Testosterone is the most important androgen in humans. This study examined the association between plasma testosterone and early death after a cancer diagnosis, and risk of cancer in a prospective general population-based study of 8771 20-94 year old men and women with 30 years of follow-up. Increased levels of plasma testosterone associated with a 30-80% increased risk of early death after cancer in men and women, but not with risk of incident cancer.
Keywords: testosterone, cancer, survival, human, prospective cohort
Abstract
Background
Testosterone is an important anabolic hormone in humans and in vitro testosterone stimulates growth of lung and colon cancer cells. We tested the hypothesis that plasma testosterone associate with increased risk of cancer and with increased risk of early death after cancer.
Materials and methods
Plasma testosterone was measured in 8771 20- to 94-year-old men and women who participated in a prospective study of the general population. Participants were included in 1981–1983 and followed for a median of 22 years (range: 0–30 years).
Results
During follow-up, 1140 men and 809 women developed cancer. For risk of early death after cancer, for men, after adjustment for age at diagnosis, tumour stage at diagnosis, and time since blood-sampling, the hazard ratio was 1.30 [95% confidence interval (CI) 1.03–1.65] for the 2nd quintile, 1.31 (1.02–1.67) for the 3rd quintile, 1.52 (1.19–1.93) for the 4th quintile, and 1.52 (1.20–1.91) for the 5th quintile, versus the 1st quintile. For women, corresponding hazard ratios were 1.09 (0.81–1.46), 1.17 (0.86–1.59), 1.03 (0.76–1.39), and 1.80 (1.32–2.46). For risk of cancer, multifactorially adjusted hazard ratios for risk of any cancer were 1.07 (95% CI 0.98–1.18) and 1.06 (0.93–1.22) for men and women, respectively, when testosterone doubled. For both men and women, a doubling of testosterone was not associated with risk of any cancer type.
Conclusions
In this prospective study of 8771 men and women from the general population followed for >30 years, increased levels of testosterone were associated with a 30%–80% increased risk of early death after cancer, but unchanged risk of incident cancer.
introduction
Testosterone is the most important androgen in humans and affects the development of many different tissues and organs [1]. Testosterone is a transcription factor regulating protein synthesis, and plays a key part in increasing intracellular protein levels. This results in tissue proliferation and heightened basal metabolism and energy consumption. As such, testosterone is among the most important anabolic hormones in the human organism. The pathophysiological effects have not been fully determined but it appears that testosterone stimulates growth of lung and colon cancer cells in vitro, a mechanism that can be halted with anti-androgens [2, 3]. Therefore, it is possible that levels of plasma testosterone may be associated with both cancer risk and survival after a cancer diagnosis.
There are only very few studies examining the association between plasma testosterone and overall cancer risk and/or cancer types. The only exception is prostate cancer where several studies have reported divergent results [4–10]. Three population-based studies including 600–3600 men showed a positive association [4, 5, 9], while four studies with 300–3300 men did not find an association. Finally, a pooled analysis of 18 prospective studies including 10 324 men did not find an association between testosterone levels and prostate cancer risk [6]. For other common cancer types, results from a study of 4165 men aged 70–88 years suggested that increased testosterone levels may be associated with increased risk of lung cancer but not with colon cancer risk [9]. Thus, the association between testosterone levels and cancer risk has only been examined for a few specific cancer types and, to our knowledge, never in women.
Although male cancer patients have lower levels of plasma testosterone [11], just one study examining the association between testosterone levels and survival in 136 male cancer patients has been carried out showing a null association [12]. Furthermore, the only observational study of plasma testosterone values and gender differences in cancer aggressiveness has suggested that the higher risk of cancer death in men may be explained by the higher testosterone levels [13]. Hence, it remains unclear whether and to what extent, testosterone is associated with early death after cancer.
