The tumour microenvironment, particularly the tumour stroma, has a key role in cancer progression and survival. In the present study, a high tumour stroma percentage (TSP) is identified as a marker of poor prognosis in colorectal cancer, independent of other host and tumour determinants of survival. Furthermore, associations between TSP and clinicopathological characteristics suggest a pertinent role in facilitating tumour growth and invasion.
Keywords: tumour stroma, tumour microenvironment, colorectal cancer, survival
Abstract
Background
Tumour stroma percentage (TSP) has previously been reported to predict survival in patients with colorectal cancer (CRC); however, whether this is independent of other aspects of the tumour microenvironment is unknown. In the present study, the relationship between TSP, the tumour microenvironment and survival was examined in patients undergoing elective, curative CRC resection.
Patients and methods
Patients undergoing resection at a single centre (1997–2008) were identified from a prospective database. TSP was measured at the invasive margin and its association with cancer-specific survival (CSS) and clinicopathological characteristics examined.
Results
Three hundred and thirty-one patients were included in the analysis. TSP was associated with CSS in patients with stage I–III disease [hazard ratio (HR) 1.84, 95% confidence interval (CI) 1.17–2.92, P = 0.009], independent of age, systemic inflammation, N stage, venous invasion and Klintrup–Mäkinen score. Furthermore, TSP was associated with reduced CSS in patients with node-negative disease (HR 2.14, 95% CI 1.01–4.54, P = 0.048) and those who received adjuvant chemotherapy (HR 2.83, 95% CI 1.23–6.53, P = 0.015), independent of venous invasion and host inflammatory responses. TSP was associated with several adverse pathological characteristics, including advanced T and N stage. Furthermore, TSP was associated with an infiltrative invasive margin and inversely associated with necrosis.
Conclusions
The TSP was a significant predictor of survival in patients undergoing elective, curative CRC resection, independent of adverse pathological characteristics and host inflammatory responses. In addition, TSP was strongly associated with local tumour growth and invasion.
introduction
Colorectal cancer (CRC) remains the second most common cause of cancer-related death in Western Europe [1]. Even in patients undergoing potentially curative resection, survival remains poor, with a 5-year survival of ∼50% [2]. It is clear that there remains a need to identify novel prognostic characteristics alongside current pathological staging, particularly in patients with node-negative disease [3].
There is now increased appreciation of the importance of the tumour microenvironment, including tumour necrosis and host local inflammatory responses, in cancer progression and survival [4, 5]. More recently, the tumour stroma itself has been identified as an important determinant of progression in a number of solid cancers [6]. The stroma facilitates the survival and proliferation of neoplastic cells and promotes epithelial–mesenchymal transition (EMT) [7], and local and metastatic dissemination [8]. Furthermore, tumour stroma may contribute towards chemoresistance in patients undergoing 5-fluorouracil (5-FU)-based chemotherapy [9].
Consistent with the above scheme, an increase in the proportion of tumour stroma has been associated with poorer survival in a number of solid cancers, including CRC [10–12]. Indeed, assessment of the proportion of tumour stroma using routine pathological specimens may act as a surrogate for tumour stroma activity and its subsequent effect on survival and chemoresistance.
It is not clear, however, whether the effect of an expanded tumour stroma on survival is independent of host inflammatory responses and other components of the tumour microenvironment. Moreover, the relationship between tumour stroma, host and tumour characteristics remain unknown. In the present study, the relationship between tumour stroma percentage (TSP), the tumour microenvironment and survival was examined in patients undergoing curative, elective CRC resection
patients and methods
Patients were identified from a prospective database of elective and emergency CRC resections in a single surgical unit at Glasgow Royal Infirmary. Patients who, on the basis of pre-operative abdominal computed tomography and laparotomy findings, were considered to have undergone potentially curative, elective resection (stage I–III) between January 1997 and May 2008 were included. Exclusion criteria were neoadjuvant therapy and death within 30 days of surgery. The study was approved by the West of Scotland Research Ethics Committee, Glasgow.
Patient demographics and pre-operative laboratory measurements (albumin and C-reactive protein) were collected prospectively. The systemic inflammatory response was assessed using the modified Glasgow Prognostic Score (mGPS) [13]. At multi-disciplinary meetings following surgery, patients with stage III and high-risk stage II disease were considered for suitability for 5-FU-based chemotherapy.
Tumours were staged using the fifth edition of the tumour, node and metastases (TNM) classification [14], with additional data taken from pathological reports issued following resection. Venous invasion was routinely identified using elastica staining as previously described [15].
