Abstract
Background
Malignant melanoma is an aggressive form of skin cancer with limited effective therapeutic options. Melanoma research concentrates on maximizing the effect on cancer cells with minimal toxicity to normal cells. AMP-activated protein kinase (AMPK) is an important regulator of cellular energy homeostasis and has been shown to control tumor progression regulating the cell cycle, protein synthesis and cell growth and/or survival. Honokiol (HNK) is a biphenolic compound derived from Magnolia officianalis, a plant that has been used in traditional Chinese and Japanese medicine for the treatment of various pathological conditions. Recent studies have shown that HNK has antitumor activity with relatively low toxicity. In this study we demonstrated that the growth inhibitory effects of HNK on melanoma and melanoma cancer stem cells (CSCs) was mediated through the activation of AMPK and hence AMPK signaling in melanoma cells.
Methods
We determined the effects of HNK treatment on various melanoma cell lines. HNK induced cell growth inhibitory effects were determined using hexosaminidase assay. Protein expression studies were done by immunoblotting. Primary spheroid assay was used to assess stemness by growing single suspension cells in ultra-low attachment plates.
Results
HNK is highly effective in inhibiting melanoma cells by attenuating AKT/mammalian target of rapamycin and AMPK signaling. HNK showed significant inhibition of the spheroid forming capacity of melanoma cells and, hence, stemness. HNK significantly decreased the number and size of melanospheres in a dose dependent manner. Western blot analyses showed enhanced phosphorylation of AMPK in melanoma cells. Furthermore, HNK decreased the cellular ATP pool in a dose-dependent manner with maximum effects observed at 48 h.
Conclusion
The results suggest that HNK can target melanoma cells and mark them for cell death through AMPK signaling. Further studies are warranted for developing HNK as an effective chemopreventive/therapeutic agent in melanoma.
Keywords: Melanoma, metabolic pathways, stem cells, ATP/ADP, AMPK
INTRODUCTION
The incidence of melanoma over the last few decades has been increasing dramatically, making it one of the fastest growing malignancies in the United States (1), (2). Melanoma is an aggressive form of skin cancer that has limited therapeutic options. Within the past decade, the paradigm for tumor development has evolved. Our increased understanding of the disease indicates that tumors contain stem cells, termed cancer stem cells (CSCs), which initiate and support tumor growth and maintenance (3). Thus, targeting the tumor associated CSC population provides the potential for more effective treatments of melanoma.
The ability of living organisms to maintain normal cellular functions and homeostasis is associated with their ability to adapt to physiological challenges. To avoid metabolic stress, cells often adapt by rapidly suppressing anabolic processes, stalling cell cycle progression, and promoting efficient energy production. Regulation of complex metabolic activities is one of the most critical steps involved in survival and growth of normal as well as cancer cells. AMP-activated protein kinase (AMPK) plays a central role in maintaining energy homeostasis by acting as a sensor of cellular energy levels in both normal and transformed cells (4) (5). AMPK exists as a heterotrimeric complex of proteins comprising of an α catalytic kinase subunit, and two regulatory β and γ subunits. In normal physiological conditions, AMPK protect cells against physiological and pathological stress by maintaining homeostatic cellular pools of ATP, AMP and ADP in cells. On the other hand, in cancer, the role of AMPK signaling is still not fully explored. Recent studies report low AMPK activity within tumors and provide some evidence that AMPK might provide protection against the development of malignancies. A study of breast cancer showed loss of AMPK activation in 90% of 350 cases with the possible mechanism being loss of activation of LKB1 kinase (a tumor suppressor) in response to metabolic stress. LKB1 and calmodulin-dependent protein kinase kinase are involved in the activation of AMP; physiologic relevance currently only exists for LKB1 (6, 7). Upon activation AMPK, metabolism focuses on the generation of ATP, while systematically blocking cellular energy outlay (8). AMPK signaling also mediates cell cycle checkpoints, thereby blocking the pro-survival pathway, and modulating mitotic progression, allowing damage repair and or cellular death if cells sustain irreparable damage (9, 10). Relevant to its ability to match cell cycle progression to energy availability, AMPK is now proposed to play a role in regulating cell polarity, mitotic progression, and cytokinesis (11–13). Activation of AMPK can be triggered by intra- or extra- cellular metabolic stress, or by physiological stimuli such as muscle contraction or by cytokines. Additionally, AMPK activation can be pharmacologically manipulated by various drugs and xenobiotic compounds like metformin, phenformin, resveratrol, and berberine. Once activated by falling energy status, ATP production increases by increasing the activity or expression of proteins involved in catabolism while conserving ATP by switching off biosynthetic pathways. Finally, recent studies also reveal that AMPK conserves ATP levels through the regulation of processes other than metabolism, such as cell cycle and neuronal membrane excitability (5).
