Abstract
Nucleoside analogs are used as chemotherapeutic options for the treatment of platinum-resistant ovarian cancers. Human concentrative nucleoside transporter 1 (hCNT1) is implicated in sensitizing solid tumors to nucleoside analogs although its role in determining drug efficacy in ovarian cancers remains unclear. Here we examined the functional expression of hCNT1 and compared its contributions towards gemcitabine efficacy in histological subtypes of ovarian cancer. Radioactivity analysis identified hCNT1-mediated 3H-gemcitabine transport in ovarian cancer cells to be significantly reduced compared with that of normal ovarian surface epithelial cells. Biochemical and immunocytochemical analysis identified that unlike normal ovarian cells which expressed high levels of hCNT1 at the apical cell surface, the transporter was either diminished in expression and/or mislocalized in cell lines of various subtypes of ovarian cancer. Retroviral expression of hCNT1 selectively rescued gemcitabine transport in cell lines representing serous, teratocarcinoma, and endometrioid subtypes, but not clear cell carcinoma (CCC). In addition, exogenous hCNT1 predominantly accumulated in intracytoplasmic vesicles in CCC suggesting defective cellular trafficking of hCNT1 as a contributing factor to transport deficiency. Despite diminution of hCNT1 transport in the majority of ovarian cancers and apparent trafficking defects with CCC, the chemotherapeutic efficacy of gemcitabine was broadly enhanced in all subtypes when delivered via engineered nanoparticles (NPs). Additionally, by bypassing the transport requirement, the delivery of a gemcitabine-cisplatin combination in NP formulation increased their synergistic interactions. These findings uncover hCNT1 as a putative determinant for nucleoside analog chemoresistance in ovarian cancer and may help rationalize drug selection and delivery strategies for various histological subtypes of ovarian cancer.
Keywords: Nucleoside transporter, hCNT1, Gemcitabine, Ovarian cancer, Chemoresistance, Nanoparticles
1. Introduction
Approximately 90% of all ovarian cancers stem from epithelial cells, including serous, endometrioid, and clear cell carcinoma (CCC) subtypes (1). While the serous subtype is the most commonly occurring ovarian cancer, treatment response and outcomes plummet as the cancer progresses. For CCCs, recurrence is common (2). Less than 2% of ovarian cancers are derived from germ cells, namely teratocarcinomas (3). Although the two have different genetic makeups, both CCCs and teratocarcinomas are extremely difficult to treat and exhibit poor response to anti-cancer drugs (2, 4–11). In fact, survival rates of patients with CCC and teratocarcinoma are often much poorer than patients with advanced serous carcinoma (2, 5, 7, 9, 12–14).
Despite the variation among ovarian cancer subtypes, treatment is not largely differentiated between cancer subtypes. The current first-line chemotherapeutic treatment for all ovarian cancer is a platinum-based therapy (e.g., cisplatin or carboplatin) primarily combined with a taxane (e.g., paclitaxel or docetaxel). Unfortunately, drug failure is common in most patients, and a second-line therapy with other classes of drugs such as nucleoside analogs becomes necessary. In 2006, the U.S. Food and Drug Administration (FDA) approved the use of gemcitabine (2′,2′-difluorodeoxycytidine; dFdC) in combination with carboplatin to treat patients with advanced, refractory, or recurrent ovarian cancer as well as patients who showed initial resistance to platin-based treatments (15–17). The growing promise of gemcitabine has also contributed to its inclusion in an increasing number of phase I and II ovarian cancer clinical trials. In fact, it has been shown that the addition of gemcitabine with either cisplatin or paclitaxel increased patient response from 13 and 24% to 53 and 40%, respectively (18). Nonetheless, while these combinations show early promising effects, they have low efficacy in CCC and tertocarcinomas (19, 20).
As a hydrophilic nucleoside analog, gemcitabine relies on nucleoside transporters to enter the cell and lack of adequate drug transport is considered a key reason for chemoresistance. While both the concentrative (hCNTs; SLC28) and equilibrative (hENTs; SLC29) nucleoside transporters are implicated in tumor uptake of gemcitabine (21, 22), recent studies are also uncovering the differential regulations of hCNTs and hENTs with respect to proliferation and differentiation states, normoxic and hypoxic influences, and migratory and invasive characteristics of tumor cells. These findings open up the possibility that hCNTs and hENTs could variably influence drug sensitivities in ovarian carcinomas, particularly in various histological subtypes of ovarian tumors and tumor microenvironments.
We have recently reported hCNT1 as putative growth suppressor with potential to render drug-resistant pancreatic cancer cells amenable to nucleoside analog chemotherapy (24). Given the increase in exploration of gemcitabine as a combined therapy regimen in multiple clinical trials, we aimed to determine whether the same relationship with nucleoside transporters affects gemcitabine therapy in ovarian cancer. In this study, we investigated the role of hCNT1 in determining gemcitabine chemosensitivity in ovarian carcinomas. We report here a significant reduction in hCNT1 activity in ovarian carcinomas with maximal reductions observed in CCC and teratocarcinoma. Intriguingly, biochemical and immunocytochemical studies identified that hCNT1 has differing roles in conferring gemcitabine sensitivity to various histological subtypes of ovarian cancer; the ability of hCNT1 to restore gemcitabine sensitivity is attributable to apparent variations in hCNT1 trafficking to the cell surface. Importantly, its defective yet required transport activity was bypassed by delivering the chemotherapeutic in NP formulation, furthering both the cytotoxic and synergistic effects of gemcitabine with cisplatin in even the most drug resistant subtypes. These findings support hCNT1 as a determinant of nucleoside analog chemoresistance in ovarian cancers and may help rationalize anti-cancer drug selection and delivery strategies for the various histological subtypes of ovarian cancer.
2. Materials and Methods
Cell Culture
The normal ovarian epithelial cell line, IOSE-80, developed by Dr. Nelly Auersperg of The University of British Columbia (Vancouver, Canada), was received from the Canadian Ovarian Tissue Bank. All ovarian cancer cell lines were obtained from the American Type Cell Culture (Manassas, VA). IOSE-80, TOV-21G, and TOV-112D were cultured in a 1:1 mixture of MCDB 105 medium and Medium 199 supplemented with 15% FBS. PA-1 was cultured in Minimum Essential Medium Eagle (MEM) with 10% FBS. ES-2 and SKOV-3 were cultured in McCoy’s 5A medium supplemented with 10% FBS. OVCAR-3 was cultured in RPMI 1640 supplemented with 0.01 g/L bovine insulin and 20% FBS. Caov-3 was cultured in DMEM supplemented with 10% FBS. All media were purchased from MediaTech (Manassas, VA) and supplemented with 100 units of penicillin/ml and 2 μg of streptomycin/mL in solution (Sigma-Aldrich, St. Louis, MO). All cells were cultured at 37°C in a 5% CO2 environment and subcultured every 48–72 h.