Peritumoural immune responses were assessed according to the Klintrup–Mäkinen (K–M) score and by examining the immune cell type and density at the invasive margin as previously described [16, 17]. Briefly, sections of the tumour at the deepest point of invasion were stained for mature (CD3), cytotoxic (CD8), memory (CD45R0) and regulatory (FOXP3) T-cells and density graded as low or high. The K–M score has previously been shown to be comparable with immune scores incorporating assessment of the type and location of immune cell infiltrates [16] and was used for subsequent survival analyses.
The assessment of TSP was carried out using H&E-stained sections of the deepest point of tumour invasion as previously described [10, 11]. Tumour sections were scanned using a Hamamatsu NanoZoomer (Welwyn Garden City, Hertfordshire, UK) at ×20 magnification, and visualisation was carried out using the Slidepath Digital Image Hub, version 4.0.1 (Slidepath, Leica Biosystems, Milton Keynes, UK). At ×5 magnification, an area representative of the tumour invasive margin was selected. Using ×10 magnification, a single field was examined, ensuring that tumour cells were present at all four sides of the image and the area of stroma was calculated as a percentage (to the nearest 5%) of the visible field. Areas of necrosis or mucin were excluded from the field. Where multiple sections were available, each section was scored and an average calculated. All tumours were scored by a single investigator (JHP), with co-scoring of 35 patients carried out by another (CSDR) to ensure consistency. The interobserver intraclass correlation coefficient was 0.783 for the assessment of TSP and 0.813 for the TSP group
Patients were routinely followed up for 5 years following surgery. Date and cause of death were crosschecked with the cancer registration system and the Registrar General (Scotland). Death records were complete until 1 December 2011 that served as the censor date. Cancer-specific survival (CSS) was measured from the date of surgery until the date of death from CRC.
statistical analysis
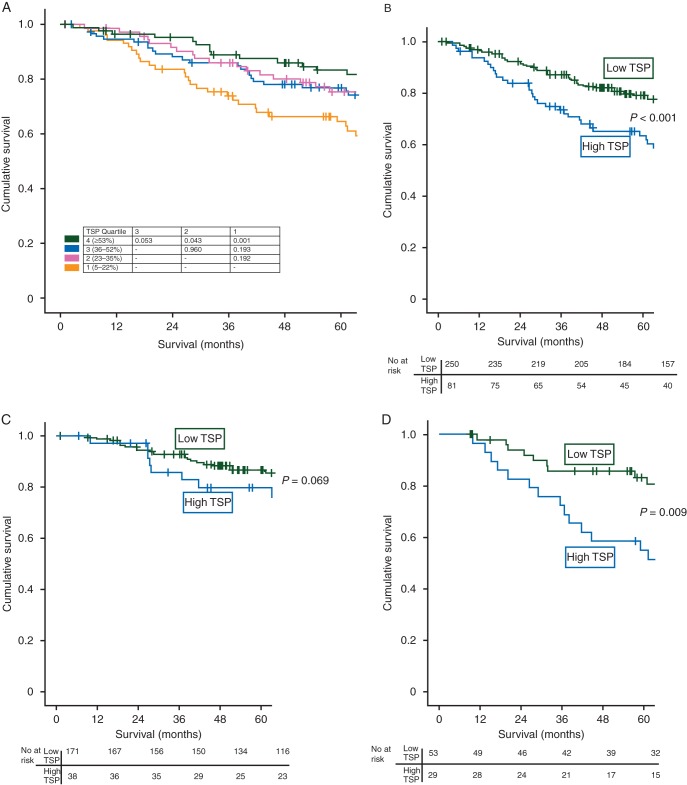
To identify a TSP threshold for survival analysis, patients were split into quartiles on the basis of TSP and survival analysed between each group using Kaplan–Meier log-rank (Mantel-Cox) pairwise comparisons (Figure 1A). Subsequently, the first, second and third quartiles (TSP <53%) were grouped as low TSP and the fourth quartile (TSP ≥53%) grouped as high TSP. To simplify the analysis, patients were subsequently grouped into low TSP (≤50%) and high TSP (>50%) (Figure 2).
Figure 1.
The relationship between tumour stroma percentage (TSP) and cancer-specific survival in patients undergoing elective resection for stage I–III colorectal cancer (CRC) (A) according to the TSP quartile, (B) in all patients with stage I–III CRC undergoing elective resection, (C) in patients with node-negative (stage I–II) CRC undergoing resection and (D) in patients undergoing elective resection followed by adjuvant chemotherapy. All P-values log-rank survival analysis.
Figure 2.
