Abstract
Plasmid DNA vaccines have been licensed for use in domesticated animals because of their excellent immunogenicity, but none have yet been licensed for use in humans. Here we report a retrospective analysis of 1218 healthy human volunteers enrolled in 10 phase I clinical trials in which DNA plasmids encoding HIV antigens were administered. Elicited T-cell immune responses were quantified by validated intracellular cytokine staining (ICS) stimulated with HIV peptide pools. HIV-specific binding and neutralizing antibody activities were also analyzed using validated assays. Results showed that, in the absence of adjuvants and boosting with alternative vaccines, DNA vaccines elicited CD8+ and CD4+ T-cell responses in an average of 13.3% (95% CI: 9.8% to 17.8%) and 37.7% (95% CI: 31.9% to 43.8%) of vaccine recipients, respectively. Three vaccinations (versus 2) improved the proportion of subjects with antigen-specific CD8+ responses (p=0.02), as did increased DNA dosage (p=0.007). Furthermore, female gender and participants having a lower Body Mass Index were independently associated with higher CD4+ T-cell response rate (p=0.001 and p=0.008, respectively). These vaccines elicited minimal neutralizing and binding antibody responses. These findings of the immunogenicity of HIV DNA vaccines in humans can provide guidance for future clinical trials.
Keywords: HIV, DNA vaccine, clinical trial, immune response
INTRODUCTION
Prevention of HIV-1 infection is a high priority for control of the HIV pandemic. The most cost-effective method to prevent HIV infection remains the development of an effective vaccine. DNA vaccines are relatively straightforward to construct, store and deploy globally. In preclinical studies, they elicit strong and sometimes protective immune responses against viruses, bacteria, parasites, autoimmune diseases, and cancer [1–5]. Various DNA vaccines have been licensed for use against West Nile virus in horses, melanoma in dogs, and infectious hematopoietic necrosis virus in salmonid fish. However, no DNA vaccine has been licensed for human use due in part to historically relatively poor immunogenicity. Whether DNA vaccines will be part of a multiple vaccine regimen for HIV is still an open question.
Individual trials have had limited sample sizes and have applied laboratory measurements of immunogenicity that are not readily comparable. The NIAID-supported HIV Vaccine Trials Network (HVTN) has performed 10 phase I or II trials of candidate DNA vaccines in which HIV-specific immunogenicity was examined using validated intracellular cytokine staining (ICS), interferon-γ enzyme-linked immunospot (IFN-γ ELISpot), binding antibody, and neutralizing antibody assays [6–12]. This report summarizes the experience with the 1218 human volunteers, who received DNA expressing HIV protein(s) or placebo, and examined potential factors that affect immunogencity.
MATERIALS AND METHODS
Criteria for trial selection
A retrospective analysis of 10 HVTN trials that included plasmid DNA vaccines encoding one or multiple HIV-1 genes was performed (Table 1). Only trials with ICS and ELISpot assays performed at HVTN laboratories were selected to minimize laboratory variability in our analyses. All trial protocols and informed consent documents were approved by Institutional Review Boards. All trial participants provided informed consent.
Table 1.
Immunogenicity outcomes of 10 HVTN human clinical trials
| Trial¶ | DNA plasmids (# plasmids) | Number of participants in each analysis (vaccine/control) | Total number participants/study | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ELISPOT | ICS | Binding antibody | Neutralizing antibody | vaccine | control | ||
| HVTN044a | Gag- Pol-Nef, Env-A, -B, -C (4) | 8/7 | 9/8 | 9/9 | 7/9 | 50 | 20 |
| HVTN052 a | Gag-Pol-Nef, Env-A, -B, -C (4) | 110/54 | 56/10 | 111/57 | 111/57 | 120 | 60 |
| HVTN060 c | Gag (1) | 0/0 | 9/7 | 1010 | 0/0 | 130 | 26 |
| HVTN063 c | Gag (1) | 0/0 | 4/1 | 9/8 | 0/0 | 100 | 20 |
| HVTN064 d | Multiple CTL epitopes | 0/0 | 19/3 | 0/0 | 0/0 | 100 | 20 |
| HVTN068 a | Gag-Pol-Nef, Env-A, -B, -C (4) | 0/0 | 21/2 | 0/0 | 0/0 | 60 | 6 |
| HVTN069 a | Gag-Pol-Nef, Env-A, -B, -C (4) | 58/0 | 72/0 | 87/0 | 0/0 | 90 | 0 |
| HVTN070 e | Gag, Pol, Env (3) | 0/0 | 26/13 | 27/17 | 27/17 | 100 | 20 |
| HVTN080 e | Gag, Pol, Env (3) | 0/0 | 9/7 | 10/8 | 10/8 | 48 | 8 |
| HVTN204 b | Gag, Pol, Nef, Env-A, -B, -C (6) | 0/0 | 27/9 | 0/0 | 0/0 | 120 | 120 |
|
| |||||||
| Subject numbers | 176/61 | 252/60 | 251/60 | 155/91 | 918 | 300 | |
Vaccine developers, construct:
VRC, with 4 plasmid DNA
VRC, with 6 plasmid DNA
Wyeth, with single DNA plasmid
Pharmexa, with single plasmid consists of multiple epitopes
PENNVAX, with 3 plasmid DNA
DNA vaccines
The plasmid DNA vaccines were designed by: Vaccine Research Center (VRC) of NIAID (Bethesda, MD), 4-plasmid or 6-plasmid mixtures; Wyeth Vaccine Research (Pearl River, New York); Pharmexa Inc. (San Diego, CA); and PENNVAX® (University of Pennsylvania, Philadelphia, PA, and Inovio Pharmaceuticals, Inc., Blue Bell, PA). The vaccine dosages ranged from 1.5–6mg, and inserts included different combinations of gag, pol, nef, env, and CTL epitopes (Table 1). The DNA vaccines were administered intramuscularly (IM) via needle with or without electroporation using the CELLECTRA® device (Inovio Pharmaceuticals, Inc.), or by a needleless device, Biojector®2000 (BJ; Bioject Medical Technologies, Inc.; Table 2).
