We compared retrospectively carboplatin/paclitaxel to cisplatinum/5-FU as dCRT treatment in esophageal cancer patients. We found comparable outcome, but lower toxicity rates and higher treatment compliance in the carboplatin/paclitaxel group. Therefore, we suggest carboplatin/paclitaxel as an alternative for cisplatinum/5-FU is a good candidate for further evaluation.
Keywords: esophageal, cancer, carboplatin, paclitaxel, definitive, chemoradiation
Abstract
Background
In esophageal cancer (EC) patients who are not eligible for surgery, definitive chemoradiation (dCRT) with curative intent using cisplatinum with 5-fluorouracil (5-FU) is the standard chemotherapy regimen. Nowadays carboplatin/paclitaxel is also often used. In this study, we compared survival and toxicity rates between both regimens.
Patients and methods
This multicenter study included 102 patients treated in five centers in the Northeast Netherlands from 1996 till 2008. Forty-seven patients received cisplatinum/5-FU (75 mg/m2 and 1 g/m2) and 55 patients carboplatin/paclitaxel (AUC2 and 50 mg/m2).
Results
Overall survival (OS) was not different between the cisplatinum/5-FU and carboplatin/paclitaxel group {[P = 0.879, hazard ratio (HR) 0.97 [confidence interval (CI) 0.62–1.51]}, with a median survival of 16.1 (CI 11.8–20.5) and 13.8 months (CI 10.8–16.9). Median disease-free survival (DFS) was comparable [P = 0.760, HR 0.93 (CI 0.60–1.45)] between the cisplatinum/5-FU group [11.1 months (CI 6.9–15.3)] and the carboplatin/paclitaxel group [9.7 months (CI 5.1–14.4)]. Groups were comparable except clinical T stage was higher in the carboplatin/paclitaxel group (P = 0.008). High clinical T stage (cT4) was not related to OS and DFS in a univariate analysis (P = 0.250 and P = 0.201). A higher percentage of patients completed the carboplatin/paclitaxel regimen (82% versus 57%, P = 0.010). Hematological and nonhematological toxicity (≥grade 3) in the carboplatin/paclitaxel group (4% and 18%) was significantly lower than in the cisplatinum/5-FU (19% and 38%, P = 0.001).
Conclusions
In this study, we showed comparable outcome, in terms of DFS and OS for carboplatin/paclitaxel compared with cisplatinum/5-FU as dCRT treatment in EC patients. Toxicity rates were lower in the carboplatin/paclitaxel group together with higher treatment compliance. Carboplatin/paclitaxel as an alternative treatment of cisplatinum/5-FU is a good candidate regimen for further evaluation.
introduction
With an increasing incidence and overall 5-year survival of about 15%, the prognosis of esophageal cancer (EC) patients remains poor [1–4]. In patients treated surgically with curative intent, 5-year survival rates are usually between 25% and 39%. In an attempt to improve prognosis, multimodality treatment has been incorporated during the last two decades. Neoadjuvant chemoradiation has shown to be superior compared with surgery alone with a gain of 12%–15%, leading to be the current standard procedure in medically fit patients with curative resectable esophageal carcinoma [5, 6].
In patients who are not eligible for curative intended surgery, due to a close relation of the tumor with- or tethered to vital structures (aorta, trachea, especially the higher lesions) or patients otherwise medically unfit for surgical resection, definitive chemoradiation (dCRT) has to be considered as an alternative option. The RTOG 85-01 trial showed that in patients not receiving surgery, chemoradiation with cisplatinum and 5-fluorouracil (5-FU) improved 5-year survival up to 26% compared with patients receiving only radiotherapy (RT) [7, 8]. Several chemotherapy regimens are currently being used as definitive regimen in EC patients. The most commonly used regimens are those consisting of cisplatinum in combination with 5-FU or paclitaxel combined with carboplatin. Current guidelines in the United States and Europe recommend the combination of cisplatinum with 5-FU as standard combined with 50.4 Gy radiation therapy [9], while the carboplatin/paclitaxel regimen is frequently used in patients with extensive comorbidity [10]. Cisplatinum has a high-toxicity profile and carboplatin is an often used alternative in platinum-based therapy regimens. However, no study has yet investigated the superiority of one of these regimens in overall survival (OS) in patients receiving dCRT.