New agents that target metabolism associated signaling pathways and effectively kill the CSC population, could, as a single therapy or in combination with current antitumor drugs, be more effective in controlling tumor growth, halting tumor progression, and improving patient outcomes. Honokiol (HNK) is a lignan isolated from the barks, seed cones, and leaves of Magnolia officianalis tree. Lignans are a class of phytoestrogens that also act as antioxidants. Extracts of various parts of the plant have been previously used in traditional Chinese and Japanese medicine for the treatment of various ailments including ulcers, muscle spasm, allergies, and bacterial infections (14, 15). Recent studies have shown that HNK also has antitumor activity with low toxicity to normal tissue (16). In this article, we have determined the effect of HNK on melanoma cancer cells and cancer stem cells, as mediated through the AMPK signaling pathway.
MATERIALS AND METHODS
Cells and reagents
B16/F10, SKMEL-28, SK103, and UAC2755 melanoma cell lines were procured from American Type Culture Collection (ATCC) and grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO) and antibiotic-antimycotic solution (Mediatech Inc., Manassas, VA) at 37°C in a humidified atmosphere containing 5% C02. Cells used in this study were within 18 passages after receipt or renewal. Growth medium was changed after every 3 days and cells were split in 1:6 ratios when they reached 80% of confluence. For HNK (Sigma Aldrich., St. Louis, MO) treatment, stock solution of HNK was prepared in DMSO, stored at −20°C in aliquots, and diluted with fresh medium immediately before use. Other general chemicals were purchased from Sigma-Aldrich, St. Louis, MO.
Cell Viability Assay
Hexosaminidase assay was used to study HNK effect on cell viability (17). In brief, cells were plated in 96 well plates, grown over night and treated next day with increasing concentrations of HNK (0–60 μM) for 24, 48 and 72 h. Cell growth was calculated as percent viability = [(A/B)×100], where A and B are the absorbance of treated and control cells, respectively. The best fit was used for further processing of data. IC50 was obtained by determining the concentration of compounds (μM) resulting in 50% of cell death after HNK treatment at desired time interval.
Melanosphere formation assay
The spheroid formation assay using melanoma cells was performed with some modifications of the previously described methodology (18). Cells were plated in ultralow attachment plates (Corning Inc., Corning, NY) at a density of 5×103 cells/ml in DMEM supplemented with 1% N2 supplement (Life Technologies., Grand Island, NY), 2% B27 supplement (Life Technologies., Grand Island, NY), 20 ng/ml human platelet growth factor (Sigma-Aldrich, St. Louis, MO) 100 ng/ml epidermal growth factor (Life Technologies., Grand Island, NY) and 1% antibiotic-antimycotic (Mediatech Inc., Manassas, VA) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Melanospheres were photographed and counted after 7 d of HNK (0–50 μM) treatment.
ATP/ADP estimation in cells
Cellular ATP pool in melanoma cells after HNK treatment was determined by a Luminescence based ATP quantification assay kit (Invitrogen) based on previously published protocol (19). For both cellular ATP and ADP levels, High Performance Liquid Chromatography (HPLC) was utilized by applying slight modifications to the previously described method (20). In brief, cells were plated in 6-well culture plates and allowed to grow over night. The next day, medium was replaced with fresh medium. Melanoma cells were treated with 30 μM HNK for 24 to 48 h. Cells were lysed with mild lysis buffer (19) and plates were incubated for 10 h at 4°C on rocker. Luminescence was measured using a 96-well microtiter plate reader. Protein content of each well was also measured using Bradford Protein Solution and quantified using the same 96-well microtiter plate reader. ATP measurement was normalized to protein content in each well and represented as percentage of control. For ATP estimation by HPLC, high purity standards for ATP and ADP (Sigma) were run on HPLC to get ATP and ADP standard curves. ATP and ADP standard curves from HPLC were used to quantify cellular ATP and ADP levels in both melanoma cell lines after HNK treatment.
Immunoblot Analyses
Cell lysates for immunoblot analyses were prepared after HNK treatment. For equal loading of samples, protein for each lysate was quantitated by using Pierce BCA protein assay kit (Thermo Scientific., Chicago, IL). Cell lysates were subjected to SDS-PAGE and blotted onto nitro cellulose membrane (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) and proteins of interest were detected by using enhanced chemiluminescence system (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Antibodies for immunoblotting were purchased from Cell Signaling Technology Inc. (Danvers, MA), and Santa Cruz Biotechnology Inc. (Dallas, TX). For demonstrating equal loading of protein, the blots were normalized for β-actin levels.
Statistical analysis
All values are expressed as the mean + SEM. Data was analyzed using an unpaired 2-tailed t test. A P value of less than 0.05 was considered statistically significant. The SPSS V17 statistical software was used for these analyses.