Importantly, there are no prospective studies of the general population including both men and women, examining the association between testosterone levels and risk of cancer, and the risk of early death after cancer. We therefore tested the hypothesis that plasma testosterone associates with increased risk of cancer and with increased risk of early death after cancer, in a prospective study of 8771 men and women from the general population with up to 30 years of follow-up.
materials and methods
setting and participants
Study participants were men and women examined in 1981 through 1983 in the Copenhagen City Heart Study [14, 15]. Participants were 20–94 years old and were randomly selected from the Danish Civil Registration System to represent the general population; the participation rate was 63% (supplementary Figure S1, available at Annals of Oncology online). All participants were white and of Danish descent. There were no losses to follow-up. The study was approved by Herlev Hospital and a Danish ethical committee (KF-100.2039/91). All participants gave written informed consent. For details see supplementary Material, available at Annals of Oncology online.
testosterone measurements and covariates
On the day of attendance, participants filled in a questionnaire which was reviewed together with an investigator. Furthermore, all participants had a physical examination carried out, and had blood samples drawn for biochemical analysis. For details on testosterone measurements and covariates, see supplementary Material, available at Annals of Oncology online.
statistical analysis
Statistical analyses were carried out using Stata 12.1 SE software. We used Cox proportional hazard regression models to analyse time to event and estimate hazard ratios with 95% confidence intervals (CIs). For details on statistical analysis, see supplementary Methods, available at Annals of Oncology online.
results
Baseline characteristics of participants in the testosterone quintiles are summarized in Table 1. We studied 4453 men and 4318 women from the Copenhagen City Heart Study. There were no losses to follow-up. Levels of testosterone associated with smoking status, number of pack-years, BMI, and alcohol consumption in men. In women, levels of testosterone associated with smoking status, cumulative smoking, BMI, alcohol consumption, level of education, menopausal status, and use of oral contraceptives. The number of cancers for men and women in each testosterone quintile are shown in supplementary Table S1, available at Annals of Oncology online.
Table 1.
Baseline characteristics of participants from the general population by quintiles of plasma testosterone
| Characteristics | Plasma testosterone levels |
|||||
|---|---|---|---|---|---|---|
| 1st quintile | 2nd quintile | 3rd quintile | 4th quintile | 5th quintile | P-trend | |
| Men | ||||||
| Testosterone levels, nmol/l | 7.2 (6.2–8.2) | 10.4 (9.7–11.2) | 13.6 (12.8–14.4) | 17.7 (16.5–18.8) | 23.8 (21.6–26.7) | |
| No. of participants | 906 | 895 | 875 | 898 | 879 | – |
| Age, years | 59 (49–66) | 59 (49–66) | 57 (48–65) | 57 (48–66) | 58 (49–65) | 0.23 |
| Current smokers, No. (%) | 509 (56) | 535 (60) | 569 (65) | 582 (65) | 648 (74) | 6 × 10−15 |
| Cumulative smoking, pack-years | 30 (14–42) | 29 (13–42) | 30 (16–41) | 30 (14–43) | 30 (18–45) | 1 × 10−3 |
| Body mass index, kg/m2 | 27 (25–29) | 26 (24–29) | 26 (24–28) | 25 (23–27) | 24 (22–26) | 3 × 10−76 |
| Alcohol consumption >252 g/week, No. (%) | 161 (18) | 164 (18) | 172 (20) | 163 (18) | 213 (24) | 3 × 10−3 |
| Less than 13 years of education, No. (%) | 606 (67) | 623 (70) | 567 (65) | 595 (66) | 625 (71) | 0.39 |
| Low income, No. (%) | 217 (24) | 216 (24) | 188 (22) | 228 (25) | 250 (28) | 0.07 |
| Women | ||||||
| Testosterone levels, nmol/l | 1.2 (1.1–1.4) | 1.6 (1.5–1.7) | 1.9 (1.8–2.0) | 2.2 (2.1–2.3) | 2.8 (2.6–3.2) | |
| No. of participants | 930 | 911 | 937 | 845 | 695 | – |
| Age, years | 58 (48–65) | 57 (47–64) | 57 (47–64) | 56 (47–64) | 57 (47–65) | 0.15 |
| Current smokers, No. (%) | 423 (45) | 474 (52) | 490 (52) | 488 (58) | 435 (63) | 5 × 10−11 |
| Cumulative smoking, pack-years | 10 (0–23) | 11 (0–24) | 13 (0–25) | 15 (3–27) | 15 (0–25) | 6 × 10−9 |
| Body mass index, kg/m2 | 24 (22–26) | 24 (22–27) | 24 (22–27) | 24 (22–27) | 24 (22–27) | 0.02 |
| Alcohol consumption >168 g/week, No. (%) | 48 (5) | 65 (7) | 62 (7) | 66 (8) | 70 (10) | 4 × 10−4 |
| Less than 13 years of education, No. (%) | 615 (66) | 599 (66) | 628 (67) | 596 (71) | 488 (70) | 0.01 |
| Low income, No. (%) | 320 (34) | 299 (33) | 317 (33) | 293 (35) | 252 (36) | 0.28 |
| Nulliparous, No. (%) | 240 (26) | 200 (22) | 207 (22) | 203 (24) | 171 (25) | 0.83 |
| Postmenopausal, No. (%) | 480 (52) | 470 (52) | 484 (52) | 459 (54) | 353 (51) | 6 × 10−3 |
| Oral contraceptive use, No. (%) | 58 (6) | 48 (5) | 29 (3) | 35 (4) | 14 (2) | 4 × 10−3 |
| Hormonal replacement therapy, No. (%) | 142 (15) | 112 (12) | 127 (14) | 93 (11) | 118 (17) | 0.32 |
Continuous variables are shown as medians (interquartile range) and categorical variables are shown as numbers (%). Cut-off values of 168 g/week for women and 252 g/week for men are from the recommendation concerning alcohol consumption from the Danish National Board of Health.