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Cytotoxicity Assay and Drug Interaction Analysis
MTT assays were performed as described earlier (23). Gemcitabine was obtained from Chemie Tek (Indianapolis, IN), and cisplatin was from LC Laboratories (Woburn, MA). DMSO and MTT were obtained from Sigma-Aldrich. To identify the interaction (i.e., additive, synergistic, or antagonistic) between gemcitabine and cisplatin in both normal and cancerous ovarian cell lines, the combination index plots were estimated for each treatment using the method developed by Chou and Talalay and the Calcusyn software (24).
3H-Nucleoside Transport Study
Cellular transport experiments were carried out as described previously (23). 3H-gemcitabine and 3H-thymidine were obtained from Moravek Radiochemicals (Brea, CA). Uridine and nitrobenzyl mercaptopurine riboside (NBMPR) were purchased from Sigma-Aldrich.
Quantitative Real-time PCR
Real-time PCR was performed as described earlier (23). Validated TaqMan primers and probes for hCNT1 (Hs_00188418_m1) and human GusB (internal control) (Hs_9999908_m1) were used (Applied Biosystems, Carlsbad, CA).
Western Blotting
Western Blotting was conducted as described previously (23). The bicinchoninic acid (BCA) protein assay kit was from Pierce Chemical (Rockford, IL). The goat anti-hCNT1 (C14 and N17) polyclonal antibodies used were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), and the anti-human rabbit polyclonal Na+/K+ ATPase antibody was purchased from Cell Signaling Technology (Danvers, MA). The mouse anti-human β-actin monoclonal antibody was from Sigma-Aldrich. Secondary antibodies were purchased from Bethyl Laboratories (Montgomery, TX). Images were developed and analyzed using FluorChem SP software (Alpha Innotech, Santa Clara, CA).
Cell Surface Protein Isolation
Isolation of the cell surface fraction of proteins was conducted using the Pierce® Cell Surface Protein Isolation Kit (Thermo Scientific) as per the manufacturer’s instructions and as previously described (25). Briefly, cells were washed twice with ice-cold PBS and subsequently biotinylated with 0.25 mg/ml Sulfo-NHS-SS-Biotin for 30 minutes on an orbital shaker at 4°C. The reaction was quenched, and cells were collected by scraping followed by centrifugation. Cells were lysed with 500 μl of Lysis Buffer containing a protease inhibitor cocktail (Roche) for 30 minutes on ice with intermittent homogenization using a 23G needle and syringe. Ten percent of the total lysate (50 μl) was retained for analysis of total proteins. The biotinylated proteins were then bound to immobilized streptavidin-agarose beads (NeutrAvidin Agarose slurry) by a one-hour incubation at room temperature using an end-over-end rotator. The unbound proteins were then collected by centrifugation of the column at 1,000 x g for 2 minutes and retained for analysis of intracellular proteins. The biotinylated proteins were eluted form the beads using SDS-PAGE buffer containing 50 mM DTT. The collected samples were then separated on SDS-PAGE for immunoblot analysis.
Immunocytochemical Analysis
Immunostaining was performed as previously described (23) using goat anti-hCNT1 (C14 and N17) (Santa Cruz Biotechnology) and goat anti-HA FITC conjugated (Bethyl Laboratories) antibodies. Secondary antibodies conjugated with Alexa 488 or 594 were used (Invitrogen, Carlsbad, CA). 4′,6-Diamidino-2-phenylindole (DAPI) was obtained from Sigma-Aldrich, ProLong Gold anti-fade mounting reagent was obtained from Molecular Probes, Invitrogen. Images were captured with a Nikon TM Eclipse fluorescence microscope and analyzed using Nikon TiE software (Nikon Instruments Inc., Melville, NY).
Retroviral Expression of hCNT1 in Cells
Expression of hCNT1 was conducted as previously described (23). Briefly, the hCNT1 full-length cDNA clone (clone ID: 8991920; accession: BC 126204) was obtained from Open Biosystems (Huntsville, AL) and cloned into the pLNCX2 vector. pLNCX2 (control) or pLNCX2-hCNT1-HA was then transfected into a packaging cell line (Phoenix) for retroviral production using X-tremeGENE (Roche, Indianapolis, IN) as per the manufacturer’s instructions. Viruses were collected after 24–48 h, and various target cells were infected in the presence of hexadimethrine bromide (polybrene; 8 μg/ml). Experiments were conducted ~48 h after infection.
Polymeric NP Formulation
NPs were created using the double emulsion technique (26) with the standard solutions of PLGA-b-PEG-OH (50 mg/mL in CH2Cl2), gemcitabine (1 mg/mL in H2O), and cisplatin (1 mg/mL in PBS) in presence of polyvinyl alcohol (PVA). Characterization was completed with transmission electron microscopy (TEM) and dynamic light scattering (DLS). For TEM, particles were stained with 2% uranyl acetate for 10 min. Gemcitabine content in the NPs was analyzed by high performance liquid chromatography (HPLC), and cisplatin in NPs was quantified by inductively coupled plasma mass spectrometry (ICP-MS). Prior to treatment in cells, the NPs were sterilized using a 0.2 μm filter.
Statistical Analysis
All experiments were performed in triplicate and repeated at least three times. In cases where only two conditions were compared, the Student’s t test was conducted in Microsoft Excel to determine significance. In cases where three or more conditions were compared, one-way ANOVA was conducted using GraphPad Prism 5.0 software. Compared with control conditions, p<0.05 and p<0.01 are represented by one and two asterisks, respectively.