Table 2.
DNA vaccine dosage and mode of administration affect HIV-specific T-cell responses elicited
| Trial | Number of participants receiving various dosages of DNA | Schedule of DNA vaccinations in months | Positive response rate (%) [95%CI]d | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1.5mg | 3mg | 4mg | 6mg | IMa | EPb | BJc | CD4+ | CD8+ | |
| HVTN044 | 10 | 0,1,2,6 | 44.4 [18.9, 73.3] | 11.1[2.0, 43.5] | |||||
| HVTN052 | 60+60e | 0,2 & 0,1,2 | 42.9 [30.8, 55.9] | 13.6 [7.0, 24.5] | |||||
| HVTN060 | 40 | 0,1,3,6,9 | 0 [0, 29.9] | 0 [0, 27.8] | |||||
| HVTN063 | 10 | 0,1,3 | 0 [0, 49.0] | 0 [0, 49.0] | |||||
| HVTN064 | 30 | 0,1,3,6 | 0 [0, 16.8] | 0 [0, 16.8] | |||||
| HVTN068 | 30 | 0,1 | 42.9 [24.5, 63.5] | 4.8 [0.8, 22.7] | |||||
| HVTN069 | 90 | 0,1,2 | 36.1 [26.0, 47.6] | 16.7 [10.2, 26.1] | |||||
| HVTN070 | 30 | 0,1,3,6 | 38.5 [22.4, 57.5] | 7.4 [2.1, 23.4] | |||||
| HVTN080 | 10 | 0,1,3 | 44.4 [18.9, 73.3] | 33.3 [12.1, 64.6] | |||||
| HVTN204 | 120 | 0,1,2 | 66.7 [47.8, 81.4] | 24.1[12.2, 42.1] | |||||
| Total subjects | 50 | 10 | 400 | 30 | |||||
|
| |||||||||
| Average response rate | 37.7 [31.9, 43.8] | 13.3 [9.8, 17.8] | |||||||
Intramuscular needle injection
IM delivery with electroporation
IM delivery by Biojector®
Post-final DNA vaccination response rate (%) and confidence intervals (CI) for both T-cell subsets were determined by ICS assay, only subjects with ICS data available were included
Sixty subjects each for 2 and 3 BJ injections, respectively
In some trials, separate DNA plasmids encoding IL-2 (HVTN-044), IL-12 (HVTN-060, -063, -070, -080), or IL-15 (HVTN-063, -070) were also used as adjuvants. Also, in 6 trials, protein or viral vector vaccines were administered as boosts after DNA priming (HVTN-052, -060, -064, -068, -069 -204). To focus on host and DNA vaccine factors that potentially influence immunogenicity, data from trial arms with adjuvants or time points after viral vector or protein boosts were excluded.
T-cell response evaluation
Either ICS or ELISpot or both were performed using HVTN validated assays with cryopreserved peripheral blood mononuclear cells (PBMC). PBMC were isolated and cryopreserved using standard procedures as described [13]. PBMC were thawed, rested overnight, and were stimulated as described [14,15] with pools of overlapping HIV-1 15-mer peptides for Env, Gag, and Nef (covering the most frequent potential T-cell epitopes (PTE-g) [16] for all studies except for HVTN 060/063 for which peptides based on a clade B consensus sequence were used (Biosynthesis, Lewisville, TX).
IFN-γ ELISpot assays were performed using a standardized, validated, bulk assay as described [17]. The ICS assays used were modified over time to expand the number of functional markers measured, however, the primary cytokine measure of CD4+ or CD8+ T cells producing IFN -γ and/or interleukin-2 was cross-validated for all versions of the assay [14,15].
Antibody response evaluation
Available binding antibody and neutralizing antibody responses ere analyzed from 7 and 4 protocols, respectively. Binding antibody titers were performed using validated ELISA; anti-Gag and anti-Env binding Ab responses were determined as described [18,19]. Neutralizing antibody titers were performed with tier 1 Env-pseudotyped viruses of subtype B HIV-1 in a validated TZM-bl assay as described [20].