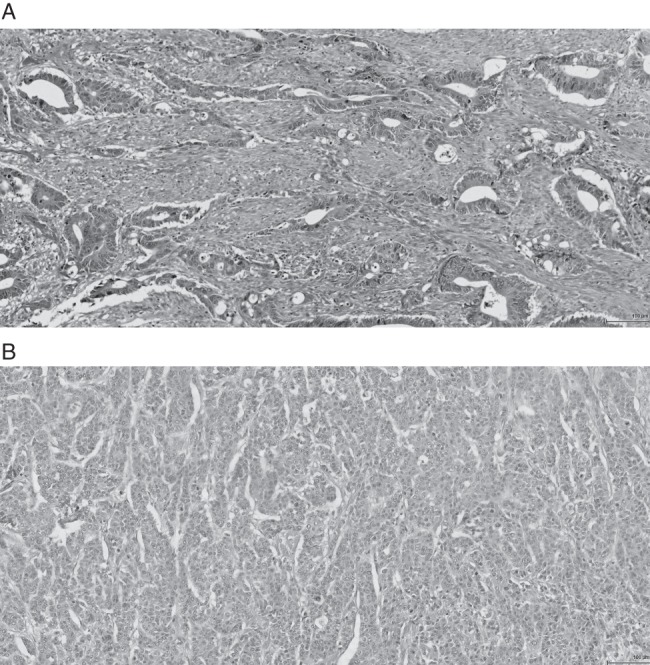
Haematoxylin and eosin-stained sections of tumour stroma at invasive margin (×200 magnification). (A) High TSP (>50%). (B) Low TSP (≤50%).
The effect of TSP on CSS was examined using Kaplan–Meier log-rank analysis. Univariable survival analysis of all patients was carried out using Cox proportional hazards regression to calculate hazard ratios (HRs) and 95% confidence intervals (95% CI). Variables with P-value of <0.1 were entered into a multivariable model using a backwards conditional method. Variables found to be significant on multivariate analysis were subsequently examined in patients with node-negative disease and those who received adjuvant chemotherapy. The association between TSP and clinicopathological characteristics was analysed using the χ2 test for linear trend. A P-value of <0.05 was considered statistically significant. All analyses were carried out using the SPSS version 21.0 (IBM SPSS, IL, USA).
results
A total of 331 patients who underwent resection of stage I–III CRC were included. Table 1 summarises clinical and pathological characteristics. Two-thirds of patients were older than 65 years at time of surgery with a similar number of males and females. The majority of patients (70%) underwent colonic resection, with pathological confirmation of stage I disease in 25 (7%) patients, stage II in 184 (56%) and stage III in 122 (37%). Eighty-two (25%) patients received adjuvant chemotherapy. A high TSP was identified in 81 (24%) patients.
Table 1.
The relationship between clinicopathological characteristics and cancer-specific survival in patients undergoing elective, curative CRC resection
| Clinicopathological characteristic |
Cancer-specific survival |
||||
|---|---|---|---|---|---|
| n (%) | Univariate analysis | P-value | Multivariate analysis | P-value | |
| All patients (n = 331) | |||||
| Age (<65/65–74/>74) | 112 (34)/110 (33)/109 (33) | 1.38 (1.07–1.77) | 0.013 | 1.42 (1.08–1.85) | 0.011 |
| Sex (female/ male) | 160 (48)/171 (52) | 0.81 (0.54–1.20) | 0.291 | – | – |
| Adjuvant therapy (no/ yes) (330)a | 248 (75)/82 (25) | 1.15 (0.73–1.80) | 0.548 | – | – |
| mGPS (0/1/2) (330) | 194 (59)/90 (27)/46 (14) | 1.75 (1.35–2.27) | <0.001 | 1.73 (1.29–2.31) | <0.001 |
| Tumour site (colon/rectum) | 232 (70)/99 (30) | 1.18 (0.77–1.81) | 0.456 | – | – |
| T-stage (1–2/3/4) | 33 (10)/208 (63)/90 (27) | 1.43 (1.07–1.92) | 0.016 | – | 0.814 |
| N stage (0/1/2) | 209 (63)/95 (29)/27 (8) | 2.19 (1.67–2.88) | <0.001 | 1.75 (1.29–2.38) | <0.001 |
| Differentiation (mod–well/poor) | 292 (88)/39 (13) | 1.59 (0.90–2.80) | 0.110 | – | – |
| Venous invasion (no/yes) | 216 (65)/115 (35) | 2.37 (1.58–3.55) | <0.001 | 1.78 (1.13–2.78) | 0.012 |
| Margin involvement (no/yes) | 310 (94)/21 (6) | 2.88 (1.54–5.42) | 0.001 | – | 0.580 |
