Abstract
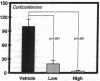
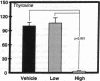
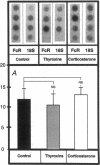
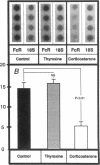
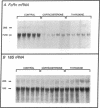
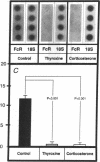
Hormonal control of immunoglobulin (Ig) absorption and of intestinal Fc receptor mRNA expression were investigated in rats to assess its potential role in the normal postsuckling inhibition of this transport system. Corticosterone and L-thyroxine therapy caused premature inhibition of the absorption of orally administered murine monoclonal antibody and of Fc receptor mRNA expression in a dose- and time-dependent manner. Low-dose corticosterone had no effect on Fc receptor mRNA synthesis after 3 d but decreased Ig transport fivefold after 7 d. High dose corticosterone resulted in a threefold reduction in Fc receptor after 3 d, and there was almost complete inhibition (> 30-fold) of transport and of Fc receptor transcript levels after 7 d. Similarly, 7 d of high-dose thyroxine decreased both serum Ig transport and Fc receptor (> 30-fold). However, adrenalectomy did not prevent the normal post-suckling declines in Ig transport or receptor synthesis. This study demonstrates that exogenous corticosteroids and thyroxine hormone inhibit Ig transport and steady-state duodenal Fc receptor mRNA levels in suckling rats. Endogenous adrenal steroids however, do not appear to be entirely responsible for the age-dependent decline in this transport system.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apodaca G., Bomsel M., Arden J., Breitfeld P. P., Tang K., Mostov K. E. The polymeric immunoglobulin receptor. A model protein to study transcytosis. J Clin Invest. 1991 Jun;87(6):1877–1882. doi: 10.1172/JCI115211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clements J. A., Matheson B. A., Wines D. R., Brady J. M., MacDonald R. J., Funder J. W. Androgen dependence of specific kallikrein gene family members expressed in rat prostate. J Biol Chem. 1988 Nov 5;263(31):16132–16137. [PubMed] [Google Scholar]
- Diamond J. M. Developmental physiology. Hard-wired local triggering of intestinal enzyme expression. Nature. 1986 Dec 4;324(6096):408–408. doi: 10.1038/324408a0. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Dussault J. H., Sack J., Chopra I. J. Ontogenesis of hypothalamic--pituitary--thyroid function and metabolism in man, sheep, and rat. Recent Prog Horm Res. 1976;33:59–116. doi: 10.1016/b978-0-12-571133-3.50010-6. [DOI] [PubMed] [Google Scholar]
- Furihata C., Kawachi T., Sugimura T. Premature induction of pepsinogen in developing rat gastric mucosa by hormones. Biochem Biophys Res Commun. 1972 May 26;47(4):705–711. doi: 10.1016/0006-291x(72)90549-9. [DOI] [PubMed] [Google Scholar]
- HALLIDAY R. The effect of steroid hormones on the absorption of antibody by the young rat. J Endocrinol. 1959 Jan;18(1):56–66. doi: 10.1677/joe.0.0180056. [DOI] [PubMed] [Google Scholar]
- Henning S. J., Leeper L. L. Coordinate loss of glucocorticoid responsiveness by intestinal enzymes during postnatal development. Am J Physiol. 1982 Feb;242(2):G89–G94. doi: 10.1152/ajpgi.1982.242.2.G89. [DOI] [PubMed] [Google Scholar]
- Henning S. J. Plasma concentrations of total and free corticosterone during development in the rat. Am J Physiol. 1978 Nov;235(5):E451–E456. doi: 10.1152/ajpendo.1978.235.5.E451. [DOI] [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Campiche M. A. Early stages of intestinal absorption of specific antibiodies in the newborn. An ultrastructural, cytochemical, and immunological study in the pig, rat, and rabbit. J Cell Biol. 1969 Aug;42(2):345–365. doi: 10.1083/jcb.42.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan J., Padbury J., Macaso T., Wang D., Beri U., Fisher D. A. Involvement of developing sympathetic nervous system in thyroxine-mediated submandibular gland nerve growth factor and epidermal growth factor responses. Pediatr Res. 1986 Mar;20(3):232–236. doi: 10.1203/00006450-198603000-00007. [DOI] [PubMed] [Google Scholar]