Statistical analysis
The immunogenicity endpoint was the binary immune response at 2 weeks post-final DNA vaccination. If there were no data available at that time point, the closest time point (such as 4 weeks post-final DNA vaccination) was included in the analysis. The response rate was defined as the proportion of participants who had a positive response to one or more peptides.
ICS assay positivity was determined by comparing the percentage of T cells making IL-2 and/or IFN-γ between the experimental and negative control wells using a one-sided Fisher’s exact test, with a Bonferroni multiplicity adjustment for the number of stimulation peptide pools [21]. Responses to any peptide pool with adjusted p-values ≤0.00001 were considered as positive. ELISpot assay positivity was determined using a published method [22]. Because each protocol used different HIV-1 antigens for antibody assays, to accommodate all data, “any response” was used, which is defined as 1 if a study participant had a positive response to any antigen, and 0 for no response to all antigens.
For analyses evaluating factors that may affect the immune response, data from 3 studies (HVTN- 060, -063, -064) that demonstrated no T-cell immunogenicity (Table 2) were excluded. The response rate and 95% confidence interval of data pooled across protocols were calculated using the score method [23]. The response rate differences by gender, age (categories: 18–30, 31–40, 41–50), and body mass index (BMI) (category: <25, 25–29, ≥30) were evaluated using Fisher exact test or chi-squared test, and a logistic regression model was fit to control for dose, and number of vaccinations.
The number of vaccinations was modeled as a continuous variable, as the data are too sparse for a fitted model. The interactions between gender and BMI, gender and age were not included because the interactions were not statistically significant and the estimations of interaction terms were unstable due to sparseness of the data. P-values ≤0.05 were considered statistically significant. No adjustment for multiple testing was performed.
RESULTS
Characteristics of the plasmid DNA vaccine clinical trials
We analyzed 10 trials including 918 vaccine recipients and 300 placebo recipients. The immunologic outcomes measured by ICS, IFN-γ ELISpot, and antibody assays and the number of participants examined are shown in Table 1. Most DNA vaccines were mixtures of multiple plasmids, but 2 trials employed a single HIV gene (gag; HVTN-060 and -063), and one was comprised of multiple discrete CTL epitopes (HVTN-064). DNA dosages ranged from 1.5–6mg per participant per vaccination and the number of subjects who received each dosage varied from 10 (3mg dose) to 400 subjects (4mg dose) (Table 2).
DNA vaccines stimulated greater CD4+ than CD8+ T-cell responses
HIV-specific CD4+ T-cell response rates were more frequently detected than that of CD8+ T-cell response in all studies (except those that had no response) as determined by ICS. The positive rates were on average 37.7% (CI: 31.9, 43.8) and 13.3% (CI: 9.8, 17.8) for CD4+ and CD8+ T cells, respectively (Table 2). Among Env, Gag and Pol peptide pools tested, the average response rate of CD4+ T cells was higher to Env at 35% (CI: 29, 41.5) than to Gag (11.8%; CI: 8.2, 16.8) and Pol (6.4%; CI: 3.8, 10.4) (data not shown).
Results of IFN-γ-ELISpot assays were comparable to ICS results. Among Env, Gag and Pol peptide pools tested by this assay, the average response rate was higher to Env at 38.1% (CI: 31.2, 45.4) and lower to Gag (4.6%; CI: 2.5, 8.3) and Pol (4.5%; CI: 2.3, 8.7) for CD4+ T cell, whereas Nef-specific responses were negligible among all participants (data not shown).
The effect of the number of DNA vaccinations on cellular immunogenicity
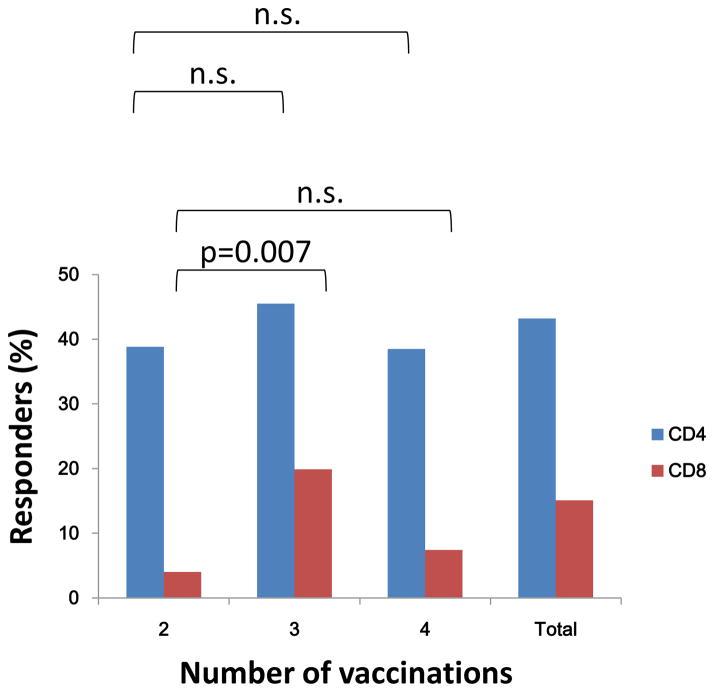
Among the 7 trials with positive T-cell responses, the effect of number of DNA vaccinations showed no difference among 2, 3, or 4 vaccinations for CD4+ T cells, each having a modest response rate of approximately 40%. However, for CD8+ T cells that had a lower response rate, responses were detected more frequently after 3 vaccinations as compared to 2 (p=0.007, Fig. 1). The multivariate logistic regression analysis after adjusting for other factors showed similar results (Table 3). Of note, this effect of number of vaccinations was observed for IM delivery by BJ only, comparative data was not available for delivery by needle/syringe with or without electroporation.