RESULTS
Honokiol affects cell viability of melanoma cells
In order to evaluate the potential use of HNK in melanoma treatment and cure, HNK growth inhibitory effects was studied by hexosaminidase assay in the aggressive melanoma cell lines, B16/F10, SKMEL-28, SK103, and UAC2755. B16/F-10 and SKMEL-28 melanoma cells were selected for subsequent studies. HNK showed significant growth inhibitory response in most of the melanoma cell lines in a dose (0–60 μM) and time (0–72 h) dependent manner. However, maximal effects were seen at 48 and 72 h of HNK treatment. We have determined that HNK induces a dose- and time-dependent decrease in growth of melanoma cells with IC50 values below 40 μM for B16/F-10, SKMEL-28 and SK103 cells at both 48- and 72 h time points (Fig. 1a). This data for B16/F-10 and SKMEL-28 cell lines has previously been published.(27) Similar results were seen in both p53 competent and deficient cell lines. All of the cell lines used do not express LKB1. IC50 values, however, were higher for UAC2755 cells as these cells did not respond well to HNK treatment (Fig. 1a). The findings indicate that HNK has cell growth inhibitory effect on melanoma cells although there might be an as yet unidentified genetic component to therapeutic response. Thus, in this initial group of experiments, HNK demonstrated the short-term effect of inhibiting cell survival/growth and long-term effect by inhibiting clonogenic potential (data not shown) of melanoma cells.
Figure 1.
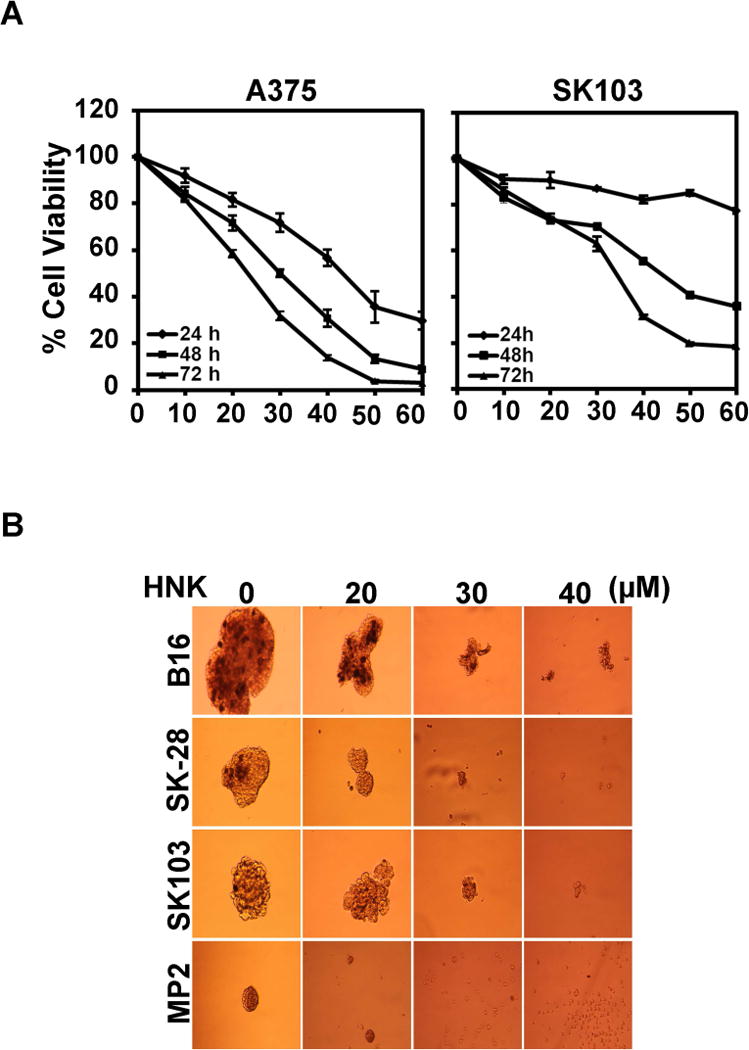
Honokiol inhibits growth and stemness of melanoma cells. (a) Cell proliferation assay showed HNK induced growth inhibitory effects on melanoma cells. Melanoma cells used in this study were incubated with increasing doses of HNK (0–60 μM) and cell viability was determined after 24, 48 and 72 h of HNK treatment. The experiments were conducted in quadruplicates, and repeated at least twice. The data were analyzed as percent of control, where the control wells were treated with equivalent amounts of DMSO alone. HNK resulted in a significant dose and time-dependent decrease in cell viability of B16/F-10, SKMEL-28, A375 and SK103 cells compared to their respective controls (P < 0.05). The results for the latter two cell lines are shown (b) HNK affects melanoma stem cells. For the melanosphere formation assay, cells were grown in ultralow attachment plates and treated with increasing concentrations of HNK (0–50 μM). After 7 days, the spheroids were photographed. HNK treatment significantly inhibited melanosphere formation in 3D culture (*p<0.05) in a dose dependent manner.