3. Results
Gemcitabine sensitivity and nucleoside transporter activity is decreased in cancerous ovarian cell lines
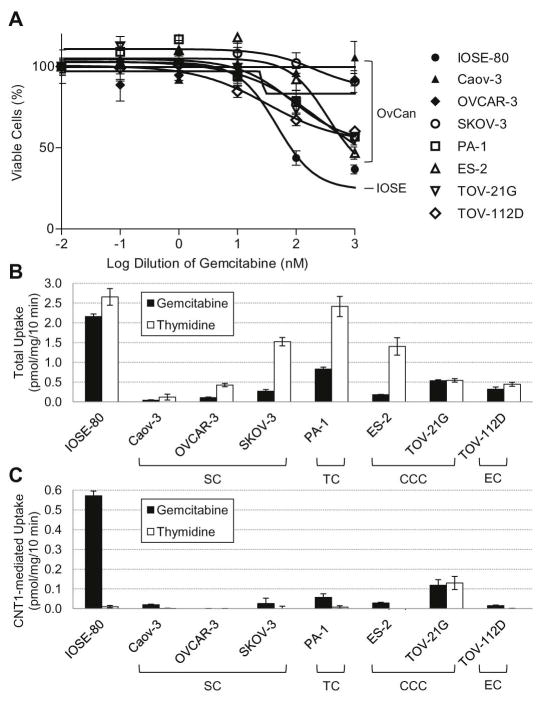
Although the expression of nucleoside transporters has been earlier tested in ovarian cancer tissues (22), the functional activity of nucleoside transporters and its relationship to drug sensitivity has not been studied. We began by investigating gemcitabine sensitivity in ovarian cells by measuring gemcitabine cytotoxicity in immortalized normal ovarian surface epithelial cells (IOSE-80) and several ovarian cancer cell lines using an MTT assay. As shown in Figure 1A, ovarian cancer cell lines exhibited less cytotoxicity to gemcitabine compared with normal IOSE-80. To test whether the decreased drug sensitivity relates to a decrease in nucleoside transport activity, we evaluated the nucleoside transporter activity by measuring the cellular uptake of tritiated gemcitabine (3H-gemcitabine) and thymidine (3H-thymidine). As shown in Figure 1B, total gemcitabine transport was reduced in ovarian cancer cell lines compared with IOSE-80, suggesting the lack of nucleoside analog drugs being adequately taken up by cancer cells. Results were similar for 3H-thymidine, although less striking than that observed with 3H-gemcitabine (Fig. 1B). To understand whether hCNT1 transportability was affected specifically, we studied 3H-gemcitabine and 3H-thymidine uptake into ovarian cells in the presence of 10 μM NBMPR (which inhibits hENT1 and hENT2) and 20 mM guanosine (which inhibits hCNT2 and hCNT3) (24). Similar to total 3H-gemcitabine cellular uptake, transport level contributed by hCNT1 was also decreased in ovarian cancer cells compared with normal (Fig. 1C). These results identify that diminished hCNT1 transportability may likely contribute to the low gemcitabine sensitivity of cancerous ovarian cell lines.
Figure 1. Gemcitabine sensitivity and nucleoside transporter activity is decreased in cancerous ovarian cell lines.
A. Cytotoxicity of gemcitabine in a panel of ovarian cell lines. Compared with normal IOSE-80 cells, all cancerous cell lines tested have decreased sensitivity to gemcitabine, with many not sensitive at all (>10 μM). All cell lines were seeded at a density of 5x103 in a 96-well plate and treated with gemcitabine for 72 h. Figure is representative of five experiments. OvCan, ovarian cancer. B. Nucleoside transporter activity in the panel of ovarian cell lines as measured by radiolabeled (3H) nucleoside uptake. Total 3H-gemcitabine uptake was reduced in all cancerous cell lines as compared with IOSE-80. C. hCNT1-mediated transport of radiolabeled (3H) nucleosides. Decrease in total nucleoside uptake of the cancerous cell lines can be possibly attributed to the significant loss of hCNT1 transport activity. Results were similar with 3H-thymidine however less striking than that obtained with 3H-gemcitabine. SC, serous carcinoma. TC, teratocarcinoma. CCC, clear cell carcinoma. EC, endometrioid carcinoma. Bars, SD. n=3.
Exogenous expression of hCNT1 improved nucleoside transporter activity and chemosensitivity in only a subset of cancerous ovarian cell lines
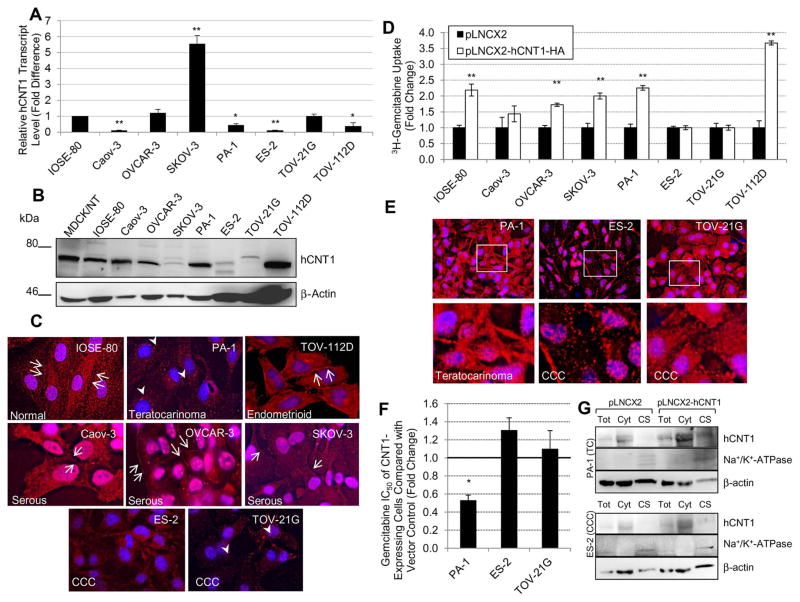
Quantitative analysis of endogenous hCNT1 expression indicated a significant reduction in hCNT1 transcripts in four out of seven cell lines tested compared with IOSE-80 (Fig. 2A). Interestingly, a significant increase in hCNT1 transcripts was seen in SKOV-3. At the protein level, changes in hCNT1 varied among the ovarian cancer cell lines when compared with IOSE-80 (Fig. 2B). This was in opposition to hENT1 expression, which remained largely unaltered in almost all of the cancerous cell lines (data not shown). Densitometry analysis indicated that hCNT1 protein was notably decreased in ES-2 and TOV-21G, with identification of a ~72 kDa hCNT1 protein band only upon increased loading of total lysates in Westerns (Fig. 2B) or overexposure of blots. hCNT1 protein is also somewhat reduced in SKOV-3 but no significant alterations in hCNT1 protein levels were seen in the remaining cancerous cell lines. While hCNT1 protein levels were decreased in certain cancerous cell lines, localization of the protein at least partially explained the decrease in transporter activity observed in all of the ovarian cancer cell lines. Compared with abundant hCNT1 expression at the apical cell surface in IOSE-80, expression in many cancerous cell lines was either reduced, mislocalized, or absent (Fig. 2C). Interestingly, all the serous cell lines (i.e., Caov-3, OVCAR-3, and SKOV-3) and the endometrioid cell line (TOV-112D) continued to display cell surface expression. TOV-21G (CCC) and PA-1 (teratocarcinoma) displayed hCNT1 expression only in the cytoplasm within vesicle-like structures in the perinuclear region. Lastly, ES-2 (CCC) showed no distinct hCNT1 immunoreactivity with only minimal and diffuse staining in the cytoplasm These results suggest that the reduced response of ovarian cancer cells to gemcitabine is at least in part due to the subtype-dependent impairment in cell surface expression (via intracellular accumulation) of hCNT1 in teratocarcinoma and CCC cell lines but not serous or endometrioid cell lines.