| Peritoneal involvement (no/yes) | 249 (75)/82 (25) | 1.86 (1.22–2.84) | 0.004 | – | 0.148 |
| Tumour perforation (no/yes) | 324 (8)/7 (2) | 2.69 (0.85–8.55) | 0.094 | – | 0.061 |
| Invasive margin (expanding/infiltrative) (312) | 178 (54)/134 (40) | 1.69 (1.11–2.56) | 0.014 | – | 0.883 |
| Tumour necrosis (low/high) (297) | 169 (51)/128 (39)/34 (10) | 1.42 (0.92–2.19) | 0.112 | – | – |
| Klintrup–Mäkinen score (weak/strong) (307) | 204 (66)/103 (34) | 0.32 (0.18–0.57) | <0.001 | 0.37 (0.21–0.66) | 0.001 |
| Tumour stroma percentage (low/high) | 250 (76)/81 (24) | 2.10 (1.38–3.19) | <0.001 | 1.84 (1.17–2.92) | 0.009 |
| Node-negative colorectal cancer (n = 209) | |||||
| Age (<65/65–74/>74) | 68 (32)/71 (34)/70 (34) | 1.41 (0.95–2.08) | 0.089 | – | 0.087 |
| mGPS (0/1/2) (208) | 123 (59)/54 (26)/31 (15) | 1.61 (1.08–2.38) | 0.018 | 1.71 (1.11–2.64) | 0.016 |
| T-stage (1–2/3/4) | 25 (12)/139 (67)/45 (21) | 0.92 (0.64–1.32) | 0.639 | – | – |
| N stage (0/1/2) | 209 (100)/0 (0)/0 (0) | – | – | – | – |
| Venous invasion (no/yes) | 151 (72)/58 (28) | 1.96 (1.03–3.74) | 0.041 | – | 0.079 |
| Margin involvement (no/yes) | 201 (96)/8 (4) | 1.55 (0.37–6.44) | 0.547 | – | – |
| Peritoneal involvement (no/yes) | 165 (79)/44 (21) | 1.32 (0.65–2.71) | 0.443 | – | – |
| Tumour perforation (no/yes) | 204 (98)/5 (2) | 1.82 (0.25–13.43) | 0.557 | – | – |
| Invasive margin (expanding/infiltrative) (198) | 122 (62)/76 (38) | 1.50 (0.78–2.89) | 0.225 | – | – |
| Klintrup–Mäkinen score (weak/strong) (194) | 124 (64)/70 (36) | 0.39 (0.17–0.89) | 0.025 | 0.41 (0.18–0.93) | 0.034 |
| Tumour stroma percentage (low/high) | 171 (82)/38 (18) | 1.88 (0.94–3.78) | 0.074 | 2.14 (1.01–4.54) | 0.048 |
| Adjuvant chemotherapy (n = 82) | |||||
| Age (<65/65–74/>74) | 48 (58)/26 (32)/8 (10) | 1.16 (0.66–2.02) | 0.613 | – | – |
| mGPS (0/1/2) | 48 (58)/25 (31)/9 (11) | 1.99 (1.19–3.32) | 0.009 | 1.95 (1.11–3.43) | 0.020 |
| T-stage (1–2/3/4) | 4 (5)/49 (60)/29 (35) | 1.55 (0.81–2.96) | 0.181 | – | – |
| N stage (0/1/2) | 23 (28)/47 (57)/ 12 (15) | 1.49 (0.80–2.77) | 0.210 | – | – |
| Venous invasion (no/yes) | 43 (52)/39 (48) | 3.01 (1.31–6.96) | 0.010 | 3.31 (1.33–8.24) | 0.010 |
| Margin involvement (no/yes) | 72 (88)/10 (12) | 4.77 (1.98–11.49) | 0.001 | – | 0.195 |
| Peritoneal involvement (no/yes) | 55 (67)/27 (33) | 2.13 (0.98–4.64) | 0.056 | – | 0.392 |
| Tumour perforation (no/yes) | 79 (96)/3 (4) | 5.82 (1.33–25.44) | 0.019 | 6.95 (1.16–41.51) | 0.034 |
| Invasive margin (expanding/infiltrative) | 37 (45)/45 (55) | 0.85 (0.39–1.84) | 0.684 | – | – |
| Klintrup–Mäkinen score (weak/strong) | 58 (71)/24 (29) | 0.17 (0.04–0.70) | 0.015 | 0.21 (0.05–0.88) | 0.033 |
| Tumour stroma percentage (low/high) | 53 (65)/29 (35) | 2.71 (1.25–5.91) | 0.012 | 2.83 (1.23–6.53) | 0.015 |
aNumber of patients when incomplete data available.
mGPS, modified Glasgow Prognostic Score.