Honokiol affects the stem cell-like properties of melanoma cells in 3D culture
Only small populations of cells in a malignancy contain tumor initiating potential, are undifferentiated, and have increased self-renewal capacity. The majority of cells within a tumor have undergone differentiation and have lost this potential (21). Melanospheres are non-adherent 3D spheroid bodies enriched in melanoma initiating cells with increased differentiation capacity in vitro and tumorigenic potential in vivo. We were interested in HNK’s potential to inhibit the growth of melanoma initiating cells. The purpose of evaluating melanospheres was to determine the role of AMPK signaling in effecting melanoma stemness. While the role and existence of true stem cells is a matter of significant debate in the literature, the role of stemness seems to have a real impact on aggressiveness and resiliency of tumor cells. Melanospheres help to identify the role that honokiol, partially mediated through the AMPK signaling pathway, may have on stemness. To evaluate this possibility, we measured melanosphere formation in 3D culture. For this assay, melanoma cells were grown in melanosphere forming medium in ultra-low attachment plates and treated with HNK (0–50 μM). After 6 days, primary spheroids in each well were photographed (Fig. 1b) and counted (data not shown). Honokiol significantly inhibited melanosphere formation for all four melanoma cell lines in 3D cultures in a dose dependent manner. This data suggests that HNK is a potent inhibitor of the melanoma stem cell population.
Honokiol affects ATP cellular pool in melanoma cells
Previous studies have demonstrated that changes in ATP levels can affect the growth and viability of cancer cells (22). Hence, changes in cellular ATP levels after HNK treatment were evaluated by using two separate techniques, a Luciferase based luminescence and HPLC. In the Luciferase based assay, no significant change in ATP levels of B16/F-10 and SKMEL-28 cells was observed after 24 h of HNK (30 μM) treatment. At 48 h of HNK treatment, however, the decline in the levels of ATP were seen in both the cell lines although it was statistically significant in only SKMEL-28 cells (Fig 2b). To further confirm the levels of ATP and compare them to the changes in ADP levels upon similar treatment, cell lysates were also analyzed by HPLC. Linear standard curves were generated using pure standards of ATP and ADP with R2 value greater than 0.99 for both ATP and ADP. Upon HPLC analysis, a similar trend to those with luciferase assay was observed. The cells did not show any significant change in ATP levels at 24 h but at 48 h there was a significant decline in ATP levels in both cell lines. Surprisingly, a corresponding change in ADP levels was not observed in both cell lines upon HNK treatment. (Figure 2a and c). This might indicate reduction in the production rate of new ATP at the time points studied as compared to increased consumption.
Figure 2.
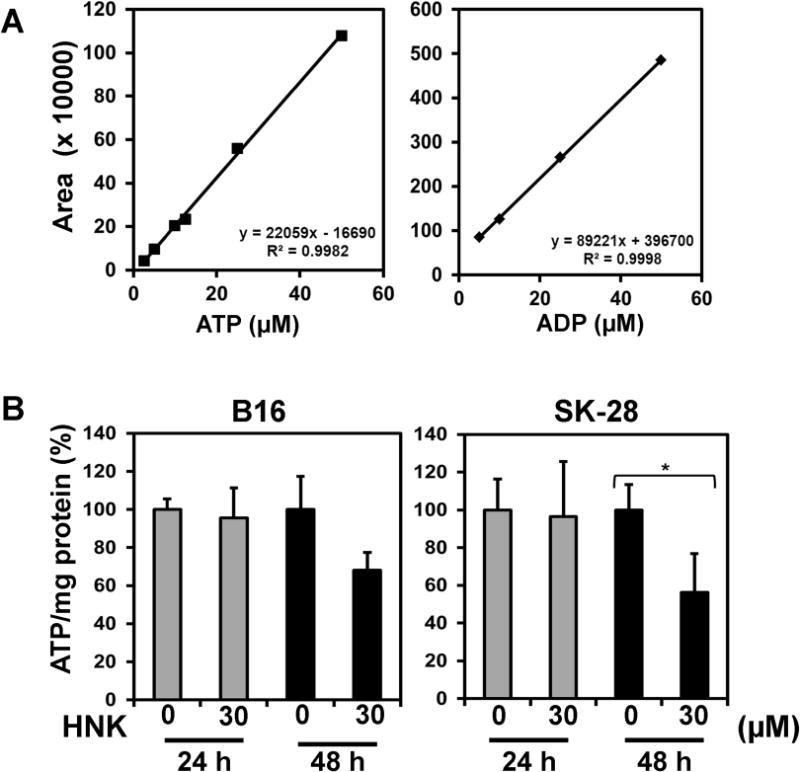
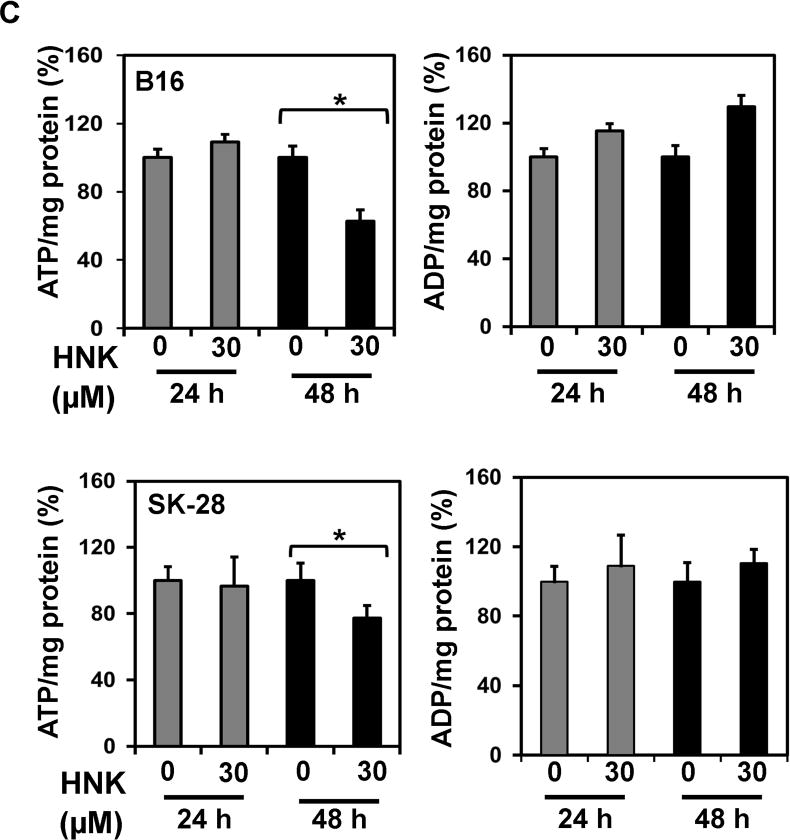