The aim of this study was to compare the differences in survival and toxicity rates between cisplatinum with 5-FU and carboplatin with paclitaxel as dCRT in a relatively large homogenous cohort of EC patients treated in Northeast Netherlands.
patients and methods
patients
In this multicenter retrospective study, we analyzed 102 EC patients without distant metastases, who were treated with curatively intended dCRT in five centers in the Northeast Netherlands from 1996 till 2008. This subgroup of patients treated with only chemoradiation as definitive treatment is part of a larger cohort described elsewhere [11]. As described in the publication of Smit et al., the indications in both dCRT regimens were technically unresectable tumors, medically unfit patients or patient's own choice. Carboplatin/paclitaxel was the standard regimen in two of the five centers and also preferred above cisplatinum/5-FU for patients with cardiovascular comorbidity. Patients with other histology than adenocarcinoma or squamous carcinoma were excluded as well as cases with missing relevant staging information or inadequate follow-up.
methods
pretreatment staging
Pretreatment staging consisted of endoscopic ultrasonography (EUS) with fine needle aspiration of suspected lymph nodes, 16–64 multidetector computed tomography (md-CT) scans of the neck, chest and abdomen and on indication cervical echographic examination. From 2002 onward, 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) was added to the staging procedure. Bronchoscopy was required when the tumor was tethered to the trachea or main stem bronchus. Patients were staged according to the Union for International Cancer Control TNM 6th edition [12].
chemotherapy regimens
The cisplatinum/5-FU dCRT (N = 47) regimen consisted of cisplatinum 75 mg/m2 (day 1) and 5-FU 1 g/m2 (day 1–4) at week 1 and 5 during RT, with two additional courses on week 8 and 11 (RTOG 85-01 scheme) [7].
In the carboplatin/ paclitaxel group (N = 55), a chemotherapy scheme was given weekly during RT at day 1, 8, 15, 22, 29 (and 35). The paclitaxel dose was 50 mg/m2 and carboplatin was administered at AUC2.
radiation scheme
RT planning was carried out after direct simulation, based on diagnostic images or 3D based on treatment planning CT images. During direct simulation, patients had to swallow barium contrast to facilitate identification and localization of the primary tumor. For the planning CT, the patients also received oral contrast.
Gross tumor volume (GTV), defined as the macroscopic primary tumor and regional lymph node metastases, was reconstructed using all available information derived from endoscopy, EUS, CT and from FDG-PET.
At direct simulation, margins from GTV to field margin were 5 cm in caudal/cranial direction and 2 cm margin in transversal plane. A margin of 4 cm in caudal/cranial direction and 1.5 cm in transversal plane was used to generate the planning target volume. If the treatment planning was based on a planning CT, the clinical target volume was obtained by adding a 3-cm margin in cranial–caudal direction and 1 cm margin in transversal plane. A 0.5–1 cm margin was used around pathological lymph nodes.
A total radiation dose of 46.8–70 Gy (median dose 50.4 Gy) was given in daily fractions of 1.8–2 Gy. One patient received a dose of 41.1 Gy as the initial neoadjuvant treatment was switched to dCRT. Generally delivered with at least 6 MV photons. Intraluminal brachytherapy was given in two fractions of 6 Gy or a single fraction of 10 Gy and administered in 5% of the patients.
data acquisition
Data was obtained using the medical records of the different centers in the North-East region of the Netherlands. Additional information from comprehensive cancer centers was acquired. The study was carried out according to national ethics guidelines (www.ccmo-online.nl).
follow-up
Patients were generally seen for regular follow-up according to national guidelines at 4–8 weeks after completion of treatment, every 6 months in the first year and thereafter annually up to 5 years or until death.
toxicity
Toxicity was measured according to the Common Terminology Criteria for Adverse Events (CTCAE 4.0). Grade 3 and 4 toxicity reactions are shown in Table 3. Grade 5 toxicity occurring up to 30 days after treatment was recorded as mortality.
Table 3.