Figure 1. T-cell response rates in relation to the number of vaccinations.
CD4+ and CD8+ T-cell responses were measured using intracellular cytokine staining assay for IFN-γ and IL-2. The total number of subjects included for CD4 and CD8 responses are 220 and 238, respectively. The number of subjects who completed 2 vaccination are 49 and 50 for CD4 and CD8 cell groups, respectively; 3 vaccinations are 145 and 161 for CD4 and CD8 cell groups, respectively, and 4 vaccination are 26 and 27 for CD4 and CD8 cell groups, respectively. The p-values were determined two-sided Fisher exact test (n.s.: no significance).
Table 3.
Odds ratio for T-cell response ratea
| Variables | Estimated odd ratio | 95% CI (Lower, Upper) | p-value |
|---|---|---|---|
| CD4 response | |||
| Gender: | |||
| Male vs. female | 0.38 | (0.21, 0.68) | 0.001 |
| Age: 18–30 | 1 | - | - |
| 31–40 | 1.57 | (0.74, 3.31) | 0.24b |
| 41–0 | 0.64 | (0.31, 1.35) | 0.24 |
| BMI | 0.92 | (0.87, 0.98) | 0.007 |
| Dose | 0.74 | (0.43, 1.27) | 0.27 |
| Number of vaccinations | 1.27 | (0.66, 2.45) | 0.47 |
|
| |||
| CD8 response | |||
| Gender: | |||
| Male vs. female | 1.83 | (0.84, 3.99) | 0.13 |
| Age: 18–30 | 1 | - | - |
| 31–40 | 0.96 | (0.35, 2.65) | 0.94c |
| 41–50 | 0.95 | (0.37, 2.41) | 0.91 |
| BMI | 0.96 | (0.88, 1.04) | 0.29 |
| Dose | 0.32 | (0.14, 0.71) | 0.005 |
| Number of vaccinations | 4.78 | (1.38, 16.61) | 0.01 |
Determined using a multivariate logistic regression model.
Comparing to Age 41–50, Age 31–40 has estimated odd ratio 2.44 (95% CI: 1.01, 5.85, p-value: 0.047) for CD4
Comparing to Age 41–50, Age 31–40 has estimated odd ratio 1.02 (95% CI: 0.31, 3.34, p-value: 0.98 for CD8
The effect of DNA vaccine administration mode on cellular immunogenicity
T-cell responses were seen in all studies that employed vaccine delivery by BJ. Participants in HVTN-068, which investigated the 4-plasmid VRC vaccine administered twice by BJ had an average response rate of 42.9% (CI: 24.5, 63.5) and 4.8% (CI: 0.8, 22.7) for CD4+ and CD8+ T cells, respectively [6]. Similarly, participants in HVTN-204 who received the 6-plasmid VRC vaccine 3 times by BJ had an average response rate of 66.7% (CI: 47.8, 81.4) and 24.1% (CI: 12.2, 42.1) for CD4+ and CD8+ T cells, respectively [7]. In comparison, participants who received DNA vaccines IM via needle/syringe had less frequent positive CD4+ and CD8+ T cell responses (HVTN-060, -063, -064 had no responses, while 38.5% [CI: 22.4, 57.5] and 7.4% [CI: 2.1, 23.4] of those in HVTN-070 had CD4+ and CD8+ T-cell responses, respectively) [8–12]. Only HVTN-080 used EP to deliver vaccine, and showed higher response rates for both CD4+ [44.4% (CI: 18.9, 73.3)] and CD8+ [33.3% (CI: 12.1, 64.6)] T cells (Table 2) [10].
The effect of DNA dosage on cellular immunogenicity
The amount of DNA administered ranged from 1.5 to 6 mg/injection (Table 2). Immune responses were absent in recipients of 1.5 mg of DNA in HVTN-060 and -063, but were similar among recipients of the 3, 4 and 6mg/doses in other trials. Of note, the vaccines that were given at the lowest dose (1.5 mg) were also composed of a plasmid encoding a single HIV protein (partial-length Gag). However, responses to Gag were detected among recipients of the VRC 4-plasmid and 6-plasmid vaccines, in which the doses of DNA-gag in the mixtures were approximately 1mg and 0.67mg, respectively, suggesting the lack of response in HVTN-060 and -063 was due to the product rather than the dose of DNA-gag. There is one exception for dosages of 3mg or more: in HVTN-064, a 4mg DNA vaccine encoding multiple CTL epitopes failed to induce any detectable responses, which was consistent with its poor immunogenicity in another clinical trial [9]. A multivariate logistic regression analysis across the 3–6mg dose range did not confirm a higher dose of DNA being associated with significantly higher T-cell response rates (Table 3).
