Abstract
Ricin toxin is an extraordinarily potent inducer of cell death and inflammation. Ricin is also a potent provocateur of the humoral immune system, eliciting a mixture of neutralizing, non-neutralizing and even toxin-enhancing antibodies. The characterization of dozens of monoclonal antibodies (mAbs) against the toxin's enzymatic (RTA) and binding (RTB) subunits has begun to reveal fundamental insights into the underlying mechanisms by which antibodies neutralize (or fail to neutralize) ricin in systemic and mucosal compartments. This information has had immediate applications in the design, development and evaluation of ricin subunit vaccines and immunotherapeutics.
1 Introduction
Bacteria and plants are known to produce protein toxins that are so potent that even trace amounts are sufficient to kill a human. Not only are these toxins extraordinarily lethal, they are incredibly diverse in terms of their mode of cytotoxicity. Nonetheless, it was recognized as early as the nineteenth century by the likes of Paul Ehrlich and others that exposure of mice and rabbits to sub-lethal doses of potent plant and bacterial toxins gives rise to “antitoxins” in serum that are capable of protecting the animals against subsequent challenges with normally lethal doses of toxins (Ehrlich 1957; Silverstein 2002). We now know, of course, that these “antitoxins” are in fact antibodies (Abs). Not only that but eliciting “antitoxin” responses remains the singular objective of all toxin vaccines in use today.
While the capacity of Abs to neutralize toxins is largely taken for granted, surprisingly little is known about the actual underlying molecular mechanisms by which this occurs. Indeed, historically there has been little incentive to investigate the nature of toxin–antibody interactions because of the success of so many toxin vaccines. That has changed, however, in the past several decades with an increased demand by the public health community and biodefense sectors for new generations of antibody-based vaccines and therapeutics against putative biothreat agents like botulinum, Shiga, abrin and ricin toxins (Mantis et al. 2011). Correspondingly, there is now a need to understand the molecular interactions by which Abs neutralize toxins and to use that information in the rational design of antibody-based countermeasures.
This chapter focuses on our current understanding of the molecular basis of antibody-mediated immunity to the Category B toxin, ricin. For the past several decades, and particularly in the past 10 years, there has been concerted effort to develop both a vaccine and an immunotherapy for ricin toxin. While considerable progress has been made in achieving these objectives, significant challenges remain, particularly with respect to rational vaccine design and immunotherapeutic optimization (Brey and Mantis 2009; Compton et al. 2011; Roche et al. 2008; Smallshaw and Vitetta 2011; Vitetta et al. 2006). Arguably, a major impediment to the development of effective countermeasures against ricin is our limited knowledge of the underlying mechanisms by which Abs impart protective immunity to the toxin following systemic and mucosal challenges. The antibody response to ricin is quite complex, as demonstrated by the fact that ricin toxoid (or toxin subunit) immunization elicits a mixture of neutralizing, non-neutralizing and toxin-enhancing Abs (Colombatti et al. 1986; Maddaloni et al. 2004; O'Hara et al. 2010). Sorting out the molecular basis by which these three classes of Abs exert their effects on ricin will undoubtedly provide unique insights into fundamental interactions between toxins and the host immune response.