Treatment compliance and major toxicities
| Cisplatinum/5-FU (N = 47) | Carboplatin/paclitaxel (N = 55) | P value | |
|---|---|---|---|
| Completed chemotherapy | 27 (57%) | 44 (82%) | 0.010 |
| Toxities (CTCAE 4.0) | |||
| Overall toxicity (≥grade 3) | 26 (55%)a | 12 (22%) | 0.001 |
| Hematological ≥grade 3 | 9 (19%) | 2 (4%) | 0.021 |
| Nonhematological ≥grade 3 | 18 (38%) | 10 (18%) | 0.028 |
| Grade 3 | 18 (38%) | 8 (15%) | 0.011 |
| Grade 4 | 7 (15%) | 3 (6%) | 0.180 |
| Mortality | 2 (4%) | 1 (2%) | 0.594 |
| Hematologicb | |||
| Febrile leucopenia | 6 (13%) | 2 (4%) | 0.139 |
| Trombocytopenia | 1 (2%) | 2 (4%) | 1.000 |
| Bleeding | 1 (2%) | 0 (0%) | 0.461 |
| Anemia | 3 (6%) | 3 (6%) | 1.000 |
| Nonhematologicb | |||
| Nauseau/vomiting | 2 (4%) | 0 (0%) | 0.210 |
| Fatigue | 1 (2%) | 0 (0%) | 0.461 |
| Diarrhea | 0 (0%) | 1 (2%) | 1.000 |
| Mucositis | 2 (4%) | 2 (4%) | 1.000 |
| Other | 14 (30%) | 7 (13%) | 0.049 |
Compared using Fisher's exact test.
aIn cisplatinum/5-FU group, one patient had both a grade 3 hematological toxicity as a grade 4 nonhematological toxicity.
bAll recorded toxicity.
P < 0.05 was considered significant, significant values presented in italics.
statistics
OS was defined as the time interval between the starting date of the chemoradiation and documentation of the day of death or last follow-up. Disease-free survival (DFS) was determined from the starting date of treatment to documented date of first recurrence or death of any cause. OS and DFS rates were calculated according to the Kaplan–Meier method and compared using the log-rank test. Patient characteristics and toxicity rates were determined and compared using Student's t-test and Fisher's exact test. Univariate and multivariate analyses were carried out using Cox-regression analyses. P values of <0.150 in the univariate analysis were included in the multivariate analysis. A P value of <0.05 [95% confidence interval (CI)] was considered as significant. The statistical analyses were carried out by using the Statistical Package for Social Sciences (SPSS, Chicago, IL) version 18.0 software.
results
patient and tumor characteristics
Patient characteristics are shown in Table 1. Age (P = 0.169), sex (P = 0.468) and WHO-performance (P = 0.334) did not differ among both groups. In both groups, comorbidity was equally present, 49% in the cisplatinum/5-FU group and 55% in the carboplatin/paclitaxel group (P = 0.691). The type of comorbidity varied between the groups as the carboplatin/paclitaxel group had more cardiovascular and pulmonary comorbidity (38%) compared with the cisplatinum/5-FU group (19%, P = 0.049). Of the tumor characteristics, localization differed with more gastroesophageal junction tumors in the cisplatinum with 5-FU group (P = 0.05). Clinical T stage did differ (P = 0.008) with a T3 stadium of 67% in the cisplatinum/5-FU group, while the majority of patients in the carboplatin/paclitaxel group (55%) had a higher stage group T4. N Stage (P = 0.465) was comparable between both groups. A higher percentage of patients in the cisplatinum with 5-FU group had a cM1a stage (23%) compared with the paclitaxel with carboplatin group which was not significant (9%, P = 0.061). Most patients received a radiation dose of 50–50.4 Gy in both treatment groups and the distribution of radiation dose did not differ between the hospitals (P = 0.181).
Table 1.