The effect of gender, BMI, and age on cellular immunogenicity
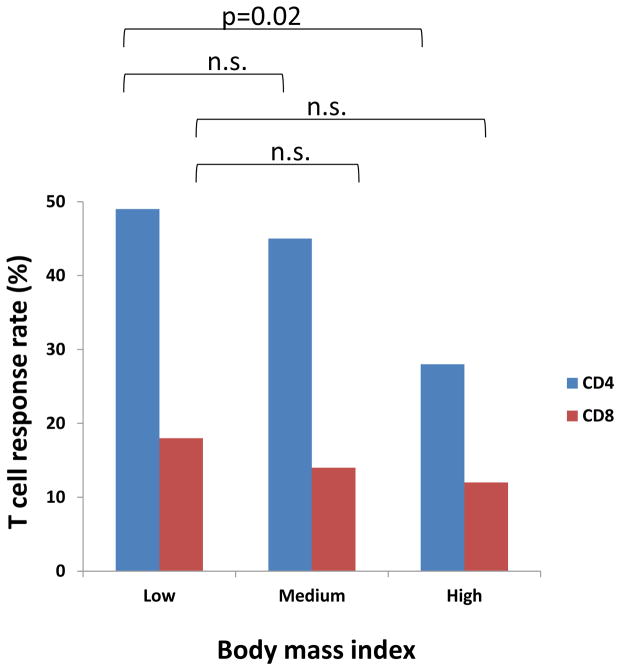
Female gender was associated with a higher HIV-specific CD4+ T-cell response ratethan male (p=0.002), but not for CD8+ T cells (Table 4). Similarly, participants with a lower BMI (BMI<25) had a higher response rate for CD4+ T cells than those with higher BMI (BMI≥30) (p=0.02), but again not for CD8+ T cells (Fig. 1). Both trends were confirmed in the multivariate logistic regression analysis (Table 3). The interaction between gender and smaller BMI is not statistically significant for CD4+ T-cell responses in the logistic regression analysis, suggesting both factors are independently associated with a better CD4+ T-cell response. In addition, the 31–40 age group has a favoring effect on the CD4+ T-cells response rate (p=0.041), but again, not for CD8+ T cells (Table S1). This was also seen in the logistic regression analysis (Table 3).
Table 4.
Gender effect on T-cell response rates
| T cell subset | Group | Gender | Number of positive | Total number | Response rate (%) | 95% CI (%) | p-valuea | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| CD4 | Control | Female | 0 | 30 | 0 | 0 | 11.4 | |
| Male | 2 | 19 | 10.5 | 2.9 | 31.4 | |||
|
| ||||||||
| Treatment | Female | 56 | 102 | 54.9 | 45.2 | 64.2 | ||
| Male | 39 | 118 | 33.1 | 25.2 | 42.0 | 0.002 | ||
|
| ||||||||
| CD8 | Control | Female | 1 | 30 | 3.3 | 0.6 | 16.7 | |
| Male | 0 | 21 | 0 | 0 | 15.5 | |||
|
| ||||||||
| Treatment | Female | 12 | 109 | 11.0 | 6.4 | 18.3 | ||
| Male | 24 | 129 | 18.6 | 12.8 | 26.2 | 0.15 | ||
Determined by Fisher exact test.
Antibody responses elicited by DNA vaccines
The primary objectives of these DNA vaccine trials were to prime T-cell immune responses, however, neutralizing and binding antibody responses were also analyzed as secondary objectives in 4 (HVTN-044, -052, -070, -080) and 7 studies (HVTN-044, -052, -060, -063, -069, -070, -080), respectively. There were very little vaccine-elicited neutralizing or binding antibody responses detected in any study (Table S2 & S3). Only in HVTN-044, which utilized an additional multiplex binding antibody assay with in-vitro antigens closely matched to the immunogen, 56% of subjects had measurable antibody responses to envelope [12].
DISCUSSION
Analysis across 10 DNA vaccine trials revealed that a number of factors affect T-cell responses. Overall, CD4+ T-cell responses was demonstrated in 60–70% individuals in some trials, but CD8+ T-cell responses were typically only detected in <25% of individuals. Factors influencing an improved response to vaccination included receipt of at least 3 doses and at least 3mg of DNA per dose. In addition, host factors of female gender and lower BMI were associated with improved responses. While binding antibodies could be demonstrated, antibody responses to HIV envelope proteins appeared to be less frequent than T-cell responses to envelope.
Both dose and the number of vaccinations appeared to influence T-cell responses, in agreement with smaller phase I studies using either HIV or HBV DNA vaccines in which a higher dose was more immunogenic, and boosting had more effect at elevating a weaker CD8+ T-cell response than a relatively stronger CD4+ T-cell response (24,25).