testosterone levels and age
When we plotted testosterone levels as a function of 10-year age groups for men and women, testosterone levels seemed to decrease slightly with increasing age (supplementary Figure S2, available at Annals of Oncology online); however, testosterone levels were not significantly associated with age in men (P-trend: 0.23) or women (P-trend: 0.15) (Table 1).
survival after a cancer diagnosis
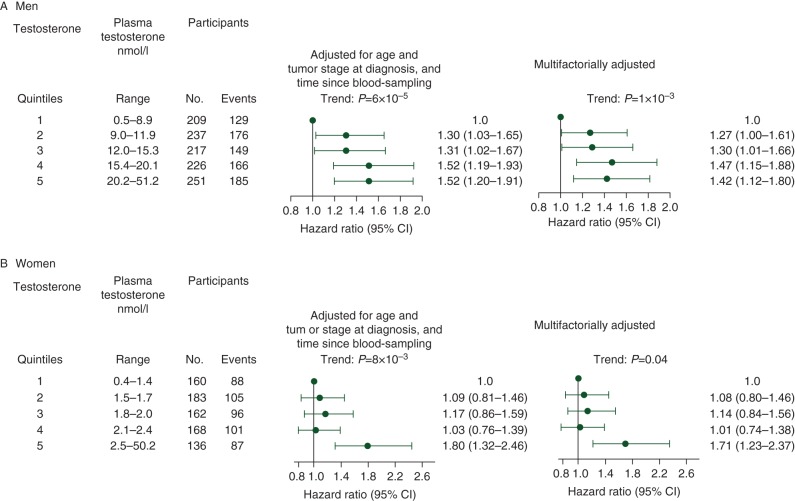
Risk of early death 5 years after cancer increased in both men and women with increasing testosterone levels (P-trend: 6 × 10−5 and 8 × 10−3, respectively) (Figure 1 A and B).
Figure 1.
Risk of early death after a cancer diagnosis by testosterone quintiles in men (A) and women (B). Based on 1140 men and 809 women diagnosed with cancer during 30 years of follow-up after the Copenhagen City Heart Study 1981–1983 examination. Multifactorially adjusted was for age and tumour stage at diagnosis, time since blood sampling, smoking status, cumulative smoking, body mass index, alcohol consumption, level of education, and level of income for both men and women. For women parity, menopausal status, oral contraceptive use, and hormone replacement therapy were also adjusted for. CI, confidence interval.
For men, after adjustment for age at diagnosis, tumour stage at diagnosis, and time since blood sampling, the hazard ratio was 1.30 (95% CI 1.03–1.65) for the 2nd quintile, 1.31 (1.02–1.67) for the 3rd quintile, 1.52 (1.19–1.93) for the 4th quintile, and 1.52 (1.20–1.91) for the 5th quintile, versus the 1st quintile (Figure 1A). For women, corresponding hazard ratios were 1.09 (0.81–1.46), 1.17 (0.86–1.59), 1.03 (0.76–1.39), and 1.80 (1.32–2.46) (Figure 1 B). When we adjusted multifactorially, hazard ratios were slightly attenuated for both men and women, but trend tests remained significant (Figure 1A and B). Hazard ratios for risk of early death after a cancer diagnosis during the entire follow-up period were similar (data not shown).