Clinical and patient characteristics
| Cisplatinum/5-FU (N = 47) | Carboplatin/paclitaxel (N = 55) | P value | |
|---|---|---|---|
| Age (mean, years) | 62.5 | 64.8 | 0.169* |
| Sex (m/f) | 39 (83%)/8 (17%) | 42 (76%)/13 (24%) | 0.468 |
| cT1 | 0 (0%) | 1/47 (2%) | 0.008 |
| cT2 | 3/46 (7%) | 2/47 (4%) | |
| cT3 | 31/46 (67%) | 18/47 (38%) | |
| cT4 | 12/46 (26%) | 26/47 (55%) | |
| cN1 (%) | 39/47 (83%) | 41/54 (76%) | 0.465 |
| cM1a (%) | 11/47 (23%) | 5/54 (9%) | 0.061 |
| Histology (AC/SCC) | 28 (60%)/19 (40%) | 23 (42%)/32 (58%) | 0.112 |
| Tumor length >5 cm | 25/36 (69%) | 27/38 (71%) | 1.000 |
| Tumor site | |||
| Upper | 9/44 (21%) | 14/54 (26%) | 0.050 |
| Mid | 4/44 (9%) | 10/54 (19%) | |
| Distal | 22/44 (50%) | 28/54 (52%) | |
| GEJ | 9/44 (21%) | 2/54 (4%) | |
| WHO performance | |||
| 0–1 | 45/47 (96%) | 47/54 (87%) | 0.334 |
| 2 | 2/47 (4%) | 5/54 (9%) | |
| 3 | 0 (0%) | 2/54 (4%) | |
| Radiation dose | |||
| <50.0 Gy | 1 (2%) | 2 (4%) | 0.062 |
| 50.0–50.4 Gy | 42 (89%) | 53 (96%) | |
| >50.4 Gy | 4 (9%) | 0 (0%) | |
| Comorbidity present | 23/47 (49%) | 30/55 (55%) | 0.691 |
| Cardiovascular and pulmonary comorbidity | 9/47 (19%) | 21/55 (38%) | 0.049 |
| Type of comorbidity | |||
| None | 24/47 (51%) | 25/55 (46%) | 0.048 |
| Pulmonary | 1/47 (2%) | 9/55 (16%) | |
| Cardiovascular | 8/47 (17%) | 12/55 (22%) | |
| Other | 14/47 (30%) | 9/55 (16%) | |
*Student's t-test, all other variables were compared using a Fisher's exact test.
GEJ, gastroesophageal junction.
TNM classification according to sixth edition.
P < 0.05 was considered significant, significant values presented in italics.
overall survival and disease-free survival
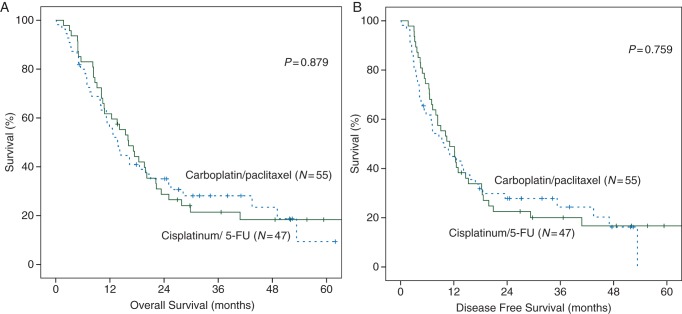
OS was comparable between the cisplatinum with 5-FU group and the carboplatin with paclitaxel group [P = 0.879, HR 0.97 (CI 0.62–1.51)], with a median survival of, respectively, 16.1 (CI 11.8–20.5) and 13.8 (CI 10.8–16.9) months (Figure 1A). DFS was also not significantly different [P = 0.76, HR 0.93 (CI 0.60–1.45)]. Median DFS was 11.1 in the cisplatinum with 5-FU group (Figure 1B, CI 6.9–15.3) and 9.7 months with carboplatin with paclitaxel (CI 5.1–14.4). OS and DFS were also not different between both chemotherapy regimens when analyzed for the two histological subtypes, adenocarcinoma and squamous cell carcinoma (supplementary Figure S2, available at Annals of Oncology online). As the Kaplan–Meier survival curves cross, the proportional hazards criterion for the log-rank test is not met. Therefore, we also tested smaller groups (OS and DFS 24 months) in the ESCC group for both regimens and found they were not significant (data not shown).
Figure 1.
Kaplan–Meier survivals estimation of the overall survival (A) and disease-free survival (B) for dCRT with cisplatinum/5-FU (N = 47) or carboplatin /paclitaxel (N = 55).
univariate and multivariate cox-regression analysis
Chemotherapy regimen was not related to OS and DFS in a univariate analysis (HR = 0.97, P = 0.879 and HR = 0.93, P = 0.760). Other factors as comorbidity, localization and completion of the chemotherapy were significantly related to OS (supplementary Table S2, available at Annals of Oncology online, P = 0.031, P = 0.028 and P = 0.046). Comorbidity, tumor localization, cN stage and completion of chemotherapy were related to DFS (P = 0.107, P = 0.014, P = 0.114, P = 0.123). When corrected for these factors in a multivariate Cox-regression analysis, OS and DFS did not change drastically for chemotherapy regimen [Table 2, P = 0.990, HR 1.00 (CI 0.61–1.64) and P = 0.641, HR 0.89 (CI 0.54–1.47)]. None of the other factors were an independent prognostic factor for OS or DFS.
Table 2.