The median survival of survivors was 107 (range 44–78) months, with 95 deaths from CRC and 66 non-cancer deaths. Mean CSS of patients with stage I–III CRC was shorter in patients with high TSP tumours compared with those with low TSP tumours (110 versus 140 months, P < 0.001) (Figure 1B).
The relationship between TSP, clinicopathological characteristics and CSS is presented in Table 1. On univariate analysis, a high TSP was associated with shorter CSS (P < 0.001). On multivariate analysis, a high TSP was associated with reduced CSS (HR 1.84, 95% CI 1.17–2.92, P = 0.009), independent of age, mGPS, N stage, venous invasion and K–M score.
In node-negative CRC, a high TSP showed a trend towards shorter mean CSS compared with a low TSP (131 versus 151 months, P = 0.069) (Figure 1C). On multivariate survival analysis (Table 1), a high TSP was independently associated with reduced CSS (HR 2.14, 95% CI 1.01–4.54, P = 0.048), independent of mGPS and K–M score.
The relationship between TSP, cliniciopathological characteristics and survival in patients who underwent adjuvant chemotherapy following CRC resection was examined. In total, 23 (12%) patients with stage II disease and 59 (48%) with stage III disease received chemotherapy. Patients receiving adjuvant therapy were more likely to be male (61% versus 48%, P = 0.048), younger than 65 years of age (58% versus 25%, P < 0.001), and have American Society of Anesthesiologists (ASA) grade I/II (73% versus 56%, P = 0.016). Adjuvant chemotherapy was associated with the presence of several high-risk pathological characteristics, including advancing T stage, lymph node positivity and venous invasion (supplementary Table S1, available at Annals of Oncology online).
A high TSP was associated with shorter mean CSS following adjuvant chemotherapy (103 versus 144 months, P = 0.009) (Figure 1D). On multivariate analysis (Table 1), a high TSP was associated with reduced CSS (HR 2.83, 95% CI 1.23–6.53, P = 0.015), independent of mGPS, venous invasion, tumour perforation and K–M score.
The relationship between TSP, clinicopathological characteristics and host inflammatory responses was examined (Table 2). TSP was not associated with age, sex or ASA grade. A high TSP was associated with adjuvant chemotherapy (P < 0.01), increasing T-stage, margin and serosal involvement (all P < 0.05), increasing N stage, an infiltrative invasive margin and was inversely associated with necrosis (all P ≤ 0.01). A high TSP showed a trend towards an association with venous invasion (P = 0.066). TSP was not associated with systemic inflammatory responses; however, showed a trend towards an inverse association with local inflammatory responses as measured by the K–M score (P = 0.069), but not by T-cell subtype density at the invasive margin.
Table 2.
The relationship between tumour stroma percentage (TSP), clinicopathological characteristics and host inflammatory responses in patients undergoing elective, curative colorectal cancer resection
| Patients, n (%) |
|||
|---|---|---|---|
| Low TSP (n = 250) | High TSP (n = 81) | P-value | |
| Clinical characteristics | |||
| Age | |||
| <65 | 82 (33) | 30 (37) | 0.197 |
| 65–74 | 80 (32) | 30 (37) | |
| >75 | 88 (35) | 21 (26) | |
| Sex | |||
| Male | 126 (50) | 34 (42) | 0.188 |
| Female | 124 (50) | 47 (58) | |
| ASA (257)a | |||
| I–II | 117 (60) | 39 (62) | 0.822 |
| III–IV | 77 (40) | 24 (38) | |
| Adjuvant therapy (330) | |||
| No | 196 (79) | 52 (64) | 0.009 |
| Yes | 53 (21) | 29 (36) | |
| Pathological characteristics | |||
| Tumour site | |||
| Colon | 179 (72) | 53 (65) | 0.293 |
| Rectum | 71 (28) | 28 (35) | |
| T-stage | |||
| 1–2 | 26 (11) | 7 (9) | 0.027 |
| 3 | 168 (67) | 40 (49) | |
| 4 | 56 (22) | 34 (42) | |
| N stage | |||
| 0 | 171 (69) | 38 (47) | 0.002 |
| 1 | 61 (24) | 34 (42) | |
| 2 | 18 (7) | 9 (11) | |
| Lymph node involvement | |||
| No | 171 (68) | 38 (47) | 0.001 |
| Yes | 79 (32) | 43 (53) | |
| Differentiation | |||
| Mod/well | 222 (89) | 70 (86) | 0.564 |
| Poor | 28 (11) | 11 (14) | |
| Venous invasion | |||
| No | 170 (68) | 46 (57) | 0.066 |
| Yes | 80 (32) | 35 (43) | |
| Margin involvement | |||
| No | 239 (96) | 71 (88) | 0.011 |
| Yes | 11 (4) | 10 (12) | |
| Peritoneal involvement | |||
| No | 196 (78) | 53 (65) | 0.019 |