When testosterone was on a continuous scale, after adjustment for age at diagnosis, tumour stage at diagnosis, and time since blood sampling, a doubling of testosterone levels was associated with a hazard ratio for risk of early death after cancer of 1.21 (95% CI 1.09–1.33) for men (supplementary Table S2, available at Annals of Oncology online). The corresponding hazard ratio for women was 1.16 (1.00–1.34). After stratification on age at diagnosis, tumour stage, and time since blood sampling, a doubling of testosterone levels was generally associated with increased risk of early death after cancer. All tests of interaction between testosterone levels and other covariates were non-significant.
risk of cancer
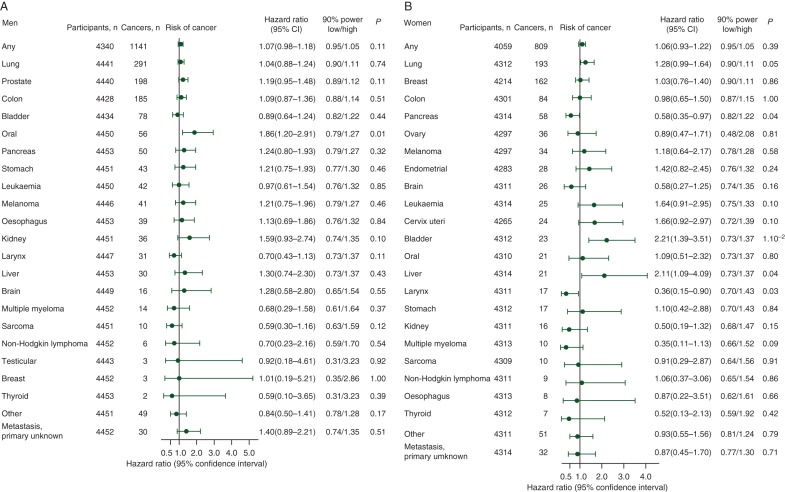
Multifactorially adjusted hazard ratios for risk of any first cancer were 1.07 (95% CI 0.98–1.18) and 1.06 (0.93–1.22) for men and women, respectively, when testosterone levels doubled (Figure 2). For both men and women, we had 90% statistical power to exclude corresponding hazard ratios at 0.95 or below and/or 1.05 or above. When we used a competing risk regression model (i.e. with death as competing event) for risk of any first cancer and cancer types, results were similar (data not shown).
Figure 2.
Risk of any cancer and cancer types in men (A) and women (B) when testosterone levels double. Based on 4453 men and 4318 women from the general population who participated in the Copenhagen City Heart Study 1981–1983 examination. Numbers at risk vary due to varying number of participants with the cancer type in question before blood sampling. Hazard ratios were multifactorially adjusted for smoking status, cumulative smoking, body mass index, alcohol consumption, level of education, and level of income for both men and women. For women parity, menopausal status, oral contraceptive use, and hormone replacement therapy were also adjusted for. Cancer types were listed according to number of cases, except other cancers and metastases/primary unknown listed last.
For men, a doubling of testosterone was not associated with risk of the majority of cancer types (Figure 2 A). The only exception was the multifactorially adjusted hazard ratio for risk of oral cancer which was 1.86 (1.20–2.91; P = 0.01). Similarly, for women, there was generally no evidence of an association between testosterone and risk of any cancer type (Figure 2B). However, a doubling in testosterone was associated with multifactorially adjusted hazard ratios for bladder and liver cancer of 2.21 (1.39–3.51; P = 0.001) and 2.11 (1.09–2.32; P = 0.04), respectively. Corresponding hazard ratios for pancreas and larynx cancer were 0.58 (0.35–0.97; P = 0.04)) and 0.36 (0.15–0.90; P = 0.03), respectively. After adjustment for the 22 multiple comparisons, these associations were no longer significant [i.e. (22 × P-value) >0.05] (data not shown), suggesting that they could represent chance findings.
For both men and women, after multifactorial adjustment, increasing testosterone quintile was not associated with increasing risk of any cancer or any cancer type (Table 2).
Table 2.