2 Ricin Toxicity, Structure and Function
2.1 Cytotoxicity
Ricin toxin is glycoprotein consisting of two distinct subunits, RTA and RTB. RTA (32 kDa) is an RNA N-glycosidase that mediates the selective depurination of a conserved adenosine residue within the so-called sarcin/ricin loop (SRL) of eukaryotic 28S ribosomal RNA (Endo et al. 1987). Hydrolysis of the SRL results in an immediate arrest in ribosome progression and a cessation in translation (Endo et al. 1987). RTB (34 kDa) is a lectin that binds to terminal α(1–3)-linked galactose (Gal) and N-acetylgalactosamine (GalNac) residues (Baenziger and Fiete 1979; Rutenber et al. 1987; Sandvig et al. 1976; Zentz et al. 1978). RTB promotes ricin internalization into cells through attachment to Gal/GalNac containing glycolipids and glycoproteins on cell surfaces. RTB also mediates ricin endocytosis and delivery of RTA via retrograde transport to the endoplasmic reticulum (ER) (Rapak et al. 1997; Spooner and Lord 2011; van Deurs et al. 1986). In the ER, RTA and RTB separate via a process involving protein disulfide isomerase (PDI) and ER degradation-enhancing α-mannosidase I-like protein 1 (EDEM1) (Slominska-Wojewodzka et al. 2006; Sokolowska et al. 2011; Spooner et al. 2004). Liberated RTA partially unfolds and is retrotranslocated across the ER membrane into the cytoplasm where refolding is facilitated by cytoplasmic chaperons (e.g., Hsc70), and possibly ribosomes themselves (Argent et al. 2000; Spooner et al. 2008, 2011). Once in the cytoplasm, it is estimated that RTA inactivates ribosomes at a rate of more than 1000 per minute (Endo and Tsurugi 1987). An overview of ricin's cytotoxic pathway is depicted in Fig. 1.
Fig. 1.
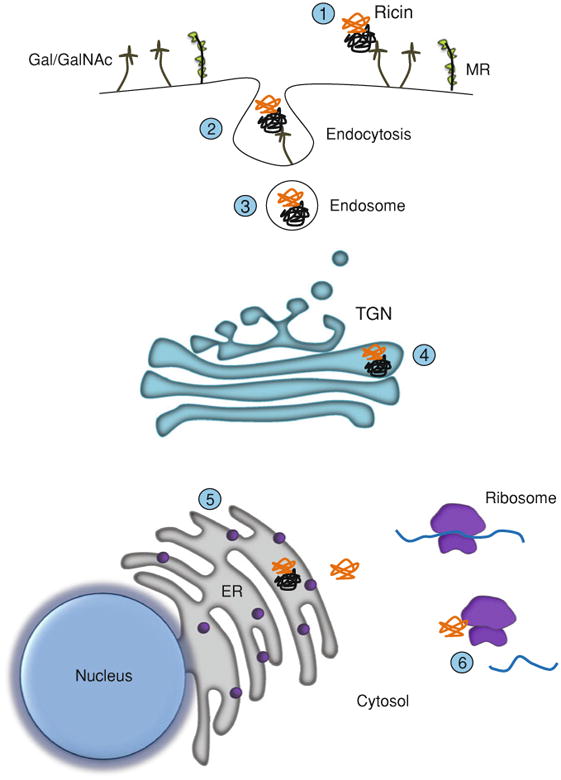
Cytotoxicity of ricin. Cartoon depicting the steps involved in cell attachment and retrograde transport of ricin. Ricin toxin's two subunits are shown in orange (RTA) and black (RTB). Step 1: RTB binds to the plasma membrane of target cells via glycoproteins and glycolipids expressing terminal Gal/GalNAc residues (stalk-like figures). Ricin is also recognized by the MR (CD206), which is expressed on macrophages and certain types of endothelial cells (see text for details). Steps 2-3: Ricin is internalized by endocytosis. Step 4: Ricin is trafficked to the trans-Golgi network (TGN). Step 5: Following delivery to the ER, RTA is liberated from RTB and then retrotranslocated into the cytoplasm. Step 6: Interaction of RTA with ribosomes results in cleavage of the SRL and arrest in protein synthesis
It has also been postulated that ricin exploits the mannose receptor (MR) as a second pathway (independent of RTB's galactose binding activity) by which to deliver RTA into the cytoplasm of host cells (Frankel et al. 1997; Simeral et al. 1980; Simmons et al. 1986; Thorpe et al. 1985). The MR (CD206) is a 175 kDa transmembrane endocytic receptor that recognizes complex oligosaccharides terminating in mannose, fucose or N-acetylglucosamine (East and Isacke 2002; Taylor et al. 2005). MR was first identified on alveolar macrophages (Largent et al. 1984; Shepherd et al. 1981), and later discovered to be expressed on a variety of cell types, including hepatic sinusoidal endothelial cells (HSEC) and Kupffer cells. It has been noted that 125I-labeled ricin accumulates in rat liver non-parenchymal cells (i.e., Kupffer cells) to a much greater extent than parenchymal cells, and that this accumulation could be inhibited by d-mannose (Magnusson and Berg 1993; Magnusson et al. 1991, 1993; Skilleter et al. 1981). While these studies support a role of the MR in promoting toxicity of ricin in vivo, recent results from ricin challenge studies of MR deficient mice revealed the opposite outcome. MR knockout mice proved to be more sensitive to ricin-induced death than their wild-type counterparts, which is consistent with a role for the MR in clearance and degradation of ricin toxin, and not enhancement of toxin uptake (Gage et al. 2011). The MR will not be discussed further in this chapter.