Figure 2. Exogenous expression of hCNT1 improved nucleoside transporter activity and chemosensitivity in only a subset of cancerous ovarian cell lines.
A. Relative transcript levels of hCNT1 in the ovarian cancer cell lines as compared with normal IOSE-80. Four of the cancerous cell lines had a significant decrease in hCNT1 transcript levels. B. Protein levels of hCNT1 in the ovarian cell lines. hCNT1 was notably decreased in ES-2 and TOV-21G, with only minor alterations in the other cancerous cell lines. For Western blotting analysis, 50 μg of whole cell lysates were subjected with the exception of ES-2, TOV-21G, and TOV-112D which utilized 150 μg for enhanced visualization of the protein of interest. β-actin was used as the loading control. C. Localization of hCNT1 (red) in the ovarian cell lines. Cell surface expression of hCNT1 (red; arrows) was abundant in IOSE-80 and moderate in the serous and endometrioid cancerous cell lines. PA-1 and TOV-21G (teratocarcinoma and CCC) expressed slight hCNT1 in the cytoplasm (red; arrowheads), while the other CCC (ES-2) showed minimal and diffuse cytoplasmic expression. Nuclei stained with DAPI are blue. D. Transient viral transduction of hCNT1 improved 3H-gemcitabine uptake in only a subset of ovarian cell lines. Uptake activity was increased in normal (IOSE-80), serous (Caov-3 and SKOV-3), teratocarcinoma (PA-1), and endometrioid (TOV-112D) cell lines. Overexpression of hCNT1 did not improve nucleoside uptake in either of the CCC cell lines. E. Exogenous expression of hCNT1 (red) partially rescued cell surface expression in PA-1 but not in ES-2 and TOV-21G. Insets are expanded and shown below. Nuclei stained with DAPI are blue. F. Exogenous expression of hCNT1 significantly decreased the gemcitabine cytotoxic IC50 of PA-1. No significant changes in IC50 were observed in the CCC cell lines. G. Western blotting analysis of total (T), cytoplasmic (Cyt), and cell surface (CS) fractions in control (pLNCX2) and hCNT1 (pLNCX2-hCNT1) expressing cells indicated some presence of hCNT1 in PA-1 but not in ES-3. Na-K ATPase was used as a cell surface marker and β-actin was used as an internal loading control. Bars, SD. n=3. *p≤0.05, **p≤0.01.
Consequently, we sought to express exogenous hCNT1 in the cancerous cell lines to test whether it would restore protein expression and activity as well as cellular chemosensitivity. As anticipated from endogenous localization of hCNT1, retroviral overexpression of hCNT1-HA in the ovarian cell lines led to an increase in 3H-gemcitabine transport in normal IOSE-80, serous Caov-3 and SKOV-3, and endometrioid TOV-112D but not in in CCC ES-2 and TOV-21G (Fig. 2D). Surprisingly, exogenous expression of hCNT1 significantly increased nucleoside analog uptake and decreased the gemcitabine IC50 in teratocarcinoma PA-1. Unlike teratocarcinoma cells, no significant changes in gemcitabine IC50 were seen with CCC (ES-2 and TOV-21G) cell lines (Fig. 2F). Further investigation into the protein’s localization by immunocytochemical analysis of HA-epitope identified exogenous hCNT1 at both the cell surface and cytoplasm in gemcitabine-transporting PA-1 but predominantly within the large, vesicle-like structures of the cytoplasm in the unaltered ES-2 and TOV-21G (Fig. 2E). Western blotting analysis of cell surface fractions confirmed the presence of hCNT1 in PA-1 but not ES-2 but presence of a control cell surface marker (Na-K ATPase) in both cell types. These results confirm that overexpression of hCNT1 in the cancerous ovarian cell lines only leads to subtype-dependent recovery of gemcitabine transport and chemosensitivity with a particular lack of recovery in CCC.
Synthesis and characterization of gemcitabine-cisplatin NPs: Polymeric NP based delivery of chemotherapeutics enhanced cytotoxicity and synergism
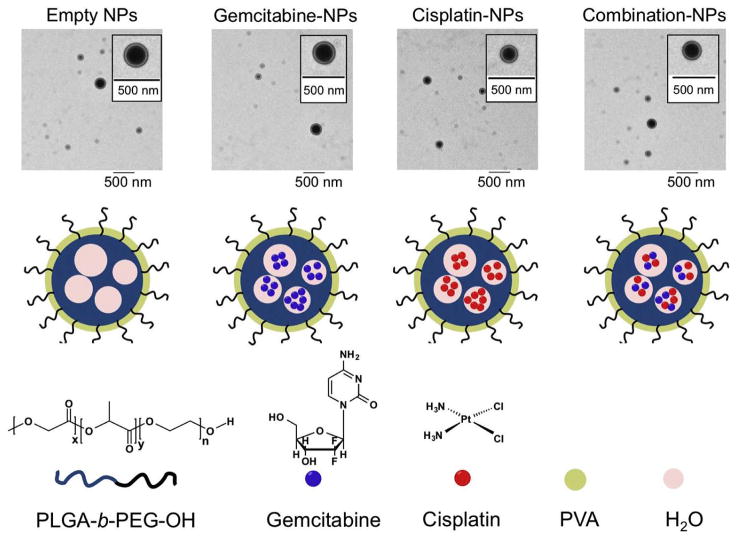
In aiming at overcoming transporter defects in ovarian cancer cells, we constructed NPs from PLGA-b-PEG-OH containing gemcitabine, cisplatin, both (combination), or neither (empty) using a double emulsion technique (Fig. 3). PLGA-PEG-OH and the drug(s) were emulsified in PVA in two separate steps using a deep probing sonicator. The final NPs were characterized with TEM and found to be around 200 nm and no more than 350 nm in size (Fig. 3, Table 1). Uniformity of the particles was verified by using dynamic light scattering and the measured polydispersity index was found to be no more than 0.35 (Table 1). HPLC measurements were conducted to determine drug loading in single agent and combination NPs as well as gemcitabine to cisplatin ratio in combination NPs (Table 1).
Figure 3. Synthesis of NPs for chemotherapeutic delivery.
Microscopic (TEM) and pictorial depictions of the NPs containing gemcitabine, cisplatin, both (combination), or neither (empty).
Table 1.