Risk of any cancer and cancer types in men and women by testosterone quintile
| Cancer type | Testosterone quintile |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st |
2nd |
3rd |
4th |
5th |
Total | ||||||
| N | HR | N | HR (95% CI) | N | HR (95% CI) | N | HR (95% CI) | N | HR (95% CI) | ||
| Men | |||||||||||
| Any | 209 | 1 | 237 | 1.13 (0.94–1.36) | 217 | 1.03 (0.85–1.25) | 226 | 1.08 (0.89–1.31) | 251 | 1.20 (0.99–1.46) | 1140 |
| Lung | 46 | 1 | 64 | 1.31 (0.89–1.91) | 58 | 1.17 (0.79–1.73) | 65 | 1.25 (0.85–1.84) | 58 | 1.06 (0.71–1.58) | 291 |
| Prostate | 39 | 1 | 31 | 0.84 (0.52–1.34) | 46 | 1.29 (0.84–1.98) | 35 | 1.04 (0.65–1.65) | 47 | 1.47 (0.95–2.30) | 198 |
| Colon | 40 | 1 | 35 | 0.92 (0.58–1.44) | 28 | 0.74 (0.45–1.20) | 44 | 1.20 (0.77–1.85) | 38 | 1.05 (0.66–1.67) | 185 |
| Bladder | 17 | 1 | 22 | 1.30 (0.69–2.45) | 10 | 0.57 (0.26–1.26) | 15 | 0.87 (0.43–1.77) | 14 | 0.77 (0.37–1.61) | 78 |
| Oral | 5 | 1 | 11 | 2.28 (0.79–6.58) | 9 | 1.97 (0.66–5.92) | 13 | 2.85 (1.01–8.09) | 18 | 4.00 (1.44–11.08) | 56 |
| Pancreas | 10 | 1 | 11 | 1.10 (0.47–2.61) | 5 | 0.49 (0.17–1.45) | 14 | 1.41 (0.62–3.23) | 10 | 1.00 (0.40–2.49) | 50 |
| Stomach | 7 | 1 | 10 | 1.44 (0.55–3.79) | 5 | 0.68 (0.22–2.17) | 8 | 1.08 (0.39–3.02) | 13 | 1.70 (0.66–4.40) | 43 |
| Leukaemia | 7 | 1 | 12 | 1.84 (0.72–4.69) | 8 | 1.18 (0.42–3.30) | 8 | 1.24 (0.44–3.48) | 7 | 1.19 (0.40–3.50) | 42 |
| Melanoma | 5 | 1 | 12 | 2.63 (0.92–7.48) | 6 | 1.26 (0.38–4.18) | 10 | 2.27 (0.77–6.72) | 8 | 2.04 (0.65–6.40) | 41 |
| Oesophagus | 6 | 1 | 10 | 1.59 (0.58–4.41) | 8 | 1.36 (0.47–3.97) | 5 | 0.77 (0.23–2.56) | 10 | 1.37 (0.47–3.96) | 39 |
| Kidney | 7 | 1 | 4 | 0.60 (0.17–2.04) | 6 | 0.89 (0.30–2.67) | 7 | 1.04 (0.36–3.02) | 12 | 1.76 (0.66–4.70) | 36 |
| Larynx | 8 | 1 | 5 | 0.61 (0.20–1.86) | 9 | 1.06 (0.40–2.81) | 4 | 0.45 (0.13–1.53) | 5 | 0.53 (0.17–1.71) | 31 |
| Liver | 6 | 1 | 6 | 1.04 (0.33–3.25) | 5 | 0.93 (0.28–3.10) | 4 | 0.72 (0.20–2.60) | 9 | 1.44 (0.48–4.31) | 30 |
| Other | 9 | 1 | 6 | 0.69 (0.24–1.93) | 10 | 1.24 (0.50–3.08) | 11 | 1.32 (0.54–3.23) | 13 | 1.62 (0.66–3.94) | 49 |
| Metastasis, primary unknown | 8 | 1 | 7 | 0.89 (0.32–2.46) | 5 | 0.64 (0.21–2.00) | 4 | 0.47 (0.14–1.59) | 6 | 0.59 (0.19–1.90) | 30 |
| Women | |||||||||||
| Any | 160 | 1 | 183 | 1.14 (0.92–1.41) | 162 | 0.99 (0.80–1.23) | 168 | 1.03 (0.83–1.28) | 136 | 1.11 (0.88–1.40) | 809 |
| Lung | 34 | 1 | 30 | 0.90 (0.55–1.46) | 44 | 1.28 (0.82–2.02) | 46 | 1.27 (0.81–1.98) | 39 | 1.37 (0.86–2.19) | 193 |
| Breast | 31 | 1 | 35 | 1.04 (0.64–1.68) | 35 | 1.05 (0.65–1.71) | 34 | 1.08 (0.66–1.77) | 27 | 1.16 (0.69–1.96) | 162 |
| Colon | 21 | 1 | 16 | 0.78 (0.41–1.51) | 18 | 0.89 (0.47–1.67) | 18 | 0.90 (0.47–1.70) | 11 | 0.78 (0.37–1.62) | 84 |
| Pancreas | 17 | 1 | 14 | 0.83 (0.41–1.69) | 11 | 0.64 (0.30–1.38) | 8 | 0.47 (0.20–1.09) | 8 | 0.62 (0.26–1.45) | 58 |
| Ovary | 12 | 1 | 5 | 0.42 (0.15–1.19) | 4 | 0.34 (0.11–1.05) | 8 | 0.68 (0.28–1.70) | 7 | 0.82 (0.32–2.14) | 36 |
| Melanoma | 5 | 1 | 9 | 1.85 (0.61–5.56) | 7 | 1.46 (0.46–4.61) | 8 | 1.85 (0.60–5.71) | 5 | 1.50 (0.43–5.25) | 34 |
| Endometrial | 3 | 1 | 7 | 2.39 (0.61–9.29) | 8 | 2.74 (0.73–10.38) | 6 | 2.24 (0.55–9.04) | 4 | 1.61 (0.34–7.72) | 28 |
| Leukaemia | 3 | 1 | 6 | 2.10 (0.52–8.43) | 8 | 2.76 (0.73–10.45) | 2 | 0.71 (0.12–4.29) | 6 | 3.09 (0.76–12.53) | 25 |