2.2 Structure of Ricin and Ricin Subunits
The X-ray crystal structure of ricin holotoxin, as well as the structures of the individual subunits were solved more than 20 years ago (Katzin et al. 1991; Montfort et al. 1987; Rutenber et al. 1991; Rutenber and Robertus 1991). In the subsequent sections, we highlight the particular structural features of RTA and RTB that are relevant to later discussions pertaining to the proposed mechanisms by which Abs neutralize (or fail to neutralize) ricin.
2.2.1 RTA
The mature form of RTA is a 267 amino acid polypeptide chain with two potential N-glycosylation sites at residues Asn10 and Asn236 (Montfort et al. 1987; Rutenber et al. 1991). RTA is highly α-helical in nature, consisting of a total of seven α-helices encompassing more than a third of the total amino acid residues (Montfort et al. 1987). X-ray crystal structure analysis by Robertus and colleagues revealed that RTA consists of three folding domains, corresponding to residues 1–117 (domain 1), 118–210 (domain 2) and 211–267 (domain 3) (Fig. 2) (Montfort et al. 1987; Rutenber et al. 1991). RTA's active site constitutes a shallow pocket or cleft formed by the interface of all three domains (Katzin et al. 1991; Rutenber et al. 1991). Site-directed mutagenesis identified five residues (i.e., Tyr80, Tyr123, Glu177, Arg180, and Trp211) within or near the active site cleft that are central to RTA's enzymatic activity (Monzingo and Robertus 1992; Rutenber et al. 1991; Rutenber and Robertus 1991). A detailed description of RTA's catalytic mechanisms can be found elsewhere in this volume (Wahome et al. 2011).
Fig. 2.
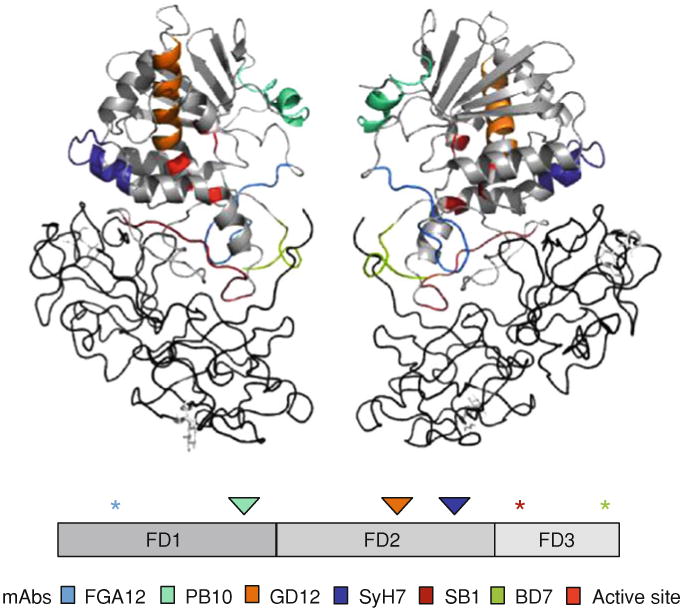
B-cell epitopes on RTA. Upper panel The crystal structure of ricin holotoxin (PDB: 2AAI) visualized using PyMOL. The epitopes recognized by neutralizing (PB10/R70, GD12, and SyH7) and non-neutralizing (FGA12, SB1, BD7) mAbs are color-coded. The active site is highlighted in red. RTB is colored in charcoal. Middle panel A linear depiction of RTA showing the subunit's three folding domains (FD1-3). The asterisks indicate the location of epitopes recognized by non-neutralizing mAbs, whereas the triangles indicate the location of epitopes recognized by neutralizing mAbs. Lower panel Color codes for the specific mAbs depicted in the upper and middle panels
While RTA's three folding domains are somewhat arbitrary, they have proven extremely valuable as topological markers for ascribing specific antibody binding locations, and we will refer to these folding domains throughout this review. The specific functions of each of RTA's three domains are discussed below.