HNK affect energy homeostasis of cancer cells by effecting cellular ATP. (a) Linear standard curves were generated using pure standards of ATP and ADP with R2 value greater than 0.99 for both ATP and ADP for HPLC. (b) ATP modulation by HNK in B16/F-10 and SKMEL-28 as measured by in a Luciferase based assay. No changes in ATP levels were seen at 24 h, but a statistically significant decrease in ATP levels was seen only in SKMEL-28 cells at 48 h. (c) HPLC was used to evaluate ATP and ADP levels in both cells lines at 24 and 48 h. No changes were seen at 24 h, but ATP levels were statistically lower in both cell lines at 48 h. No changes in ADP levels were observed.
Honokiol induces AMPK activation in melanoma cells
To further examine the mechanism by which HNK induces growth inhibition of melanoma cells and melanoma stem cells and also changes in ATP cellular pools, we studied AMPK signaling in melanoma cells as had been seen in other cell types. The AMPK pathway has been shown to play a central role in cellular energy homeostasis and act as an energy sensor in cells. AMPK is a heterotrimeric complex that consists of a catalytic α subunit and two regulatory subunits, β and γ. For AMPK activation, phosphorylation of Threonine172 in the α-subunit is required (3, 4) in cells. HNK (0–30 μM) showed dose and time dependent induction in phosphorylation of Thr172 in the α-subunit of AMPK in melanoma cells (Figure 3). We further confirmed these changes by using compound C (AMPK inhibitor) in combination with HNK. We observed that Compound C showed significantly attenuated HNK induced changes and partially rescued melanoma cells from HNK induced cell death (data not shown). As Compound C was not able to rescue cells completely from HNK’s effects, it raised the possibility of involvement of other pathways in these changes.
Figure 3.

Western blot analysis for AMPK in melanoma cells. Cells were treated with increasing concentration of HNK showed increased activation of AMPK in a concentration dependent manner (representative blot shown).
DISCUSSION
Honokiol is a putative candidate for both chemoprevention and therapeutic interventions because of its diverse pharmacological properties with intense antitumor effects, good bioavailability and absorption in addition to rapid excretion in pre-clinical models. In this series of experiments, we studied the effects of HNK on growth and stemness of melanoma cells. Honokiol was effective in reducing the cell viability of melanoma cells in a dose and time dependent manner with maximal changes observed at 48 and 72 h of treatment. The growth inhibitory effects of HNK were also reported by us and others in different cancer types including colon, breast, and glioblastoma with minimal or no toxic effects in normal counterparts (23) (24) (25) (26) (18) (27) (28) (16). As was discussed earlier, CSCs could be an important target for the treatment and cure of melanoma. We further studied the effects of HNK on stemness of melanoma cells studied by 3D melanosphere formation assay. Honokiol was very effective in inhibiting the growth of melanospheres in 3D culture (data not shown).