Also, the combined data suggested that intramuscular delivery by BJ may be superior to injection by needle and syringe, in agreement with a previous malaria DNA vaccine phase I study, where BJ IM elicited more IFN-γ responders than needle IM (26). The HVTN-070 and HVTN-080 trials clearly demonstrated the impact of CELLECTRA® EP in enhancing DNA vaccine CD4+ and CD8+ T-cell responses, either by itself or in combination with a cytokine plasmid adjuvant. When supplementing HIV DNA delivery with electroporation and IL-12 DNA plasmid in HVTN-080 approximately 80% of vaccinees had HIV-specific CD4+ responses and 50% for CD8+ after 3 vaccinations (Table 2 does not include groups that received cytokine adjuvants) [10]. This finding is supported in another clinical trial using an HPV vaccine with CELLECTRA® EP [27]. Unfortunately, in our studies, the total number of participants who received vaccine by EP was insufficient for a direct comparison to those who received DNA delivered by BJ.
We also explored the influence of gender, BMI, and age on the immunogenicity outcomes. Female gender and lower BMI were found to be associated with higher CD4+ T-cell response rates than male gender and persons with a higher BMI. This is consistent with previous studies showing that in comparison to those with normal body weight, overweight adults had poorer responses to hepatitis A and B vaccinations [28], reduced response to influenza vaccination [29,30], and less robust cellular immune responses to exogenous stimulation by mitogens [31]. This BMI effect may be related to better cellular uptake for IM administered vaccines or other physiological factors that currently are unclear. Similarly, the gender effect could be related to multifactorial determinants that are yet to be determined. While DNA vaccines have been known to stimulate robust immune responses in small animals and nonhuman primates [1,32,33], they have been less immunogenic in humans. When comparing the T-cell response rates in these human trials to the similar prototype constructs evaluated in non-human primates, the T-cell response rates in non-human primates were 80–100% [34–36] compared to 0–67% in human trials. In an outbred human population, factors such as high BMI may help explain some of the differences between animals and humans. It is possible that DNA vaccines may be more immunogenic in children with their generally lower BMI and should be considered for use in this population. The precise mechanism of how higher BMI affects CD4+ T-cell responses to vaccination is unknown, but obesity has been associated with altered cytokine production, altered monocyte and lymphocyte function, and reduced macrophage and dendritic cell function [37–40]
Because of the complexity of data analysis and interpretation of results, we opted not to address the effect of boosting with other vaccine types and co-administration of adjuvants. Recent human data, however, suggests that DNA priming can help shape what may be a more desirable profile of antibody and T-cell responses after boosting with MVA- or Ad5-vectored candidate HIV vaccines [8,41,42]. Furthermore, it appears that co-administration of DNA vaccines with adjuvants can augment immune responses [12,42]. Indeed, the use of plasmid DNA vaccines as a prime in combination vaccine regimens has become a common practice over the past decade and all 10 trials analyzed here employed DNA vaccines as a prime in a prime-boost regimen or combined with plasmids expressing cytokine adjuvants.
While previous experience has shown that DNA vaccines by themselves have not been highly immunogenic in humans, HIV-specific CD4+ T-cell responses were seen in up to two-thirds of study participants who received DNA vaccines in recent HVTN trials, perhaps explained in part by improved modes of delivery (i.e., IM via Biojector or with electroporation) and an evolving understanding of how to construct plasmids to increase immunogenicity.
To our knowledge, this is the first time that immunogenicity assessed by standardized laboratory assays from a variety of DNA vaccines has been analyzed in such a large number of healthy human volunteers, allowing us to explore additional features that might influence their immunogenicity, such as dose, schedule, and characteristics of the vaccine recipient. One limitation is that the associations (or lack of) observed in our retrospective analyses may be due to confounding factors such as different trial design and insufficient subject number for comprehensive comparison between the effect of 2, 3, and 4 vaccinations and between IM and BJ mode of vaccine delivery. Despite these difficulties in retrospective data analysis, we have made some tantalizing findings that may need to be formally verified in prospective studies in the future. Despite the noted caveat, these findings can be instructive in interpreting and assessing differences in immune profiles in animals vs. humans, designing future DNA vaccine candidates for HIV and other pathogens, and identifying optimal vaccination regimens with respect to mode of immunogen delivery and frequency of vaccination.
Supplementary Material
Figure 2. BMI affects vaccine elicited HIV-specific human CD4+ T-cell responses, but not CD8+ T-cell responses.
In 7 clinical trials that demonstrated vaccine-elicited immune responses, the distribution of T-cell responses were plotted by low (BMI < 25), medium (25 ≤ BMI <30) and high groups (BMI ≥ 30). Results show that BMI affects the CD4+ T-cell response more than the CD8+ T-cell response. Results show that BMI significantly affects the CD4+ T-cell response between high and low BMI groups (p=0.02), but not for the CD8+ T-cell response (n.s.: no significance).
Acknowledgments
We thank the vaccine developers, trial site staff, as well as volunteers who made these studies possible.