Domain I (residues 1–117) is dominated by a five stranded β sheet that terminates in a solvent exposed loop-helix-loop motif that spans residues Tyr91-Phe108 (Lebeda and Olson 1999). The exact function(s) of domain I remains unknown. Mimimally, it serves a structural role in maintaining the proper orientation of the active site and at least one residue, Tyr80, is known to be essential for RTA's catalytic activity (Katzin et al. 1991; Ready et al. 1991). The role of the solvent exposed loop-helix-loop is also not defined, despite the fact that it is conserved among all other structurally similar RIPs and is known to be a primary target of ricin neutralizing Abs (Lebeda and Olson 1999; Lemley et al. 1994; Neal et al. 2010; O'Hara et al. 2010). A recent study has suggested that the loop-helix-loop motif may influence the side chain orientation of Glu177, a residue that is essential for RTA's depurination activity (Dai et al. 2011).
Folding domain 2 (residues 118–210) is associated with domain I primarily through hydrophobic interactions (Katzin etal. 1991). Domain 2 is remarkable in that it is dominated by five α-helices, referred to as helices C through G. Of particular note is helix E (residues 161–180), which runs through the center of RTA and which terminates with two residues(Glu177 and Arg180) that are involved in RTA catalytic activity. Domain 2 also contains an Arg-rich stretch spanning Glu187 to Ser198 (Glu-Met-Arg-Thr-Arg-Ile-Arg-Tyr-Asn-Arg-Arg-Ser) that forms a positively charged patch on the “backside” of RTA. Katzin speculated that this positively charged patch is likely responsible for the initial contacts of RTA to rRNA (Katzin et al. 1991). This hypothesis has been substantiated, inpart, by recent work by Li et al. (Li et al. 2009). The authors have proposed a two-step electrostatic interaction model for how RTA engages with the SRL, likely involving the Arg-rich stretch spanning Glu187 to Ser198.As will be discussed below, a mAb against this Arg-rich region has been shown to neutralize ricin in vitro and to be protective in vivo (O'Hara et al. 2010). It is intriguing to speculate that this mAb may function by interfering with RTA-ribosome interactions
Folding domain 3 (211-267) makes relatively few contacts with domain 2, and the two domains have been proposed to be independent entities (Katzin et al. 1991). Nonetheless, two important functions have been ascribed to domain 3. Its first function is to interact with RTB, as evidenced by the fact that residues 211–267 form a protruding element that slides into the cleft between RTB's two domains (Montfort et al. 1987). This interaction is likely due to the highly hydrophobic nature of this domain 3, but further fortified by a disulfide bond between Cys259 of RTA and Cys20 of RTB (Lewis and Youle 1986).
Domain 3's second recognized function is to facilitate RTA retrotranslocation across the ER membrane. RTA's hydrophobic C-terminus (i.e., residues Val245–256) becomes exposed following PDI-mediated liberation of RTA from RTB and is then proposed to mediate the association of RTA with the ER membrane (Chaddock et al. 1996; Day et al. 2002; Mayerhofer et al. 2009; Olson et al. 2004; Sokolowska et al. 2011). The C-termius may further drive the unfolding of RTA, which is intrinsically thermally unstable, and thereby facilitate ERAD-dependent translocation into the cytosol. A more detailed discussion of these events is provided elsewhere in this volume (Spooner and Lord 2011).