| Cervix uteri | 3 | 1 | 10 | 3.53 (0.97–12.89) | 4 | 1.32 (0.29–5.94) | 3 | 1.12 (0.22–5.61) | 4 | 1.84 (0.40–8.39) | 24 |
| Bladder | 2 | 1 | 4 | 2.33 (0.42–12.75) | 6 | 3.59 (0.72–17.87) | 4 | 2.48 (0.45–13.61) | 7 | 5.97 (1.22–29.12) | 23 |
| Oral | 3 | 1 | 4 | 0.99 (0.21–4.61) | 5 | 1.36 (0.32–5.75) | 3 | 0.82 (0.16–4.16) | 6 | 1.99 (0.48–8.19) | 21 |
| Other | 10 | 1 | 16 | 1.75 (0.79–3.88) | 10 | 1.06 (0.44–2.56) | 8 | 0.95 (0.37–2.41) | 7 | 1.07 (0.40–2.87) | 51 |
| Metastasis, primary unknown | 7 | 1 | 11 | 1.56 (0.60–4.06) | 2 | 0.28 (0.06–1.36) | 6 | 0.86 (0.28–2.59) | 6 | 0.91 (0.29–2.91) | 32 |
Only cancer types with a total >20 cases and >0 cases per quintile were included. Multifactorially adjusted for smoking status, cumulative smoking, body mass index, alcohol consumption, level of education, and level of income for men and women. For women, parity, menopausal status, oral contraceptive use, and hormone replacement therapy were also adjusted for.
HR, hazard ratio; CI, confidence interval.
discussion
The key finding of this study is that increased levels of plasma testosterone are associated with a 30%–80% increased risk of early death after cancer in both men and women from the general population. This is a novel finding. However, results do not support an association between testosterone levels and cancer risk.
A possible mechanism behind our findings for risk of early death after cancer could be that increased levels of testosterone stimulate intracellular protein synthesis, which is needed for rapid proliferation both in healthy and cancerous cells. Also, it is possible that cancer cells may benefit from the higher basal metabolism due to increased testosterone. However, the effect of testosterone on cancer cells and the possible inhibitory potential of anti-androgens remain unclear.
For risk of early death after cancer, increasing testosterone was associated with increased risk of death in both men and women. To our knowledge, this has not been examined previously in population-based studies, but only in one small study of 136 male cancer patients, which did not find an association between plasma testosterone and risk of early death [12]. However, that study cohort consisted of a selected group of cancer patients and maximum follow-up was ∼4 years. Furthermore, testosterone was measured after cancer diagnosis and, thus, may have decreased during the time leading up to diagnosis, making comparison to our findings difficult.
Previous studies on the association between testosterone and overall mortality have produced diverging results [16]. Two studies including 1568 and 2314 participants showed that low levels of testosterone were associated with increased risk of overall mortality [17, 18]. In contrast, two studies with 254 and 4255 participants did not find such an association [19, 20]. Finally, a study using genetic data on 1882 participants (i.e. with a lower risk of confounding and reverse causation) indicated that testosterone was not a causal factor in overall mortality [21]. Taken together, while the present study indicates that testosterone is associated with early death after a cancer diagnosis further research is needed to elucidate the role of testosterone in overall mortality.