Multivariate Cox-regression analysis
| Multivariate analysis | HR (95% CI) | P value |
|---|---|---|
| OS (N = 97) | ||
| Chemotherapy regimen | 1.00 (0.61–1.64) | 0.990 |
| Comorbidity presenta | 0.67 (0.39–1.13) | 0.135 |
| Localization | 0.149 | |
| Upper | 0.62 (0.25–1.57) | 0.314 |
| Mid | 1.49 (0.57–3.87) | 0.418 |
| Distal | 1.20 (0.52–2.75) | 0.669 |
| GEJ | Reference | |
| Completed chemotherapy | 1.28 (0.73–2.24) | 0.388 |
| DFS (N = 96) | ||
| Chemotherapy regimen | 0.89 (0.54–1.47) | 0.641 |
| Comorbidity present | 0.78 (0.46–1.32) | 0.357 |
| cN | 0.63 (0.32–1.22) | 0.168 |
| Localization | 0.301 | |
| Upper | 0.90 (0.35–2.34) | 0.829 |
| Mid | 1.61 (0.62–4.16) | 0.330 |
| Distal | 1.59 (0.70–3.65) | 0.270 |
| GEJ | Reference | |
| Completed chemotherapy | 1.45 (0.82–2.56) | 0.198 |
aDue to the large number of subgroups, comorbidity was only included as dichotomous variable.
GEJ, gastroesophageal junction.
toxicity and mortality
Table 3 shows the treatment compliance and toxicity grades. A higher percentage of patients with carboplatin/paclitaxel completed their treatment compared with the cisplatinum/5-FU group (82% versus 57%, P = 0.010). The occurrence of side events was significantly lower in the carboplatin/paclitaxel group compared with the cisplatinum/5-FU group (P = 0.001). In the cisplatinum/5-FU group, 38% (N = 18/47) experienced a grade 3 toxicity and 15% (N = 7/47) a grade 4 toxicity. In the carboplatin/paclitaxel group, toxicity rates were lower, 15% (N = 8/55) experienced a grade 3 toxicity and 6% (N = 3/55) a grade 4 toxicity. Hematologic toxicity was most common and higher in the cisplatinum/5-FU group (19% versus 4%, P = 0.021). Nonhematologic adverse events were also more common in the cisplatinum/5-FU group (38% versus 18%, P = 0.028).
In the cisplatinum/5-FU group, two patients died due to treatment-related events. One patient had atrial fibrillation resulting in brain infarction and death. The other patient developed severe bone marrow depletion and died from neutropenic sepsis based on a pneumonia. In the carboplatin/paclitaxel group, one patient with previous hepatocellular carcinoma died due to liver failure and severe diarrhea.
discussion
In this study, we described comparable outcomes in terms of OS and DFS between EC patients treated with cisplatinum/5-FU and with carboplatin/paclitaxel as part of dCRT. Severe toxicity rates including hematological and nonhematological events (both ≥grade 3) were significantly lower for the carboplatin/paclitaxel group (P = 0.001). Furthermore, a significantly higher percentage of patients completed their therapy in the carboplatin with paclitaxel group which could be due to fewer and milder adverse events (P = 0.010). However, completion of chemotherapy was not an independent prognostic factor for OS or DFS in a multivariate analysis.
Literature concerning the effectiveness and side-effects of carboplatin with paclitaxel compared with standard cisplatinum with 5-FU is limited. Supplementary Table S3, available at Annals of Oncology online gives an overview of the literature reporting on one or both of the schemes in dCRT in EC. Polee et al. [13] were the first to show the use of carboplatin with paclitaxel as dCRT scheme in a phase I study. Median survival was 11 months, and myelotoxicity was regarded acceptable as only 5% of the 77% with neutropenia developed fever. Wang et al. [14] showed a good response rate in a small group of EC patients with locally advanced disease (N = 16) treated with dCRT with carboplatin and paclitaxel with an overall 3-year survival rate of 60%. However, they do not compare this regimen with other chemotherapy regimens. Courrech Staal et al. [15] compared carboplatin with paclitaxel and cisplatinum with 5-FU, in both a curative dCRT and a neoadjuvant setting. The median OS was 15 months for the dCRT group (N = 49), which is similar as to our study. No survival distinction was made between the two therapy regimens in this study. A recent study of Blom et al. [16] compared cisplatinum/5-FU and carboplatin/paclitaxel in the neoadjuvant setting in EC patients and showed comparable overall toxicity (≥grade 3) as in our study and no difference in survival.