2.2.2 RTB
RTB consists of two globular domains with identical folding topologies and has been compared to an elongated “dumbbell” (Fig. 3) (Lord et al. 1994; Montfort et al. 1987). The two domains, residues 1–135 (domain 1) and residues 136–262 (domain 2), are each roughly 30 Å spheres (Montfort et al. 1987). Domains 1 and 2 themselves comprise three homologous subdomains (α, β, γ) that probably arose by gene duplication from a primordial carbohydrate recognition domain (CRD) (Rutenber et al. 1987). Only subdomains 1α or 2γ retain functional carbohydrate recognition activity (Rutenber et al. 1987; Swimmer et al. 1992). Subdomain 1α binds Gal exclusively and is considered a “low affinity” CRD. Subdomain 2γ, on the other hand, binds both Gal and GalNac and is considered a “high affinity” CRD (Baenziger and Fiete 1979; Newton et al. 1992; Rutenber et al. 1991; Zentz et al. 1978). It should be noted that RTB's overall affinity for monosaccharides is quite low (Kd in the range 10−3 to 10−4), whereas its affinity for complex sugars on the surface of cells is 3–4 magnitudes greater (Baenziger and Fiete 1979). Selective ablation of domains 1α and 2γ by genetic or biochemical methods has revealed that both domains must be inactivated to abolish RTB's ability to attach to cells (Sphyris et al. 1995; Swimmer et al. 1992).
Fig. 3.

B-cell epitopes on RTB. Upper panel The crystal structure of ricin holotoxin (PDB code 2AAI) visualized using PyMOL. The epitope recognized by neutralizing (24B11) and non-neutralizing (JB11, CB12, SA3, TFTB-1) mAbs are color-coded. RTA is colored in gray. RTB's mannose side chains are colored in cyan, while lactose moieties in RTB's CRDs are shown in white. Middle panel A linear depiction of RTB highlighting the six subdomains as originally described by Rutenber et al. (1987). The asterisks indicate the location of epitopes recognized by non-neutralizing mAbs, whereas the triangles indicate the location of epitopes recognized by neutralizing mAbs. Lower panel: Color codes for the specific mAbs depicted in the upper and middle panels
RTB's CRDs each form a shallow pocket created by a sharp bend in the polypeptide backbone associated with the three consecutive residues, Asp, Val and Arg, plus a more distal fourth variable aromatic residue that provides the binding platform for the sugar (Montfort et al. 1987; Rutenber et al. 1987). In subdomain 1α, the key residues are Asp22, Val23, Arg24 and Trp37. In subdomain 2γ, the CRD is defined by residues Asp234, Val235, Arg236 and Tyr248. The galactose moieties in CRDs 1 and 2 are stabilized by hydrogen bonding with the amide groups of Asn46 and Asn255, respectively. Finally, a Gln-X-Trp sequence is present in five of RTB's six subdomains and is thought to stabilize each of the subdomains. The conserved folds of RTB's two domains are characteristic of a superfamily known as the ricin-type (R-type) lectins, which are found in plants, animals and bacteria (Cummings and Etzler 2009). For example, toxins produced by Campylobacter jejuni, Haemophilus ducreyi, and Clostridium botulinum all show homology to RTB's CRDs (Cao et al. 2006; Inoue et al. 2003; Lara-Tejero and Galan 2001; Nesic et al. 2004; Nesic and Stebbins 2005).
RTB has two functions in ricin cytotoxicity. First, RTB mediates holotoxin attachment and entry into host cells. RTB binds to terminal Gal and GalNac glycoproteins and glycolipids on cell surfaces, thereby facilitating toxin endocytosis (Rutenber et al. 1987; Sandvig et al. 1976; van Deurs et al. 1986). Unlike Shiga or cholera toxins, which are very selective in terms of their receptor utilization, RTB is highly promiscuous. As a consequence, RTB is capable of gaining entry into virtually all known cell types. RTB's second function is to mediate the retrograde trafficking of ricin holotoxin from the early endosome to the ER (Rapak et al. 1997; Sandvig et al. 1976, 2010; Skanland et al. 2007; Spooner and Lord 2011; van Deurs et al. 1988). Specific molecules have been identified that are associated with RTA's delivery across the ER membrane in a process known as retrotranslocation, however, none of these have been shown to directly associate with RTB (Skanland et al. 2007; Utskarpen et al. 2006; Wu et al. 1994). Nor has RTB been shown to specifically associate with cellular components involved in movement of cargo through cells. Furthermore, although ricin passes through the trans-Golgi network and eventually gains entry to the ER, ricin lacks a KDEL-retrieval sequence that is present on other toxins (e.g., Shiga and cholera toxins) that exploit the retrograde pathway as a means to gain access to the cytosol (Sandvig et al. 2010). Thus, much remains to be learned regarding RTB's role in ricin retrograde trafficking.