Recently, we reported that HNK induces autophagic cell death in melanoma cells was evident from intracellular autophagic vacuole formation (29). Autophagy is a self-degradative process involved in maintaining energy homeostatic during cellular crisis and in response to nutrient stress. In normal physiological conditions, autophagy is generally thought to a survival mechanism in cells (30) (31) (32). However, persistent or excessive autophagy is shown to promote cell death following treatment with pharmacological agents, either by induction of apoptosis or by autophagic cell death (32). Therefore, to explore HNK induced effects in melanoma cells, we studied the effects of HNK (30 μM) on intracellular ATP and ADP pools in B16/F-10 and SKMEL-28 cells. During initial 24 h of HNK treatment, we did not note any noticeable changes in ATP and ADP levels in both cell lines. However, depletion of ATP levels in both melanoma cells were noticed post 48 h of HNK treatment. In contrast to this, no change in cellular ADP pools were detected at any of the time point in both the cell lines. In living cells, ATP is the most important direct source of energy of most of the biological processes by the hydrolysis reaction of ATP into ADP and/or AMP. The ratio of ATP to ADP and AMP is a barometer of cellular energy status, and is therefore tightly monitored by the cell. Therefore, these subtle changes in the ratio of ATP, ADP, and AMP may have profound consequences.
Mechanisms through which autophagy mediates its effects on both normal and cancer cells are not completely understood. Various signaling pathways have been implicated in the upregulation or downregulation of autophagy and the most commonly studied ones are Phosphatidylinositol 3-kinase/mammalian target of rapamycin (PI3K/mTOR) and AMPK signaling. Interestingly, AMPK serves as a key cellular energy sensor and a master energy regulator in cells (33) (34). AMP-activated protein kinase (AMPK) is activated during low energy conditions, particularly when the ratio of AMP/ATP or ADP/ATP increases. AMPK senses energy levels by directly binding of AMP, ADP or ATP via the adenine nucleotide-binding sites of the γ subunit. Binding of AMP or ADP leads to conformational change of the enzyme and activates AMPK through several mechanisms, including allosteric activation, promotes the phosphorylation of the conserved threonine in the activation loop of AMPK (34). In this study, we have observed AMPK activation post HNK treatment in dose and time dependent manner in melanoma cells. We have also seen decreases in the phosphorylation of mTOR in both the melanoma cell lines (data not shown). AMPK activity is capable of responding to the differing levels of energy (34).
The results suggest that HNK can target melanoma cancer cells and mark them for cell death through AMPK signaling. Further studies are warranted for developing HNK as an effective chemopreventive/therapeutic agent in melanoma. While honokiol has been implicated in AMPK signaling in other cancer types, this is the first publication implicates AMPK in effecting melanoma cell survival and stemness. As we have observed by evaluating other pathways implicated in honokiol related signaling (particularly the Notch signaling pathway), honokiol has a differential effect in terms of downstream signaling depending on the tumor type.
DISCUSSION
DISCUSSANT
DR. MARIA ALLO (Los Altos, CA): Honokiol is a substance derived from the magnolia plant and has been widely used in South Korea, China, and Japan to treat many maladies that include anxiety, cough, allergies, and minor GI disorders without any appreciable toxicity. Recent studies have noted antitumor activity that is thought to be related to targeting of the apoptosis pathways.
The authors have sought to more precisely determine the mechanism by which honokiol exerts its antitumor effect. They studied four strains of cultured melanoma cells and the effects of honokiol on cell viability, melanosphere formation, and changes in the cellular ATP pool. From these results, the authors conclude that honokiol inhibits cell survival and growth short term and clonogenic potential long term by inducing the AmpK activation on melanoma cells.
I have a couple of questions for the authors.
In your paper, you note a statistically significant difference in the depletion of ATP levels in the SK-28 versus the B16/F10 cells. I wonder if that might be related to greater inhibition of the RAS signaling that’s associated with defective P53, which is present, of course, in the MS cells but not in the B16 cells.
The second question is, did any or all of the cell lines you studied lack or show nonexpression of tumor suppressor protein LKB-1, which has been cited previously to be affected by these Magnolia extracts?
Thirdly, do you think that addition of calcium ionophore would amplify the differences that you saw among the cell lines?
DR. KAUSHIK (FNU) GAURAV: I agree that the observed difference in ATP depletion may, in fact, be cell type dependent.. As you know, B16/F10 are aggressive and metastatic in nature whereas SKMEL-28 are aggressive but non metastatic in nature. There certainly is the possibility of differential regulation of Ras and p53 signaling by Honokiol in these various melanoma cell types. However, we have not yet studied this aspect of Honokiol effect in our cells. Hopefully we will also try to explore this aspect in the future. Currently, we have focused on Notch signaling. We have seen inhibition the Notch signaling in melanoma cells by honokiol. The mode of cell killing by honokiol is via autophagy (data not shown) and not apoptosis. Honokiol is significantly inhibits the cells by inducing cell cycle arrest and inducing autophagy. We have also studied other proteins associated with cell death, cell growth and notch signaling (data not shown). Honokiol is significantly inhibiting the expression of these proteins especially cyclinD1, Hes-1 in melanoma cells. As for the use of a calcium ionophore, using it in combination with honokiol is an intriguing suggestion that we will try.