| Yes | 54 (22) | 28 (35) | |
| Tumour perforation | |||
| No | 244 (98) | 80 (99) | 0.527 |
| Yes | 6 (2) | 1 (1) | |
| Invasive margin (312) | |||
| Expansive | 152 (64) | 26 (34) | <0.001 |
| Infiltrative | 84 (36) | 50 (66) | |
| Tumour necrosis (297) | |||
| Low grade | 115 (51) | 54 (74) | 0.001 |
| High grade | 109 (49) | 19 (26) | |
| Local inflammatory response | |||
| CD3 margin (249) | |||
| Weak | 105 (57) | 36 (56) | 0.944 |
| Strong | 80 (43) | 28 (44) | |
| CD8 margin (245) | |||
| Weak | 103 (57) | 38 (59) | 0.732 |
| Strong | 78 (43) | 26 (41`) | |
| CD45R0 margin (251) | |||
| Weak | 100 (53) | 35 (57) | 0.628 |
| Strong | 89 (47) | 27 (43) | |
| FOXP3 margin (248) | |||
| Weak | 108 (58) | 40 (64) | 0.476 |
| Strong | 77 (42) | 23 (36) | |
| Klintrup–Mäkinen grade (307) | |||
| Weak | 147 (64) | 57 (75) | 0.069 |
| Strong | 84 (36) | 19 (25) | |
| Systemic inflammatory response | |||
| CRP >10 mg/L (330) | |||
| No | 143 (57) | 51 (63) | 0.380 |
| Yes | 106 (43) | 30 (37) | |
| Albumin <35 g/L (330) | |||
| No | 197 (79) | 70 (86) | 0.147 |
| Yes | 52 (21) | 11 (14) | |
| mGPS | |||
| 0 | 143 (57) | 51 (64) | 0.177 |
| 1 | 67 (27) | 23 (28) | |
| 2 | 39 (16) | 7 (9) | |
aNumber of patients when incomplete data available.
ASA, American Society of Anesthesiologists; CRP, C-reactive protein; mGPS, modified Glasgow Prognostic Score.
discussion
In the present study of patients undergoing elective, curative resection for stage I–III CRC, a high TSP was independently associated with reduced CSS, independent of adverse pathological characteristics and host inflammatory responses. Indeed, given that the present threshold of 50% TSP is consistent with previous reports [10, 11], these results suggest that this simple, rapid assessment of the tumour stroma using routine pathological specimens may improve risk stratification of patients undergoing curative CRC resection.
Despite being associated with increasing T stage, TSP was inversely associated with tumour necrosis. The basis of this observation was not clear; however, expansion of the stroma may obviate the development of tumour necrosis through increased angiogenesis [18] and resistance to tissue hypoxia [19]. Furthermore, the tumour stroma may reciprocate in tumour cell metabolism by facilitating recycling of products of anaerobic metabolism for further use by tumour cells [20]. Moreover, the inverse association between TSP and necrosis may also explain the lack of any perceived effect of TSP on the host systemic inflammatory response, as necrosis has been shown to promote systemic inflammation through interleukin-6 and other circulating pro-inflammatory cytokines [21].
Taken together, the present results suggest that an expanded tumour stroma may influence disease progression through a direct effect on tumour growth and invasive capabilities. Indeed, the presence of a tumour-supporting stroma may overcome barriers such as a lack of suitable energy substrate and build-up of metabolic waste [20], tissue hypoxia [19] and host-tissue integrity [8], all of which may otherwise prevent tumour growth and invasion. It was of interest that the proportions of T1–2 and T3 tumours with a high TSP were similar (21% and 19%, respectively), whereas 38% of T4 tumours had a high TSP. Therefore, the present results suggest that although a high TSP may be identified in early-stage tumours, expansion of the stromal compartment is also a characteristic of more locally advanced, aggressive disease, perhaps facilitating tumour budding and host-tissue infiltration [22].
Indeed, the present study found an association between TSP and the presence of an infiltrative invasive margin. Furthermore, lymphatic metastases and venous invasion were also more likely in patients with a high TSP. The tumour stroma has previously been shown to facilitate EMT [8, 23] and tumour cell migration into normal tissue at the host–tumour interface, characteristic of an infiltrative invasive margin [22]. Similarly, the presence of an immature stroma and a high density of stromal myofibroblasts have both been associated with tumour budding [24, 25]. Therefore, the present findings further support a pertinent role for the tumour stroma in facilitating tumour cell de-differentiation and dissemination.