3 Ricin–Antibody Interactions
Exploiting ricin's extraordinary capacity to provoke an immune response, Paul Erhlich and others in the late 1880s were the first to demonstrate the potential of Abs (or “antitoxins”, as they were known at the time) to completely inactivate the toxin (Olsnes 2004; Silverstein 2002). Since those early studies, dozens of reports using antisera and polyclonal antibody preparations derived from different animal species (e.g., mouse, rabbit) and tested on a diversity of cell types (e.g., human, non-human primate and mouse) and animal models (e.g., mice, rats, rabbits) have confirmed that Abs directed against the holotoxin are generally sufficient to neutralize ricin in vitro and, in most instances, confer passive immunity in vivo (Chanh et al. 1993; Dai et al. 2011; Dertzbaugh et al. 2005; Foxwell et al. 1985; Godal et al. 1983; Griffiths et al. 1995, 1999; Hazen 1927; Hewetson et al. 1993; Houston 1982; Lemley and Wright 1992; Maddaloni et al. 2004; Mantis et al. 2006; Olsnes et al. 1974; Olsnes and Saltvedt 1975; Prigent et al. 2011; Smallshaw et al. 2005, 2007; Yermakova and Mantis 2011). Abs against either subunit have been shown to be protective, although RTA-specific Abs are generally considered to be more effective than RTB-specific Abs (Maddaloni et al. 2004; Olsnes et al. 1974; Prigent et al. 2011; Yermakova and Mantis 2011).
Advances in our understanding of the molecular details underlying antibody-mediated neutralization of ricin have largely come about through the study of ricin-specific mAbs. A list of reports describing ricin-specific mAbs is provided in Table 1. Of particular note are two studies by Colombatti et al. (Colombatti et al. 1987; Colombatti et al. 1986). In 1986, Colombatti et al. were the first to report the production and characterization of a collection of ricin-specific mAbs. That study was significant in that it demonstrated that toxoid immunization gives rise to a mixture of ricin neutralizing (NAbs), non-neutralizing (non-NAbs) and toxin-enhancing mAbs, an observation that has since been confirmed by others and that reveals the complexity of the antibody response to ricin (Maddaloni et al. 2004; O'Hara et al. 2010; Yermakova and Mantis 2011). That study also revealed that there are three general classes of ricin-specific mAbs; those that bind RTA, those that bind RTB, and those that only bind the holotoxin (not either of the individual subunits). In a second study a year later, Colombatti et al. documented that protective immunity to ricin in a mouse model can be achieved by passive administration of a Nab (Colombatti et al. 1987), an observation that has also been confirmed by numerous other investigators (Table 1). While NAbs do not impart the same degree of protection that can be achieved by polyclonal Abs, the fact that a mAb against a single epitope on ricin is sufficient to completely neutralize the toxin in vitro and in vivo has profound implications for the design of vaccines and immunotherapeutics, as we will discuss later in this chapter.
Table 2. Murine IgG mAbs against RTA and RTB tested for protection against ricin challenge.