For risk of cancer types, we observed an elevated risk of oral cancer in men. Due to the large number of cancer types (n = 22), we would expect to find at least one positive association by chance alone, and thus, cannot determine whether this association is true or a play of chance. Similarly, the results for decreased risk of pancreas and larynx cancer and increased risk of bladder and liver cancer in women are likely to be chance findings based on the small number of cases and the large number of parallel tests. Also, risk estimates for these four cancer types are symmetrically distributed around 1.00, also suggesting that these could be spurious results. Finally, when we corrected for multiple comparisons, these results were no longer statistically significant.
Supporting the validity of our findings, testosterone levels in our cohort were similar to those previously described, both overall and in age-specific categories [22]. We also found associations between testosterone and smoking and BMI, as seen in other studies [18, 23].
Our findings on cancer type risk are supported by results from other studies. First, our results on prostate cancer risk were similar to those of both a recent large prospective study of 3255 men from the Reduction by Dutasteride of Prostate Cancer (REDUCE) trial and a pooled analysis of 18 studies on testosterone and prostate cancer risk that reported a null association [6, 10]. In contrast to these findings, other studies have shown that risk of prostate cancer increases with increasing testosterone levels [4, 5, 9]. However, these studies used a case–control design, had fewer participants and prostate cancer cases, and/or older participants, which may explain the difference in results. Second, also in support of the results from our study, a population-based study of 3635 men did not find an association between testosterone levels and risk of colorectal cancer [9]. Conversely, Hyde et al. reported increased risk of lung cancer with increasing testosterone levels, even after exclusion of current smokers, i.e. who both have increased testosterone levels and increased risk of lung cancer. In the present study, we did not observe an increased risk of lung cancer in men despite having 291 lung cancer cases and >30 years of follow-up compared with 84 lung cancer cases and 7 years of follow-up in the study by Hyde et al. Additionally, we did not find an increased risk of lung cancer in women, and we were also able to adjust for both smoking status and cumulative smoking, limiting the possibility of influence from residual confounding. Taken together, our results suggest that increased testosterone levels are not associated with risk of incident cancer in men or women.
Our study has several strengths. The study population is representative of the general population, follow-up was up to 30 years without losses to follow-up, and the study population is well characterized. Furthermore, we had 8771 participants with 1950 cancer cases which overall gave us considerable statistical power. Yet another strength is that we were able to examine the association between testosterone levels and risk of death after a cancer diagnosis and risk of cancer separately in men and women.
The limited number of events for each individual cancer type reduced our statistical power. Nonetheless, we had 90% power to exclude clinically relevant changes of risk for most cancer types. Another potential limitation of our study is that our study population only included white participants of Danish descent, and thus our results may not necessarily apply to other ethnic groups. On the other hand, we are not aware of any data to suggest that results like the present should not be valid for men and women of all races. Finally, a potential limitation may be that we examined overall mortality after a cancer diagnosis and not cancer-specific mortality. However, 86% of all deaths after a cancer diagnosis were cancer related when we looked at the first three causes of death listed for each participant. This was even higher if we included all nine causes of death listed.
In conclusion, in this prospective study of 8771 men and women from the general population followed for up to 30 years, increased levels of testosterone were associated with a 30%–80% increased risk of early death after cancer, but with unchanged risk of incident cancer.
funding
This work was supported by Herlev Hospital, Copenhagen University Hospital (no grant number); the JaschaFoundation (grant 1992 to DDØ); and the University of Copenhagen (grant 573 to DDØ).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We thank laboratory technician Anitta Pedersen for excellent technical assistance, as well as the participants and team of the Copenhagen City Heart Study.