Characterization of NPs
| NP | Size (nm) | PDI | ZP (mV) | Concentration (μg/mL) | Loading (%) | Encapsulation Efficiency |
|---|---|---|---|---|---|---|
| Gemcitabine | 225.8 ± 2.4 | 0.197 ±0.05 | −14.6 ± 1.2 | 35.4 | 1.4 | 7.08 |
| Cisplatin | 248.2 ± 3.0 | 0.262 ± 0.02 | −12.6 ± 0.1 | 3.0 | 0.2 | 0.49 |
| Combination | 341.5 ± 2.4 | 0.342 ± 0.01 | −14.9 ± 1.5 | G: 31.4; C: 24.6 | G: 1.3; C: 2.0 | G: 6.28; C: 1.31 |
| Empty | 205.6 ± 1.8 | 0.121 ± 0.02 | −17.75 ± 0.5 | - | - | - |
NP, NP; PDI, polydispersity index; ZP, zeta potential.
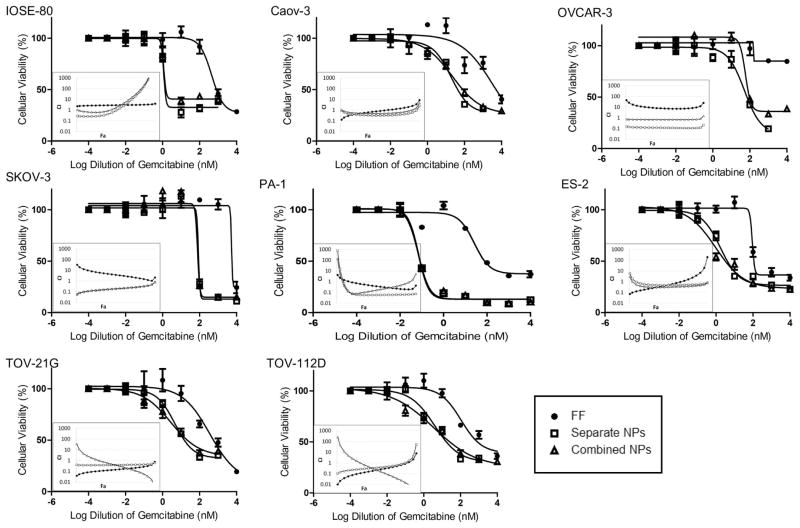
Normal and cancerous ovarian cell lines were treated with gemcitabine, cisplatin, or a combination of both at a 1:0.8 (G:C) concentration ratio in free formulation or by NP delivery for 72 h. NP treatment of gemcitabine plus cisplatin was achieved by utilizing a combination of gemcitabine NPs and cisplatin NPs at a 1:0.8 concentration ratio (separate) as well as by NPs encapsulating both drugs at a 1:0.8 concentration ratio (combination). The drug concentrations were chosen by the amount loaded into the combination NPs, which were created to be as close to 1:1 as possible. While our current NP design cannot exactly control for the relative loading of the two drugs, we were able to fine tune the ratio of polymer and PVA to produce a relatively consistent drug ratio. In all cell lines, including the CCC cell lines, NP delivery of either gemcitabine or cisplatin resulted in significantly lower IC50s as compared with free formulation (Table 2), indicating that the NPs can bypass transporter defects and improve chemosensitivity. For gemcitabine plus cisplatin treatments, both separate and combination NPs were equally effective and dramatically increased cytotoxicity in all cell lines compared with free formulation of the drug combination (Fig. 4). Control experiments with increasing concentrations of empty NPs were also performed with no significant change in cellular viability seen (data not shown).
Table 2.
Cytotoxicity of gemcitabine and cisplatin when delivered as free formulation or by NP formulation
| G | G NPs | C | C NPs | |
|---|---|---|---|---|
| IOSE-80 | 80.9 ± 14.5 nM | 3.5 ± 1.9 nM | 7.4 ± 3.3 μM | 9.5 ± 0.5 nM |
| Caov-3 | >10 μM | 726.8 ± 132.8 nM | 3.6 ± 0.4 μM | 68.2 ± 5.0 nM |
| OVCAR-3 | >10 μM | 9.9 ± 0.9 μM | >10 μM | 374.1 ± 74.2 nM |
| SKOV-3 | >10 μM | 473.2 ± 10.2 nM | >10 μM | 141.6 ± 8.2 nM |
| PA-1 | >10 μM | 0.1 ± 0.0 nM | 372.4 ± 4.3 nM | 4.3 ± 0.9 nM |
| ES-2 | 0.8 ± 0.0 μM | 3.5 ± 0.3 nM | 3.2 ± 0.3 μM | 105.2 ± 12.0 nM |
| TOV-21G | 1.3 ± 0.0 μM | 18.1 ± 4.3 nM | 6.5 ± 0.2 μM | 67.6 ± 10.3 nM |
| TOV-112D | >10 μM | 313.4 ± 104.4 nM | >10 μM | 18.8 ± 3.7 nM |
G, gemcitabine; C, cisplatin; NPs, NPs.
For each cell line, the NP IC50 was statistically significant (p<0.01) compared with its free-formulation
IC50 as determined by the Student’s t test.
Figure 4. NP delivery of gemcitabine (G) plus cisplatin (C), whether as separate or combination NPs (NPs), enhanced cytotoxicity and synergism between the two drugs in the panel of ovarian cell lines.
In all cell lines, NP delivery increased the cytotoxicity of the drug combination as compared with free formulation (FF). Both separate and combination NPs were equally effective. Bars, SD. As depicted by the combination index (CI) plots, synergism increased between gemcitabine and cisplatin when delivered by NP formulation (white data points), regardless of whether they were separate or combination NPs, compared with free formulation (black data points). CI>1, antagonism; CI=1, additivity; CI<1, synergism.
Interactions between the two drugs were identified by combination index (CI) plots. Analyses revealed distinct alterations in interactions between the formulations. Overall, NP delivery of the two drugs, whether as separate or combination, increased the synergism between the two compounds as compared with free formulation in all cell lines (Fig. 4). Notable increases in synergism were observed in Caov-3, OVCAR-3, and SKOV-3 as well as in CCC (ES-2) cell types. These results demonstrate the potential of using NPs to overcome inherent biological (transporter) defects of cancer cells, increase chemotherapeutic response, and augment synergism between cisplatin and gemcitabine drug combination.
4. Discussion
Ovarian cancer is the fifth leading cause of cancer-related deaths in women, causing more deaths than any other cancer of the female reproductive system (27). With no proven screening methods and few early symptoms, diagnosis usually occurs at a more severe, advanced stage (~60% of all cases) when the 5-year survival rate is only 27% (27). Various subtypes of ovarian cancer further complicate both diagnosis and treatment. In this study, we characterized the gemcitabine sensitivity of normal human ovarian surface epithelial cells compared with those of ovarian carcinoma cells and identified a significant reduction in gemcitabine cytotoxicity response in the cancer cells tested. While all the other cancerous cell lines had an IC50 >10 μM, the CCC cell lines ES-2 and TOV-21G showed cytotoxicity comparable to the normal cells only at high levels of gemcitabine. Their IC50s at therapeutic levels of gemcitabine (in the nM range) remained 6–16-fold greater than their normal counterparts suggesting the occurrence of severe resistance phenomena. We also found diminished nucleoside analog uptake by the cancerous cell lines compared with normal IOSE-80. In particular, hCNT1 transport levels were significantly reduced, suggesting that it may likely contribute to the decrease in total uptake levels. Although there was no perfect correlation between cytotoxicity and gemcitabine transport levels, both aspects were significantly decreased in the cancer cells. It is quite likely that other intrinsic determinants, perhaps including those that determine gemcitabine cellular activation and efficacy, are contributing to gemcitabine response in the ovarian cells as well.