Toxicity rates for the carboplatin with paclitaxel group of our study were comparable with the rates in the CROSS trial. In our study, 4% of patients experienced hematological events (≥grade 3) and 18% nonhematological events (≥grade 3) compared with 7.6% and 13%, respectively, in the CROSS trial [6].
Our study is limited by the number of patients included and the retrospective design. An important limitation is that patients were not randomized, which could lead to differences in patient characteristics and treatment per hospital between both treatment groups. Between our groups, we showed no differences in outcome but a larger number of patients would be required to make these conclusions more robust. A phase III trial would be suitable for that purpose. However, the number of EC patients receiving dCRT is limited, and we do not expect groups to become significant with larger numbers. As toxicity rates are lower in the carboplatin/paclitaxel group an evaluation of the quality of life would also be of interest in future studies.
Another important difference between our groups is that patients in the carboplatin/paclitaxel group had more cardiovascular and pulmonary comorbidity. This could suggest a possible selection bias to include patients in better physical condition in the cisplatinum/5-FU group. However, most patients (78%) receiving carboplatin/paclitaxel were treated in the two centers were this was the standard regimen thereby arguing against a selection bias.
In conclusion, the present study suggests that OS and DFS are similar in both treatment regimens in dCRT in EC. Carboplatin with paclitaxel has fewer adverse events with higher treatment compliance. These results suggest carboplatin/paclitaxel could be used as an alternative for cisplatinum/5-FU in dCRT for EC patients which should be further evaluated.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.Crane LM, Schaapveld M, Visser O, et al. Oesophageal cancer in The Netherlands: increasing incidence and mortality but improving survival. Eur J Cancer. 2007;43:1445–1451. doi: 10.1016/j.ejca.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Dutch Cancer Registration. 2005. Survival of esophageal cancer patients in the Netherlands www.cijfersoverkanker.nl/selecties/Dataset_2/img512d09a0bce07. 8 September 2013, data last accessed.
- 3.Lepage C, Rachet B, Jooste V, et al. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am J Gastroenterol. 2008;103:2694–2699. doi: 10.1111/j.1572-0241.2008.02191.x. [DOI] [PubMed] [Google Scholar]
- 4.Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381:400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 5.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–692. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 8.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 9.Stahl M, Budach W, Meyer HJ, et al. Esophageal cancer: Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21(Suppl 5):v46–v49. doi: 10.1093/annonc/mdq163. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Clinical Practical Guidelines in Oncology Esophageal and Esophagogastric Junction Cancers (excluding the proximal 5 cm of the stomach) Version 2.2013. 2013: ESOPH-E 6.
- 11.Smit JK, Muijs CT, Burgerhof JG, et al. Survival after definitive (chemo)radiotherapy in esophageal cancer patients: a population-based study in the north-East Netherlands. Ann Surg Oncol. 2013;20:1985–1992. doi: 10.1245/s10434-012-2824-2. [DOI] [PubMed] [Google Scholar]
- 12.Sobin LH. TNM, sixth edition: new developments in general concepts and rules. Semin Surg Oncol. 2003;21:19–22. doi: 10.1002/ssu.10017. [DOI] [PubMed] [Google Scholar]
- 13.Polee MB, Sparreboom A, Eskens FA, et al. A phase I and pharmacokinetic study of weekly paclitaxel and carboplatin in patients with metastatic esophageal cancer. Clin Cancer Res. 2004;10:1928–1934. doi: 10.1158/1078-0432.ccr-03-0319. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Ryu J, Gandara D, et al. A phase II study of paclitaxel, carboplatin, and radiation with or without surgery for esophageal cancer. J Thorac Oncol. 2007;2:153–157. doi: 10.1097/JTO.0b013e31802bff75. [DOI] [PubMed] [Google Scholar]
- 15.Courrech Staal EF, Aleman BM, van Velthuysen ML, et al. Chemoradiation for esophageal cancer: institutional experience with three different regimens. Am J Clin Oncol. 2011;34:343–349. doi: 10.1097/COC.0b013e3181dbbafe. [DOI] [PubMed] [Google Scholar]
- 16.Blom RL, Sosef MN, Nap M, et al. Comparison of two neoadjuvant chemoradiotherapy regimens in patients with potentially curable esophageal carcinoma. Dis Esophagus. 2013 doi: 10.1111/dote.12110. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.