Although the interrelationships between the tumour stroma, tumour microenvironment and gross pathological characteristics are likely complex, TSP remained independently associated with CSS in patients undergoing potentially curative CRC resection. Furthermore, alongside host local and systemic inflammatory responses, TSP was more strongly associated with reduced CSS than pathological characteristics currently used to identify high-risk, node-negative disease [26]. Indeed, the present results further confirm the importance of both tumour-based factors, such as the tumour microenvironment, and host factors, such as systemic inflammation [13], in determining oncological outcome.
Consistent with previous reports of the role of tumour stroma in chemoresistance [9], survival was significantly shorter in patients undergoing adjuvant therapy for high TSP tumours. In addition to identifying high-risk patients, TSP may also select patients less likely to benefit from standard therapy and who should be considered for additional adjunctive treatment, potentially targeted at the stroma itself [27]. Indeed, given the potential role of the tumour stroma in angiogenesis [18], TSP may be a biomarker of response to anti-angiogenic therapies. Such agents, however, are not currently licensed for use in CRC within our healthcare system. Furthermore, relatively few patients in the present study received adjuvant chemotherapy, with <50% of patients with stage III undergoing adjuvant treatment. Therefore, TSP remains to be investigated in a larger cohort of patients undergoing adjuvant chemotherapy for high-risk CRC, as does the impact of TSP on the efficacy of anti-angiogenic therapies.
Despite recognition of the importance of the tumour stroma in cancer progression, its relationship with other components of the tumour microenvironment has yet to be fully characterised. In the present study, there appeared to be a trend towards a weaker peritumoural inflammatory infiltrate, as measured by the K–M score but not by T-cell subtypes, in patients with high TSP tumours. It has previously been proposed that the tumour stroma may prevent effective tumour infiltration by adaptive immune cells [24, 28]. In the present study, the effect of TSP on survival remained independent of local inflammatory responses, suggesting the presence of other mechanisms rather than a direct effect on adaptive immunity. Although the T-cell markers examined in the present study were chosen on the basis of a recent systematic review confirming their relevance in CRC [5], the relationship between TSP and other cellular components of both the adaptive and innate local inflammatory responses remains to be examined. Indeed, tumour stroma may promote the development of a pro-tumour rather than anti-tumour immune infiltrate [29]. Therefore, further characterisation of the inflammatory infiltrate and its association with tumour stroma is warranted.
The present study is limited by the small number of patients with stage I disease (25 patients). As such, it was not possible to examine the effect of TSP on clinicopathological characteristics and survival separately in stage I and II CRC and gain further insights into the natural history of tumour stroma development. Despite this limitation, the present study provides comprehensive assessment of the associations between TSP and the tumour microenvironment and, in a cohort of patients with mature survival data, further confirms the prognostic relevance of assessment of the tumour microenvironment in patients undergoing curative CRC resection.
In summary, the present study shows the importance of the tumour microenvironment, and in particular TSP, in determining outcome in patients with CRC. Owing to its relatively simple assessment, TSP may be readily incorporated into routine clinical pathology reporting to improve risk stratification following curative CRC resection.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1.UK CR. Bowel Cancer Mortality Statistics. UK: Cancer Research UK; http://info.cancerresearchuk.org/cancerstats/types/bowel/mortality. 3 April 2013, 1 June 2013. [Google Scholar]
- 2.Oliphant R, Nicholson GA, Horgan PG, et al. Deprivation and colorectal cancer surgery: longer-term survival inequalities are due to differential postoperative mortality between socioeconomic groups. Ann Surg Oncol. 2013;20:2132–2139. doi: 10.1245/s10434-013-2959-9. [DOI] [PubMed] [Google Scholar]
- 3.Horgan PG, McMillan DC. Surgeons and selection of adjuvant therapy for node‐negative colonic cancer. Br J Surg. 2010;97:1459–1460. doi: 10.1002/bjs.7254. [DOI] [PubMed] [Google Scholar]
- 4.Richards CH, Roxburgh CS, Anderson JH, et al. Prognostic value of tumour necrosis and host inflammatory responses in colorectal cancer. Br J Surg. 2012;99:287–294. doi: 10.1002/bjs.7755. [DOI] [PubMed] [Google Scholar]