Funding
This work is supported in part by NIAID award to the HIV Vaccine Trials Network (UM1-AI-068618), Fred Hutchinson Cancer Research Center Core (2 UM1 AI068614-08), University of Rochester (UM1-AI-069511), SCHARP (2 UM1AI068635-08), and HVTN Laboratory Center (2 UM1 AI068618-08).
Footnotes
Conflict of Interest Statement
The authors do not have potential conflicts of interest with the work presented in this article.
ClinicalTrials.gov identifier numbers for parent protocols included in these analyses are: HVTN 044, NCT00069030; HVTN 052 NCT00071851; HVTN 060 NCT00111605; HVTN 063 NCT00115960; HVTN 064 NCT00141024; HVTN 068 NCT00270218; HVTN 069 NCT00384787; HVTN 070 NCT00528489; HVTN 080 NCT00991354; HVTN 204 NCT00125970
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Xia Jin, Email: xjin@ips.ac.cn.
Cecilia Morgan, Email: cmorgan@fredhutch.org.
Xuesong Yu, Email: xyu@scharp.org.
Stephen DeRosa, Email: sderosa@fredhutch.org.
Georgia D. Tomaras, Email: georgia.tomaras@duke.edu.
David C. Montefiori, Email: david.montefiori@duke.edu.
James Kublin, Email: jkublin@fredhutch.org.
Larry Corey, Email: lcorey@fredhutch.org.
Michael C. Keefer, Email: michael_keefer@urmc.rochester.edu.
References
- 1.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239(1):62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 2.Mann JF, McKay PF, Fiserova A, et al. Enhanced immunogenicity of an HIV-1 DNA vaccine delivered with electroporation via combined intramuscular and intradermal routes. J Virol. 2014;88(12):6959–69. doi: 10.1128/JVI.00183-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibui A, Nakae S, Watanabe J, et al. Screening of novel malaria DNA vaccine candidates using full-length cDNA library. Exp Parasitol. 2013;135(3):546–50. doi: 10.1016/j.exppara.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Kang TH, Mao CP, La V, Chen A, Hung CF, Wu TC. Innovative DNA vaccine to break immune tolerance against tumor self-antigen. Hum Gene Ther. 2013;24(2):181–8. doi: 10.1089/hum.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad G1, Zhang W, Torben W, et al. Preclinical prophylactic efficacy testing of Sm-p80-based vaccine in a nonhuman primate model of Schistosoma mansoni infection and immunoglobulin G and E responses to Sm-p80 in human serum samples from an area where schistosomiasis is endemic. J Infect Dis. 2011;204(9):1437–49. doi: 10.1093/infdis/jir545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Rosa SC, Thomas EP, Bui J, et al. HIV-DNA priming alters T cell responses to HIV-adenovirus vaccine even when responses to DNA are undetectable. J Immunol. 2011;187(6):3391–3401. doi: 10.4049/jimmunol.1101421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churchyard GJ, Morgan C, Adams E, et al. A phase IIA randomized clinical trial of a multiclade HIV-1 DNA prime followed by a multiclade rAd5 HIV-1 vaccine boost in healthy adults (HVTN204) PLoS One. 2011;6(8):e21225. doi: 10.1371/journal.pone.0021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalams SA, Parker S, Jin X, et al. Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults. PloS One. 2012;7(1):e29231. doi: 10.1371/journal.pone.0029231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin X, Newman MJ, De-Rosa S, et al. A novel HIV T helper epitope-based vaccine elicits cytokine-secreting HIV-specific CD4+ T cells in a phase I clinical trial in HIV-uninfected adults. Vaccine. 2009;27(50):7080–6. doi: 10.1016/j.vaccine.2009.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalams SA, Parker SD, Elizaga M, et al. Safety and comparative immunogenicity of an HIV-1 DNA vaccine in combination with plasmid interleukin 12 and impact of intramuscular electroporation for delivery. J Infect Dis. 2013;208(5):818–829. doi: 10.1093/infdis/jit236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koblin BA, Casapia M, Morgan C, Qin L, et al. Safety and immunogenicity of an HIV adenoviral vector boost after DNA plasmid vaccine prime by route of administration: A randomized clinical trial. PLoS One. 2011;6(9):e24517. doi: 10.1371/journal.pone.0024517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baden LR, Blattner WA, Morgan C, et al. Timing of plasmid cytokine (IL-2/Ig) administration affects HIV-1 vaccine immunogenicity in HIV-seronegative subjects. J Infect Dis. 2011;204(10):1541–1549. doi: 10.1093/infdis/jir615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bull M, Lee D, Stucky J, et al. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J Immunol Methods. 2007;322(1–2):57–69. doi: 10.1016/j.jim.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horton H, Thomas EP, Stucky JA, et al. Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods. 2007;323(1):39–54. doi: 10.1016/j.jim.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McElrath MJ, DeRosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Malhotra U, Gilbert PB, et al. Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine. 2006;24(47–48):6893–6904. doi: 10.1016/j.vaccine.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Dubey S, Clair J, Fu TM, et al. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J Acquir Immune Defic Syndr. 2007;45:20–27. doi: 10.1097/QAI.0b013e3180377b5b. [DOI] [PubMed] [Google Scholar]