Studies in the past have identified high levels of ENT transport in late-stage (stage IV, grade III) serous ovarian carcinoma with pharmacological inhibition of ENTs to greatly decrease araC uptake (28). Unlike ENTs, the precise roles of hCNTs in gemcitabine resistance in various histological ovarian cancers remain undetermined. In a study by Farré et al., 90 ovarian carcinoma tissue samples were analyzed for nucleoside transporter expression (29). Although there was high heterogeneity among the samples, hENT1 mRNA expression was observed to be significantly retained in the tumor samples, with a moderate increase in protein levels was also found. Conversely, hCNT1 transcript levels were extremely low in most of the cancer tissue samples as compared with normal. At the protein level, immunohistochemical analysis indicated hCNT1 expression was absent in over half of the CCC samples, suggesting the possibility of subtype-dependent involvement of transporter defects. When present in the remaining ovarian subtype samples, hCNT1 staining was found to be predominantly intracellular, possibly rendering them nonfunctional. These observations prompted us to further examine the expressional and functional characteristics of hCNT1 in the panel of cancerous ovarian cell lines representing various histological subtypes. Interestingly, at the transcriptional level, we found decreased hCNT1 but increased hENT1 in the cancerous ovarian cell lines compared with normal IOSE-80. At the protein level, both transporters showed variable expression depending on the cell line. These results are consistent with the previous study which revealed that nucleoside transporter mRNA levels and function do not necessarily correlate (29). Furthermore, protein levels also may not necessarily correlate with activity depending on the expression level of the transporter at the plasma membrane (29). To this, we looked at the localization of hCNT1 in the panel of cell lines and found the transporter highly expressed and at the cell surface in normal IOSE-80. Conversely, expression at the cell surface was slightly decreased in serous cell lines but not in the endometrioid cell line (TOV-112D). The transporter was internalized in the teratocarinoma cell line (PA-1) as well as one CCC cell line (TOV-21G) and was almost absent in the other CCC (ES-2) cell line. These results demonstrate that due to the aberrant expression of hCNT1, the transporter may have differing roles in conferring gemcitabine sensitivity to various histological subtypes of ovarian cancer. In particular, reduction, loss and/or intracellular localization of hCNT1 may likely contribute to the low gemcitabine response in the teratocarinoma and CCC subtypes.
As previously suggested, the opposite expressions of hCNT1 and hENT1 may be due to their functional dichotomy (23, 29). That is, hCNTs have been implicated in cellular differentiation, activation, and apoptosis, while hENTs have been implicated in cellular proliferation and growth advantages. Furthermore, since transcriptional downregulation of hCNT1 was observed in the majority of cancer cells despite lack of significant changes in protein expression, it is likely that the efficiency of translation of hCNT1 may be enhanced in tumor cells. Therefore, we attempted to rescue hCNT1 activity in the cancerous ovarian cell lines using a retroviral cDNA construct of the hCNT1 transporter. In cell lines with highly aberrant hCNT1 expression (i.e., PA-1, ES-2, and TOV-21G), our repeated attempts to continually express exogenous hCNT1 failed, and we were unable to generate stable clones. Although this supports our earlier studies that demonstrated a putative tumor suppressive role of hCNT1 in pancreatic cancers; it is unclear why stable hCNT1 expression could be lethal in CCC cell lines despite lack of rescue of functional nucleoside transport activity. It is possible that hCNT1 can impart a growth suppressive role irrespective of its role as a plasma membrane nucleoside transporter; although direct evidence for this phenomenon is unavailable. Intriguingly, while this manuscript was in preparation, a study by Pérez-Torras et al. demonstrated hCNT1 can alter cell cycle progression and induce cell death in pancreatic cancer cells independent of its nucleoside translocation properties (39). In this regard, growth suppressive mechanisms of hCNT1 in ovarian cancer subtypes warrants further studies. Nonetheless, for mechanistic explorations on drug transport activities, we used transient viral transduction of hCNT1 and were able to successfully restore and enhance gemcitabine uptake in a subset of other cell lines (i.e., the normal IOSE, serous Caov-3 and SKOV-3, teratocarcinoma PA-1, and endometrioid TOV-112D). With the localization of exogenous hCNT1 (i.e., HA-epitope tagged hCNT1), we confirmed that exogenous hCNT1 was also predominantly localized at the cell surface in serous-type (SKOV-3 and Caov-3) cancerous cell lines. Cell surface expression of the transporter was moderately enhanced in teratocarcinoma PA-1 but not in CCC ES-2 or TOV-21G. Consistently, only teratocarcinoma PA-1 displayed a significant decrease in gemcitabine IC50 and not in CCC ES-2 or TOV-21G. Further investigation into the CCC cell lines indicated predominant accumulation of hCNT1 in intracytoplasmic vesicles visualized as large-sized punctate dots relative to other cell lines where hCNT1 is localized as fine dots at the apical surface (e.g., PA-1) or as continuous staining at cell-to-cell contacts (e.g., SKOV-3, TOV-112D). While further studies are needed, based on these observations, it is tempting to speculate that some of the trafficking components(s) or chaperone protein(s) essential for plasma membrane insertion and functional activity of hCNT1 are defective in CCC cells.