- 5.Roxburgh CSD, McMillan DC. The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev. 2012;38:451–466. doi: 10.1016/j.ctrv.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Freeman MR, Li Q, Chung LW. Can stroma reaction predict cancer lethality? Clin Cancer Res. 2013;19:4905–4907. doi: 10.1158/1078-0432.CCR-13-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu R, Li J, Xie K, et al. FGFR4 promotes stroma-induced epithelial-to-mesenchymal transition in colorectal cancer. Cancer Res. 2013;73:5926–5935. doi: 10.1158/0008-5472.CAN-12-4718. [DOI] [PubMed] [Google Scholar]
- 8.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 9.Petty RD, Samuel LM, Murray GI, et al. APRIL is a novel clinical chemo-resistance biomarker in colorectal adenocarcinoma identified by gene expression profiling. BMC Cancer. 2009;9:434. doi: 10.1186/1471-2407-9-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesker WE, Junggeburt JM, Szuhai K, et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol. 2007;29:387–398. doi: 10.1155/2007/175276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huijbers A, Tollenaar RA, v Pelt GW, et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: validation in the VICTOR trial. Ann Oncol. 2013;24:179–185. doi: 10.1093/annonc/mds246. [DOI] [PubMed] [Google Scholar]
- 12.Dekker TJ, van de Velde CJ, van Pelt GW, et al. Prognostic significance of the tumor-stroma ratio: validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854) Breast Cancer Res Treat. 2013;139:371–379. doi: 10.1007/s10549-013-2571-5. [DOI] [PubMed] [Google Scholar]
- 13.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Fleming ID American Joint Committee on Cancer, American Cancer Society, American College of Surgeons. AJCC Cancer Staging Manual. Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 15.Roxburgh CS, McMillan DC, Richards CH, et al. The clinical utility of the combination of t stage and venous invasion to predict survival in patients undergoing surgery for colorectal cancer. Ann Surg. 2013 doi: 10.1097/SLA.0000000000000229. http://dx.doi.org/10.1097/SLA.0000000000000229 . [DOI] [PubMed] [Google Scholar]
- 16.Richards CH, Roxburgh CSD, Powell AG, et al. The clinical utility of the local inflammatory respinse in colorectal cancer. Eur J Cancer. 2013 doi: 10.1016/j.ejca.2013.09.008. http://dx.doi.org/10.1016/j.ejca.2013.09.008 . [DOI] [PubMed] [Google Scholar]
- 17.Roxburgh C, Salmond J, Horgan P, et al. Tumour inflammatory infiltrate predicts survival following curative resection for node-negative colorectal cancer. Eur J Cancer. 2009;45:2138–2145. doi: 10.1016/j.ejca.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Koshida Y, Kuranami M, Watanabe M. Interaction between stromal fibroblasts and colorectal cancer cells in the expression of vascular endothelial growth factor. J Surg Res. 2006;134:270–277. doi: 10.1016/j.jss.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Moserle L, Casanovas O. Anti-angiogenesis and metastasis: a tumour and stromal cell alliance. J Intern Med. 2013;273:128–137. doi: 10.1111/joim.12018. [DOI] [PubMed] [Google Scholar]
- 20.Koukourakis MI, Giatromanolaki A, Harris AL, et al. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66:632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 21.Guthrie GJ, Roxburgh CS, Richards CH, et al. Circulating IL-6 concentrations link tumour necrosis and systemic and local inflammatory responses in patients undergoing resection for colorectal cancer. Br J Cancer. 2013;109:131–137. doi: 10.1038/bjc.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zlobec I, Lugli A. Invasive front of colorectal cancer: dynamic interface of pro-/anti-tumor factors. World J Gastroenterol. 2009;15:5898–5906. doi: 10.3748/wjg.15.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemmings C. Is carcinoma a mesenchymal disease? The role of the stromal microenvironment in carcinogenesis. Pathology (Phila) 2013;45:371–381. doi: 10.1097/PAT.0b013e328360b600. [DOI] [PubMed] [Google Scholar]
- 24.Ueno H, Jones AM, Wilkinson KH, et al. Histological categorisation of fibrotic cancer stroma in advanced rectal cancer. Gut. 2004;53:581–586. doi: 10.1136/gut.2003.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsujino T, Seshimo I, Yamamoto H, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–2090. doi: 10.1158/1078-0432.CCR-06-2191. [DOI] [PubMed] [Google Scholar]
- 26.Petersen VC, Baxter KJ, Love SB, et al. Identification of objective pathological prognostic determinants and models of prognosis in Dukes’ B colon cancer. Gut. 2002;51:65–69. doi: 10.1136/gut.51.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engels B, Rowley DA, Schreiber H. Targeting stroma to treat cancers. Semin Cancer Biol. 2012;22:41–49. doi: 10.1016/j.semcancer.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieubeau B, Heymann MF, Henry F, et al. Immunomodulatory effects of tumor-associated fibroblasts in colorectal-tumor development. Int J Cancer. 1999;81:629–636. doi: 10.1002/(sici)1097-0215(19990517)81:4<629::aid-ijc20>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 29.Fridman WH, Galon J, Pages F, et al. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71:5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.