DR. RICHARD GRAY (Phoenix, AZ): In terms of anecdotal use of honokiol in terms of serum levels that are able to be achieved by the way it’s being used now, is it feasible that it will have these types of effects that you are observing in vitro?
DR. KAUSHIK (FNU) GAURAV: We are in the next phase of honokiol use and hope this will also work in vivo model in a same way it worked in in vitro. Our group, led by Dr. Shrikant Anant, has performed similar in vivo studies in colon cancer. In those studies, honokiol is effective in reducing tumor size and has been shown to have good bioavailability.
DR. BARBARA POCKAJ (Phoenix, AZ): You said it decreased stem cells, but these were stem cells in a cell line, and that seems inconsistent to me. Did you prove those were indeed stem cells by doing any staining that demonstrate these cells are stem cells?
DR. KAUSHIK (FNU) GAURAV: DR. Pockaj, you are certainly correctly pointing out the controversy that exists with regards to stem cells. Perhaps, referring to the effect that honokiol has on “stemness” might be more accurate. We have performed stem cell marker staining in both B16 and SKMEL-28 cells. We also studied CD271 and CD166 expression in melanoma cells by western blotting (data not shown). The honokiol significantly inhibited the expression of melanoma stem cells markers in melanoma cells. We also did site population through flow cytometry (data not shown, as it needs to be repeated two more times). From these preliminary studies, honokiol showed inhibition in site population of both the cells types which we interpret as decreasing the stem cell population or “stemness.”
DR. BARBARA POCKAJ (Phoenix, AZ): Are your plans, then, prior to doing any kind of clinical studies, trying to do this in an animal model?
DR. KAUSHIK (FNU) GAURAV: Absolutely, we are the process of writing an animal protocol for just that reason.
DR. MARTIN MCCARTER (Aurora, CO): Just wondering if you have any ideas regarding potential biomarkers for response in human specimens using a honokiol agent.
DR. KAUSHIK (FNU) GAURAV: As for biomarkers, we certainly would consider using stem cell markers, because stem cells are there in tumor and we can monitor for changes in stemness. That certainly should be a component of our in vivo studies.
DR. MARTIN MCCARTER (Aurora, CO): Those are often difficult to pick out in a human specimen.
DR. KAUSHIK (FNU) GAURAV: We have had luck in primary tissue culture obtained from resected metastatic melanomas in identifying stem cell markers so we are hopeful that we will be able to identify stem cell markers in the more aggressive melanoma tumors that we hope to treat.
Acknowledgments
Grant Support: This work was supported by NIH grants CA109269, CA182872 (SA), CA168524 (RAJ), and the Department of Surgery, KUMC (JMVM).
ABBREVIATIONS
- AKT
known as Protein Kinase B
- BCA
Bicinchoninic acid
- ERK
Extracellular signal-regulated kinases
- LC 3
Light Chain 3
- mTOR
Mammalian target of rapamycin
- PTEN
Phosphatase and tensin homologue deleted from chromosome 10
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- PBS
Phosphate Buffer Saline
- CSC
Cancer stem cells
- AMPK
AMP-activated protein kinase
- HNK
Honokiol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Linos E, Swetter SM, Cockburn MG, et al. Increasing burden of melanoma in the United States. The Journal of Investigative Dermatology. 2009 Jul;129(7):1666–74. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perlis C, Herlyn M. Recent Advances in Melanoma Biology. The oncologist. 2004;9(2):182–7. doi: 10.1634/theoncologist.9-2-182. [DOI] [PubMed] [Google Scholar]
- 3.Hendrix MJ, Seftor EA, Seftor RE, et al. Reprogramming metastatic tumour cells with embryonic microenvironments. Nature Reviews Cancer. 2007 Apr;7(4):246–55. doi: 10.1038/nrc2108. [DOI] [PubMed] [Google Scholar]
- 4.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews Molecular Cell Biology. 2012 Apr;13(4):251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen CH, Yuan P, Perez-Lorenzo R, et al. Phosphorylation of BRAF by AMPK impairs BRAF-KSR1 association and cell proliferation. Molecular Cell. 2013 Oct 24;52(2):161–72. doi: 10.1016/j.molcel.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawley SA, Boudeau J, Reid JL, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. Journal of Biology. 2003;2(4):28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawley SA, Pan DA, Mustard KJ, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metabolism. 2005 Jul;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Oakhill JS, Scott JW, Kemp BE. Structure and function of AMP-activated protein kinase. Acta Physiologica. 2009 May;196(1):3–14. doi: 10.1111/j.1748-1716.2009.01977.x. [DOI] [PubMed] [Google Scholar]
- 9.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature Cell Biology. 2011 Sep;13(9):1016–23. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw RJ. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiologica. 2009 May;196(1):65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bettencourt-Dias M, Giet R, Sinka R, et al. Genome-wide survey of protein kinases required for cell cycle progression. Nature. 2004 Dec 23;432(7020):980–7. doi: 10.1038/nature03160. [DOI] [PubMed] [Google Scholar]