references
- 1.Guyton AC, Hall JE. Textbook of Medical Physiology. Philadelphia, PA, USA: W.B. Saunders,; 2000. [Google Scholar]
- 2.Tutton PJ, Barkla DH. The influence of androgens, anti-androgens, and castration on cell proliferation in the jejunal and colonic crypt epithelia, and in dimethylhydrazine-induced adenocarcinoma of rat colon. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;38:351–355. doi: 10.1007/BF02892830. [DOI] [PubMed] [Google Scholar]
- 3.Maasberg M, Rotsch M, Jaques G, et al. Androgen receptors, androgen-dependent proliferation, and 5 alpha-reductase activity of small-cell lung cancer cell lines. Int J Cancer. 1989;43:685–691. doi: 10.1002/ijc.2910430424. [DOI] [PubMed] [Google Scholar]
- 4.Gann PH, Hennekens CH, Ma J, et al. Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst. 1996;88:1118–1126. doi: 10.1093/jnci/88.16.1118. [DOI] [PubMed] [Google Scholar]
- 5.Parsons JK, Carter HB, Platz EA, et al. Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev. 2005;14:2257–2260. doi: 10.1158/1055-9965.EPI-04-0715. [DOI] [PubMed] [Google Scholar]
- 6.Roddam AW, Allen NE, Appleby P, et al. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels NA, Nielson CM, Hoffman AR, et al. Sex hormones and the risk of incident prostate cancer. Urology. 2010;76:1034–1040. doi: 10.1016/j.urology.2010.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawada N, Iwasaki M, Inoue M, et al. Plasma testosterone and sex hormone-binding globulin concentrations and the risk of prostate cancer among Japanese men: a nested case-control study. Cancer Sci. 2010;101:2652–2657. doi: 10.1111/j.1349-7006.2010.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyde Z, Flicker L, McCaul KA, et al. Associations between testosterone levels and incident prostate, lung, and colorectal cancer. A population-based study. Cancer Epidemiol Biomarkers Prev. 2012;21:1319–1329. doi: 10.1158/1055-9965.EPI-12-0129. [DOI] [PubMed] [Google Scholar]
- 10.Muller RL, Gerber L, Moreira DM, et al. Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the reduction by dutasteride of prostate cancer events trial. Eur Urol. 2012;62:757–764. doi: 10.1016/j.eururo.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JM, Li H, Mann D, et al. Hypogonadism in male patients with cancer. Cancer. 2006;106:2583–2591. doi: 10.1002/cncr.21889. [DOI] [PubMed] [Google Scholar]
- 12.Utech AE, Tadros EM, Hayes TG, et al. Predicting survival in cancer patients: the role of cachexia and hormonal, nutritional and inflammatory markers. J Cachexia Sarcopenia Muscle. 2012;3:241–251. doi: 10.1007/s13539-012-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shahabi S, He S, Kopf M, et al. Free testosterone drives cancer aggressiveness: evidence from US population studies. PLoS One. 2013;8:e61955. doi: 10.1371/journal.pone.0061955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orsted DD, Bojesen SE, Tybjaerg-Hansen A, et al. Tumor suppressor p53 Arg72Pro polymorphism and longevity, cancer survival, and risk of cancer in the general population. J Exp Med. 2007;204:1295–1301. doi: 10.1084/jem.20062476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orsted DD, Nordestgaard BG, Jensen GB, et al. Prostate-specific antigen and long-term prediction of prostate cancer incidence and mortality in the general population. Eur Urol. 2012;61:865–874. doi: 10.1016/j.eururo.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Araujo AB, Dixon JM, Suarez EA, et al. Clinical review: endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:3007–3019. doi: 10.1210/jc.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vikan T, Schirmer H, Njolstad I, et al. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromso Study. Eur J Endocrinol. 2009;161:435–442. doi: 10.1530/EJE-09-0284. [DOI] [PubMed] [Google Scholar]
- 18.Khaw KT, Dowsett M, Folkerd E, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 19.Haring R, Teng Z, Xanthakis V, et al. Association of sex steroids, gonadotrophins, and their trajectories with clinical cardiovascular disease and all-cause mortality in elderly men from the Framingham Heart Study. Clin Endocrinol (Oxf) 2013;78:629–634. doi: 10.1111/cen.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips AC, Gale CR, Batty GD. Sex hormones and cause-specific mortality in the male veterans: the Vietnam Experience Study. QJM. 2012;105:241–246. doi: 10.1093/qjmed/hcr204. [DOI] [PubMed] [Google Scholar]
- 21.Haring R, Teumer A, Volker U, et al. Mendelian randomization suggests non-causal associations of testosterone with cardiometabolic risk factors and mortality. Andrology. 2013;1:17–23. doi: 10.1111/j.2047-2927.2012.00002.x. [DOI] [PubMed] [Google Scholar]
- 22.Harman SM, Metter EJ, Tobin JD, et al. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 23.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.