- Lu R. B., Chaichanwatanakul K., Lin C. H., Lebenthal E., Lee P. C. Thyroxine effect on exocrine pancreatic development in rats. Am J Physiol. 1988 Mar;254(3 Pt 1):G315–G321. doi: 10.1152/ajpgi.1988.254.3.G315. [DOI] [PubMed] [Google Scholar]
- Macy E., Kemeny M., Saxon A. Enhanced ELISA: how to measure less than 10 picograms of a specific protein (immunoglobulin) in less than 8 hours. FASEB J. 1988 Nov;2(14):3003–3009. doi: 10.1096/fasebj.2.14.3263291. [DOI] [PubMed] [Google Scholar]
- Morris B., Morris R. The effects of corticosterone and cortisone on the uptake of polyvinyl pyrrolidone and the transmission of immunoglobulin G by the small intestine in young rats. J Physiol. 1976 Jan;254(2):389–403. doi: 10.1113/jphysiol.1976.sp011237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Simister N. E. Transcytosis. Cell. 1985 Dec;43(2 Pt 1):389–390. doi: 10.1016/0092-8674(85)90166-7. [DOI] [PubMed] [Google Scholar]
- Rodewald R., Abrahamson D. R. Receptor-mediated transport of IgG across the intestinal epithelium of the neonatal rat. Ciba Found Symp. 1982;(92):209–232. doi: 10.1002/9780470720745.ch11. [DOI] [PubMed] [Google Scholar]
- Rodewald R. Intestinal transport of antibodies in the newborn rat. J Cell Biol. 1973 Jul;58(1):189–211. doi: 10.1083/jcb.58.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R., Kraehenbuhl J. P. Receptor-mediated transport of IgG. J Cell Biol. 1984 Jul;99(1 Pt 2):159s–164s. doi: 10.1083/jcb.99.1.159s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R. pH-dependent binding of immunoglobulins to intestinal cells of the neonatal rat. J Cell Biol. 1976 Nov;71(2):666–669. doi: 10.1083/jcb.71.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder R. J., Henning S. J. Roles of plasma clearance and corticosteroid-binding globulin in the developmental increase in circulating corticosterone in infant rats. Endocrinology. 1989 May;124(5):2612–2618. doi: 10.1210/endo-124-5-2612. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Stevens F. J. Modification of an ELISA-based procedure for affinity determination: correction necessary for use with bivalent antibody. Mol Immunol. 1987 Oct;24(10):1055–1060. doi: 10.1016/0161-5890(87)90073-3. [DOI] [PubMed] [Google Scholar]
- Tseng C. C., Schmidt K. L., Johnson L. R. Hormonal effects on development of the secretory apparatus of chief cells. Am J Physiol. 1987 Sep;253(3 Pt 1):G274–G283. doi: 10.1152/ajpgi.1987.253.3.G274. [DOI] [PubMed] [Google Scholar]
- Tseng C. C., Schmidt K. L., Johnson L. R. Hormonal effects on development of the secretory apparatus of parietal cells. Am J Physiol. 1987 Sep;253(3 Pt 1):G284–G289. doi: 10.1152/ajpgi.1987.253.3.G284. [DOI] [PubMed] [Google Scholar]
- Wong H. C., Walsh J. H., Yang H., Taché Y., Buchan A. M. A monoclonal antibody to somatostatin with potent in vivo immunoneutralizing activity. Peptides. 1990 Jul-Aug;11(4):707–712. doi: 10.1016/0196-9781(90)90185-8. [DOI] [PubMed] [Google Scholar]
- Wu S. V., Giraud A., Mogard M., Sumii K., Walsh J. H. Effects of inhibition of gastric secretion on antral gastrin and somatostatin gene expression in rats. Am J Physiol. 1990 May;258(5 Pt 1):G788–G793. doi: 10.1152/ajpgi.1990.258.5.G788. [DOI] [PubMed] [Google Scholar]
- Yeh K. Y., Yeh M., Holt P. R. Differential effects of thyroxine and cortisone on jejunal sucrase expression in suckling rats. Am J Physiol. 1989 Mar;256(3 Pt 1):G604–G612. doi: 10.1152/ajpgi.1989.256.3.G604. [DOI] [PubMed] [Google Scholar]
- Yeh K. Y., Yeh M., Holt P. R. Thyroxine and cortisone cooperate to modulate postnatal intestinal enzyme differentiation in the rat. Am J Physiol. 1991 Mar;260(3 Pt 1):G371–G378. doi: 10.1152/ajpgi.1991.260.3.G371. [DOI] [PubMed] [Google Scholar]
- Yeh K., Moog F. Intestinal lactase activity in the suckling rat: influence of hypophysectomy and thyroidectomy. Science. 1974 Jan 11;183(4120):77–79. doi: 10.1126/science.183.4120.77. [DOI] [PubMed] [Google Scholar]