Chemoresistance of the cancerous ovarian cell lines is likely due to multiple factors. Our findings brought into light the reduced expression or mislocalization of hCNT1 as a possible contributor of poor gemcitabine response in specific ovarian cancer subtypes. Since enhancement of hCNT1 expression and activity cannot be clinically conducted using pharmacological agents but rather only by gene therapy, we bypassed the transporter requirement by delivering the nucleoside analog drug using engineered NPs. Since self-assembled polymeric NPs constructed from poly(lactic-co-glycolic acid) (PLEG) (b)-polyethyleneglycol (PEG) (PLGA-b-PEG) copolymer hold promise as delivery vehicles for various therapeutics, we developed PLGA-b-PEG NPs to bypass the transporter requirement and enhance delivery of chemotherapeutic drugs. We also looked at the effects of a gemcitabine-cisplatin combination since gemcitabine is increasingly being used in conjunction with cisplatin, and the combination has been shown to operate in synergy (30–37). Using a double-emulsion technique, we encapsulated gemcitabine, cisplatin, or both in NP formulations. In all cell lines and drug conditions (i.e., gemcitabine alone, cisplatin alone, and gemcitabine in combination with cisplatin), the use of NP drug delivery resulted in significantly increased cytotoxicity. This was indeed the case regardless of whether the combination was delivered separately (i.e., gemcitabine NPs plus cisplatin NPs) or in combination (i.e., gemcitabine plus cisplatin within the same NPs). In addition to bypassing the transporter requirement, NP delivery of the drugs increased cellular chemosensitivity far beyond that of free formulation in hCNT1-expressing cells (>20–200 folds for Gem NP). This suggests that in addition to overcoming the observed transport deficiencies, the NPs are most likely imparting other consequences such as delayed deamination (inactivation), enhanced phosphorylation (activation), or propelled self-potentiation of gemcitabine (38–40). Consistent with previous studies, gemcitabine and cisplatin as free formulations demonstrated dose-dependent synergistic activity in most of the ovarian cell lines. However, synergism between the two drugs was increased in various cell lines with NP delivery, furthering cytotoxic effects in even the most drug-resistant cell lines and subtypes. Previous studies using the A2780 ovarian cancer cell line suggest that the synergism between the two drugs is due to increased platinum-DNA adduct formation, increased gemcitabine triphosphate incorporation into DNA, as well as hindered DNA replication and repair via gemcitabine inhibition of ribonucleotide reductase, which is responsible for the generation of the dNTP pool (30–34, 36, 37). It is reasonable to expect that NPs may further enhance these complementing mechanisms by improving exposure of the drugs at the sites of action.
In summary, we found gemcitabine cytotoxicity to be reduced in ovarian cancer cells and that reduction in hCNT1 transport to likely contribute to diminished chemosensitivity. Among various ovarian cancer subtypes tested, cell surface expression of hCNT1 was maximally diminished in teratocarcinoma and CCC, which also happens to exhibit severe refractoriness to chemotherapeutic agents in clinics. Reintroduction of hCNT1 restored hCNT1 transport activity in only teratocarcinoma and not CCC cells. Interestingly, NP mediated delivery of gemcitabine bypassed the transport requirement and improved gemcitabine chemosensitivity and synergistic interactions with cisplatin in all ovarian cancer subtypes tested including teratocarcinoma and CCC. Further preclinical and clinical studies are required to test if this strategy can bring improvement in ovarian cancer patient sensitivity to nucleoside analog-based chemotherapy.
Acknowledgments
Financial Support: This study was supported by NIH grant 1R15CA176528-01A1 funds and the University of Georgia College of Pharmacy funds (R.G.). This work was also supported by a start-up grant from the National Institutes of Health (P30 GM 092378) to UGA and by the Office of the Vice President for Research, UGA (S.D.).
We would like to thank Haesung Lee, Madeline Krentz, Caitlin Gilbert, Toan Thanh Hoang, and Kineta Naidu for their assistance with this project.
Footnotes
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colombo N, Peiretti M, Parma G, Lapresa M, Mancari R, Carinelli S, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2010;21:v23–v30. doi: 10.1093/annonc/mdq244. [DOI] [PubMed] [Google Scholar]
- 2.Pectasides D, Pectasides E, Psyrri A, Economopoulos T. Treatment issues in clear cell carcinoma of the ovary: a different entity? The oncologist. 2006;11:1089–94. doi: 10.1634/theoncologist.11-10-1089. [DOI] [PubMed] [Google Scholar]
- 3.Ovarian Cancer. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 4.Jain A, Seiden MV. Rare epithelial tumors arising in or near the ovary: a review of the risk factors, presentation, and future treatment direction for ovarian clear cell and mucinous carcinoma. American Society of Clinical Oncology educational book / ASCO American Society of Clinical Oncology Meeting. 2013 doi: 10.14694/EdBook_AM.2013.33.e200. [DOI] [PubMed] [Google Scholar]
- 5.Takano M, Kikuchi Y, Yaegashi N, Kuzuya K, Ueki M, Tsuda H, et al. Clear cell carcinoma of the ovary: a retrospective multicentre experience of 254 patients with complete surgical staging. Br J Cancer. 2006;94:1369–74. doi: 10.1038/sj.bjc.6603116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crotzer DR, Sun CC, Coleman RL, Wolf JK, Levenback CF, Gershenson DM. Lack of effective systemic therapy for recurrent clear cell carcinoma of the ovary. Gynecol Oncol. 2007;105:404–8. doi: 10.1016/j.ygyno.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer science. 2008;99:653–8. doi: 10.1111/j.1349-7006.2008.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le-Ruppert K, Masters JR, Knuechel R, Seegers S, Tainsky MA, Hofstaedter F, et al. The effect of retinoic acid on chemosensitivity of PA-1 human teratocarcinoma cells and its modulation by an activated N-ras oncogene. International journal of cancer Journal international du cancer. 1992;51:646–51. doi: 10.1002/ijc.2910510423. [DOI] [PubMed] [Google Scholar]
- 9.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary. Cancer. 2000;88:2584–9. [PubMed] [Google Scholar]
- 10.Ho C-M, Huang Y-J, Chen T-C, Huang S-H, Liu F-S, Chang Chien C-C, et al. Pure-type clear cell carcinoma of the ovary as a distinct histological type and improved survival in patients treated with paclitaxel-platinum-based chemotherapy in pure-type advanced disease. Gynecologic oncology. 2004;94:197–203. doi: 10.1016/j.ygyno.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Takano M, Tsuda H, Sugiyama T. Clear cell carcinoma of the ovary: Is there a role of histology-specific treatment? Journal of Experimental & Clinical Cancer Research. 2012;31:53. doi: 10.1186/1756-9966-31-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGowan-Jordan IJ, Speevak MD, Blakey D, Chevrette M. Suppression of tumorigenicity in human teratocarcinoma cell line PA-1 by introduction of chromosome 4. Cancer Res. 1994;54:2568–72. [PubMed] [Google Scholar]
- 13.Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecologic Oncology. 2008;109:370–6. doi: 10.1016/j.ygyno.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Mizuno M, Kikkawa F, Shibata K, Kajiyama H, Ino K, Kawai M, et al. Long-term follow-up and prognostic factor analysis in clear cell adenocarcinoma of the ovary. Journal of surgical oncology. 2006;94:138–43. doi: 10.1002/jso.20251. [DOI] [PubMed] [Google Scholar]
- 15.Pazdur R. FDA Approval for Gemcitabine Hydrochloride: National Cancer Institute at the National Institutes of Health. 2011 [cited 2012 Aug 6]. Available from: http://www.cancer.gov/cancertopics/druginfo/fda-gemcitabine-hydrochloride.