- 12.Mirouse V, Swick LL, Kazgan N, et al. LKB1 and AMPK maintain epithelial cell polarity under energetic stress. The Journal of Cell Biology. 2007 May 7;177(3):387–92. doi: 10.1083/jcb.200702053. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The active form of the metabolic sensor: AMP-activated protein kinase (AMPK) directly binds the mitotic apparatus and travels from centrosomes to the spindle midzone during mitosis and cytokinesis. Cell Cycle. 2009 Aug;8(15):2385–98. doi: 10.4161/cc.8.15.9082. [DOI] [PubMed] [Google Scholar]
- 14.Fujita M, Itokawa H, Sashida Y. Studies on the components of Magnolia obovata Thunb. 3. Occurrence of magnolol and honokiol in M. obovata and other allied plants. Yakugaku zasshi: Journal of the Pharmaceutical Society of Japan. 1973 Apr;93(4):429–34. doi: 10.1248/yakushi1947.93.4_429. [DOI] [PubMed] [Google Scholar]
- 15.Lee YJ, Lee YM, Lee CK, et al. Therapeutic applications of compounds in the Magnolia family. Pharmacology & therapeutics. 2011 May;130(2):157–76. doi: 10.1016/j.pharmthera.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Fried LE, Arbiser JL. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxidants & Redox Signaling. 2009 May;11(5):1139–48. doi: 10.1089/ars.2009.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. Journal of Immunological Methods. 1984 Mar 16;67(2):379–88. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 18.Ponnurangam S, Mammen JM, Ramalingam S, et al. Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Molecular Cancer Therapeutics. 2012 Apr;11(4):963–72. doi: 10.1158/1535-7163.MCT-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo S, Pal D, Shah SJ, et al. Effect of HEPES buffer on the uptake and transport of P-glycoprotein substrates and large neutral amino acids. Molecular Pharmaceutics. 2010 Apr 5;7(2):412–20. doi: 10.1021/mp900193e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson FS, Murphy RC. Isocratic separation of some purine nucleotide, nucleoside, and base metabolites from biological extracts by high-performance liquid chromatography. Journal of Chromatography. 1976 Jun 23;121(2):251–62. doi: 10.1016/s0021-9673(00)85021-9. [DOI] [PubMed] [Google Scholar]
- 21.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annual Review of Medicine. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 22.Kwatra D, Venugopal A, Standing D, et al. Bitter melon extracts enhance the activity of chemotherapeutic agents through the modulation of multiple drug resistance. Journal of Pharmaceutical Sciences. 2013 Dec;102(12):4444–54. doi: 10.1002/jps.23753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang QQ, Fan LY, Yang GL, et al. Improved therapeutic effectiveness by combining liposomal honokiol with cisplatin in lung cancer model. BMC Cancer. 2008;8:242. doi: 10.1186/1471-2407-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou W, Chen L, Yang G, et al. Synergistic antitumor effects of liposomal honokiol combined with adriamycin in breast cancer models. Phytotherapy Research: PTR. 2008 Aug;22(8):1125–32. doi: 10.1002/ptr.2472. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Liu Y, Zhao X, et al. Honokiol, a natural therapeutic candidate, induces apoptosis and inhibits angiogenesis of ovarian tumor cells. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2008 Sep;140(1):95–102. doi: 10.1016/j.ejogrb.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 26.Chen F, Wang T, Wu YF, et al. Honokiol: a potent chemotherapy candidate for human colorectal carcinoma. World Journal of Gastroenterology: WJG. 2004 Dec 1;10(23):3459–63. doi: 10.3748/wjg.v10.i23.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Z, Subramaniam D, Ramalingam S, et al. Honokiol radiosensitizes colorectal cancer cells: enhanced activity in cells with mismatch repair defects. American Journal of Physiology Gastrointestinal and Liver Physiology. 2011 Nov;301(5):G929–37. doi: 10.1152/ajpgi.00159.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Arora S, Bhardwaj A, Srivastava SK, et al. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PloS One. 2011;6(6):e21573. doi: 10.1371/journal.pone.0021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaushik G, Ramalingam S, Subramaniam D, et al. Honokiol induces cytotoxic and cytostatic effects in malignant melanoma cancer cells. American Journal of Surgery. 2012 Dec;204(6):868–73. doi: 10.1016/j.amjsurg.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nature Cell Biology. 2010 Sep;12(9):814–22. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Current Topics in Microbiology and Immunology. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klionsky DJ, Abdalla FC, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012 Apr;8(4):445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carling D. The AMP-activated protein kinase cascade–a unifying system for energy control. Trends in Biochemical Sciences. 2004 Jan;29(1):18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Yuan HX, Xiong Y, Guan KL. Nutrient sensing, metabolism, and cell growth control. Molecular Cell. 2013 Feb 7;49(3):379–87. doi: 10.1016/j.molcel.2013.01.019. Epub 2013/02/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]