- 18.Goepfert PA, Tomaras GD, Horton H, et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine. 2007;25(3):510–518. doi: 10.1016/j.vaccine.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 19.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82(24):12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, Gao F, Mascola JR, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westfall P, Wolfinger R. Multiple tests with discrete distributions. Am Stat. 1997;51:3–8. [Google Scholar]
- 22.Moodie Z, Huang Y, Gu L, Hural J, Self SG. Statistical positivity criteria for the analysis of ELISpot assay data in HIV-1 vaccine trials. J Immunol Methods. 2006;315(1–2):121–132. doi: 10.1016/j.jim.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Agresti A, Coull BA. Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat. 1998;52:119–126. [Google Scholar]
- 24.Boyer JD, Cohen AD, Vogt S, et al. Vaccination of seronegative volunteers with a Human Immunodeficiency Virus Type 1 env/rev DNA vaccine induces antigen-specific proliferation and lymphocyte production of β-chemokines. J Infect Dis. 2000;181:476–483. doi: 10.1086/315229. [DOI] [PubMed] [Google Scholar]
- 25.Roy MJ, Wu MS, Barr LJ, et al. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of hepatitis B virus DNA vaccine. Vaccine. 2001;19:764–778. doi: 10.1016/s0264-410x(00)00302-9. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, Epstein J, Baraceros FM, et al. Induction of CD4+ T cell-dependent CD8+ type 1 responses in humans by a malaria DNA vaccine. PNAS. 2001;98(19):10817–10822. doi: 10.1073/pnas.181123498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagarazzi ML, Yan J, Morrow MP, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012;4(155):155ra138. doi: 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Wielen M, Van Damme P, Chlibek R, Smetana J, von Sonnenburg F. Hepatitis A/B vaccination of adults over 40 years old: comparison of three vaccine regimens and effect of influencing factors. Vaccine. 2006 Jun 29;24(26):5509–5515. doi: 10.1016/j.vaccine.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 29.Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes (Lond) 2012;36(8):1072–1077. doi: 10.1038/ijo.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White SJ, Taylor MJ, Hurt RT, et al. Leptin-based adjuvants: an innovative approach to improve vaccine response. Vaccine. 2013;31:1666–72. doi: 10.1016/j.vaccine.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han SN, Jeon KJ, Kim MS, et al. Obestity with a body mass index under 30 does not significantly impair the immune response in young adults. Nutr Res. 2011;31:362–9. doi: 10.1016/j.nutres.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Williams JA. Improving DNA vaccine performance through vector design. Curr Gene Ther. 2014;14(3):170–89. doi: 10.2174/156652321403140819122538. [DOI] [PubMed] [Google Scholar]
- 33.Flingai S, Czerwonko M, Goodman J, Kudchodkar SB, Muthumani K, Weiner DB. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front Immunol. 2013 Nov 4;4:354. doi: 10.3389/fimmu.2013.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Letvin NL, Huang Y, Chakrabarti BK, et al. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J Virol. 2004;78(14):7490–7. doi: 10.1128/JVI.78.14.7490-7497.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barouch DH, Yang ZY, Kong WP, et al. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol. 2005;79(14):8828–34. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chong SY, Egan MA, Kutzler MA, et al. Comparative ability of plasmid IL-12 and IL-15 to enhance cellular and humoral immune responses elicited by a SIVgag plasmid DNA vaccine and alter disease progression following SHIV(89. 6P) challenge in rhesus macaques. Vaccine. 2007;25(26):4967–82. doi: 10.1016/j.vaccine.2006.11.070. [DOI] [PubMed] [Google Scholar]
- 37.Wirtz PH, Ehlert U, Emini L, Suter T. Higher body mass index (BMI) is associated with reduced glucocorticoid inhibition of inflammatory cytokine production following acute psychosocial stress in men. Psychoneuroendocrinology. 2008;33(8):1102–10. doi: 10.1016/j.psyneuen.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Vendrell J, Broch M, Vilarrasa N, et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: Relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:2322–30. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian V, Ferrante AW. Obesity, inflammation, and macrophages. Nestle Nutr Workshop Ser Pediatr Program. 2009;63:151–9. doi: 10.1159/000209979. [DOI] [PubMed] [Google Scholar]
- 40.O’Shea D, Corrigan M, Dunne MR, et al. Changes in human dendritic cell number and function in severe obesity may contribute to increased susceptibility to viral infection. Int J Obes (Lond) 2013;37(11):1510–3. doi: 10.1038/ijo.2013.16. [DOI] [PubMed] [Google Scholar]
- 41.Goepfert PA, Elizaga ML, Sato A, et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis. 2011;203:610–9. doi: 10.1093/infdis/jiq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koup RA, Roederer M, Lamoreaux L, et al. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS One. 2010;5:e9015. doi: 10.1371/journal.pone.0009015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.