gerritse FL, Meulenbeld HJ, Roodhart JML, et al. Analysis of docetaxel therapy in elderly (70 years old) castration resistant prostate cancer patients enrolled in the Netherlands Prostate Study. Eur J Cancer. 2013;49:3176–3183. doi: 10.1016/j.ejca.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Hamaker ME, Seynaeve C, Wymenga ANM, et al. Slow accrual of elderly patients with metastatic breast cancer in the Dutch multicentre OMEGA study. Breast. 2013;22:556–559. doi: 10.1016/j.breast.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Crivellari D, Gray KP, Dellapasqua S, et al. Adjuvant pegylated liposomal doxorubicin for older women with endocrine nonresponsive breast cancer who are NOT suitable for a “standard chemotherapy regimen”: the CASA randomized trial. Breast. 2013;22:130–137. doi: 10.1016/j.breast.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard R, Ballinger R, Cameron D, et al. Adjuvant chemotherapy in older women (ACTION) study: what did we learn from the pilot phase? Br J Cancer. 2011;25:1260–1266. doi: 10.1038/bjc.2011.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wildiers H, Mauer M, Pallis A, et al. End point and trial design in geriatric oncology research: a joint European Organisation for Research and Treatment of Cancer—alliance for clinical trials in oncology—International Society of Geriatric Oncology position article. J Clin Oncol. 2013;31:3711–3718. doi: 10.1200/JCO.2013.49.6125. [DOI] [PubMed] [Google Scholar]