- 16.Gemzar Patient Therapy Guide. Eli LIlly; 2014. Choosing your own path: Advanced recurrent ovarian cancer. [Google Scholar]
- 17.Lorusso D, Di Stefano A, Fanfani F, Scambia G. Role of gemcitabine in ovarian cancer treatment. Annals of Oncology. 2006;17:v188–v94. doi: 10.1093/annonc/mdj979. [DOI] [PubMed] [Google Scholar]
- 18.Hansen SW. Gemcitabine in the treatment of ovarian cancer. Int J Gynecol Cancer. 2001;11 (Suppl 1):39–41. [PubMed] [Google Scholar]
- 19.Herzog TJ. Recurrent Ovarian Cancer: How Important Is It to Treat to Disease Progression? Clinical Cancer Research. 2004;10:7439–49. doi: 10.1158/1078-0432.CCR-04-0683. [DOI] [PubMed] [Google Scholar]
- 20.Jelovac D, Armstrong DK. Recent progress in the diagnosis and treatment of ovarian cancer. CA: a cancer journal for clinicians. 2011;61:183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackey JR, Mani RS, Selner M, Mowles D, Young JD, Belt JA, et al. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Research. 1998;58:4349. [PubMed] [Google Scholar]
- 22.Mackey JR, Yao SYM, Smith KM, Karpinski E, Baldwin SA, Cass CE, et al. Gemcitabine transport in xenopus oocytes expressing recombinant plasma membrane mammalian nucleoside transporters. Journal of the National Cancer Institute. 1999;91:1876. doi: 10.1093/jnci/91.21.1876. [DOI] [PubMed] [Google Scholar]
- 23.Bhutia YD, Hung SW, Patel B, Lovin D, Govindarajan R. CNT1 Expression Influences Proliferation and Chemosensitivity in Drug-Resistant Pancreatic Cancer Cells. Cancer research. 2011;71:1825. doi: 10.1158/0008-5472.CAN-10-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Advances in enzyme regulation. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 25.Bhutia YD, Hung SW, Patel B, Lovin D, Govindarajan R. CNT1 expression influences proliferation and chemosensitivity in drug-resistant pancreatic cancer cells. Cancer Research. 2011;71:1825–35. doi: 10.1158/0008-5472.CAN-10-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung SW, Mody H, Marrache S, Bhutia YD, Davis F, Cho JH, et al. Pharmacological Reversal of Histone Methylation Presensitizes Pancreatic Cancer Cells to Nucleoside Drugs: In Vitro Optimization and Novel Nanoparticle Delivery Studies. PLoS ONE. 2013;8:e71196. doi: 10.1371/journal.pone.0071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cancer Facts and Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 28.Jamieson G, Snook M, Bradley T, Bertoncello I, Wiley J. Transport and metabolism of 1-β-D-arabinofuranosylcytosine in human ovarian adenocarcinoma cells. Cancer Research. 1989;49:309–13. [PubMed] [Google Scholar]
- 29.Farré X, Guillén-Gómez E, Sánchez L, Hardisson D, Plaza Y, Lloberas J, et al. Expression of the nucleoside-derived drug transporters hCNT1, hENT1 and hENT2 in gynecologic tumors. International Journal of Cancer. 2004;112:959–66. doi: 10.1002/ijc.20524. [DOI] [PubMed] [Google Scholar]
- 30.Bergman AM, van Haperen VWR, Veerman G, Kuiper CM, Peters GJ. Synergistic interaction between cisplatin and gemcitabine in vitro. Clinical Cancer Research. 1996;2:521–30. [PubMed] [Google Scholar]
- 31.Kawaguchi H, Terai Y, Tanabe A, Sasaki H, Takai M, Fujiwara S, et al. Gemcitabine as a molecular targeting agent that blocks the Akt cascade in platinum-resistant ovarian cancer. Journal of ovarian research. 2014;7:38. doi: 10.1186/1757-2215-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moufarij MA, Phillips DR, Cullinane C. Gemcitabine potentiates cisplatin cytotoxicity and inhibits repair of cisplatin-DNA damage in ovarian cancer cell lines. Molecular pharmacology. 2003;63:862–9. doi: 10.1124/mol.63.4.862. [DOI] [PubMed] [Google Scholar]
- 33.Peters GJ, Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Braakhuis BJ. Interaction between cisplatin and gemcitabine in vitro and in vivo. Seminars in oncology. 1995;22:72–9. [PubMed] [Google Scholar]
- 34.Peters GJ, Van Moorsel C, Lakerveld B, Smid K, Noordhuis P, Comijn EC, et al. Effects of gemcitabine on cis-platinum-DNA adduct formation and repair in a panel of gemcitabine and cisplatin-sensitive or-resistant human ovarian cancer cell lines. International journal of oncology. 2006;28:237. [PubMed] [Google Scholar]
- 35.Rose PG, Mossbruger K, Fusco N, Smrekar M, Eaton S, Rodriguez M. Gemcitabine reverses cisplatin resistance: demonstration of activity in platinum-and multidrug-resistant ovarian and peritoneal carcinoma. Gynecologic oncology. 2003;88:17–21. doi: 10.1006/gyno.2002.6850. [DOI] [PubMed] [Google Scholar]
- 36.Van Moorsel C, Pinedo H, Veerman G, Bergman A, Kuiper C, Vermorken JB, et al. Mechanisms of synergism between cisplatin and gemcitabine in ovarian and non-small-cell lung cancer cell lines. British journal of cancer. 1999;80:981. doi: 10.1038/sj.bjc.6690452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Moorsel CJ, Smid K, Voorn DA, Bergman AM, Pinedo HM, Peters GJ. Effect of gemcitabine and cis-platinum combinations on ribonucleotide and deoxyribonucleotide pools in ovarian cancer cell lines. Int J Oncol. 2003;22:201–7. doi: 10.3892/ijo.22.1.201. [DOI] [PubMed] [Google Scholar]
- 38.Chung WG, Sandoval MA, Sloat BR, Lansakara PD, Cui Z. Stearoyl gemcitabine nanoparticles overcome resistance related to the over-expression of ribonucleotide reductase subunit M1. Journal of controlled release : official journal of the Controlled Release Society. 2012;157:132–40. doi: 10.1016/j.jconrel.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couvreur P, Stella B, Reddy LH, Hillaireau H, Dubernet C, Desmaële D, et al. Squalenoyl nanomedicines as potential therapeutics. Nano letters. 2006;6:2544–8. doi: 10.1021/nl061942q. [DOI] [PubMed] [Google Scholar]
- 40.Lansakara PD, Rodriguez BL, Cui Z. Synthesis and in vitro evaluation of novel lipophilic monophosphorylated gemcitabine derivatives and their nanoparticles. International journal of pharmaceutics. 2012;429:123–34. doi: 10.1016/j.ijpharm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]