| Subunit | mAb | Protecta | Domain | Epitope | IEDBb | Reference |
|---|---|---|---|---|---|---|
| RTA | FGA12 | * | I | D37-R48 | 139713 | O'Hara et al. (2010) |
| PB10 | + | I | N97-F108 | 137759 | O'Hara et al. (2010) | |
| R70c | + | I | N 97-F108 | 137759 | Lemley et al. (1994) | |
| GD12 | + | II | T163-M174 | 137770 | Neal et al. (2010) | |
| SyH7 | + | II | E187-S198 | 139725 | O'Hara et al. (2010) | |
| SB1 | – | III | Q223-F240 | 139866 | O'Hara et al. (2010) | |
| BD7 | * | III | C259-F267 | 139698 | O'Hara et al. (2010) | |
| RAC18 | + | II/III | (Q173, A178, W211) | 77497 | Maddaloni et al. (2004) | |
| RAC-23 | – | II or III | n.d. | – | Maddaloni et al. (2004) | |
| RTB | 24B11 | + | 1α | P38-T43 | 149403 | {McGuinness and Mantis 2006) |
| JB11 | – | 1β | T50-L64 | 149498 | {Yermakova and Mantis 2011) | |
| CB12 | – | 1β | Y78-I92 | 149530 | {Yermakova and Mantis 2011) | |
| SA3 | – | 1β | T85-Y99 | 149491 | {Yermakova and Mantis 2011) | |
| TFTB-1 | – | 2α | A169-Q184 | 149247 | {McGuinness and Mantis 2006) | |
| SylH3 | + | n.d. | n.d. | – | {Yermakova and Mantis 2011) | |
| JB4 | + | 2β | C190-I204 | – | unpublished | |
| 75/3B12 | + | n.d. | n.d. | – | Colombatti et al. (1987) |
Protection based on a mouse model of systemic and/or mucosal challenge
Immune Epitope Database epitope identification number
R70 was originally named UNIVAX 70/138
indicate that the mAbs are non-neutralizing in vitro and were therefore not tested for protection in vivo
n. d. not determined
In the following sections, we highlight what is known about ricin–mAb interactions. We first describe the specific B-cell epitopes on RTA and RTB that have been identified to date. We then discuss the mechanisms by which Abs to specific epitopes may function to neutralize the toxin and attempt to link epitope specificity with mechanism of neutralization. It should be underscored that “neutralizing activity” and “protection” are not necessarily synonymous. Neutralizing activity is defined as the degree to which an antibody (or mixture of Abs) can reduce ricin's cytotoxicity in an in vitro cell-based assay. Protection, on the other hand, is defined as the degree to which an antibody (or mixture of Abs) can reduce or prevent the morbidity and/or mortality of an animal in response to ricin toxin challenge. While there is generally a good correlation between the two, it is not absolute as there are examples of neutralizing Abs that are not protective (A. Yermakova, D. Vance and N. Mantis, manuscript in preparation).
3.1 Discovery and Characterization of B-Cell Epitopes on Ricin
Two general strategies have been used in the discovery of B-cell epitopes on ricin: pepscan analysis (Carter and Loomis-Price 2004) and phage-displayed peptide libraries (Brissette and Goldstein 2007). Pepscan analysis, which can be applied to antisera or individual mAbs, entails screening in an enzyme-linked immunosorbant assay (ELISA) format a collection of overlapping peptides that span the length of a protein of interest for antibody reactivity. Pepscan analysis has been used to localize linear B-cell epitopes on other toxins, including anthrax (Abboud et al. 2009; Kelly-Cirino and Mantis 2009; Nguyen et al. 2009a, b) and botulinum neurotoxin (Scotcher et al. 2009a; Scotcher et al. 2009b). Phage displayed peptide libraries are often used in conjunction with pepscan analysis to selectively enrich for peptide(s) that have affinity for a mAb of interest (Mullaney et al. 2001; Smith and Petrenko 1997). One of the caveats associated with the use of phage library screening for epitope identification is that the candidate peptide may mimic only part of the recognition area that constitutes the epitope on the surface of the native antigen (Barlow et al. 1986).
Following the establishment of the Immune Epitope Database (IEDB) by the National Institutes of Allergy and Infectious Diseases (NIAID) in 2004, all B (and T) cell epitopes identified on the Category A-C biothreat agents and toxins list are now curated (Vita et al. 2010). The IEDB (www.immuneepitope.org) provides a publically accessible database of all experimentally characterized B and T cell epitopes on the select agents, including ricin. Each epitope is assigned a unique identification number that can be used to links that epitope to its original citation in PubMed. As such, the IEDB serves as a powerful resource for epitope analysis and comparison for the biodefense community. In this review, we have included IEDB identifier numbers wherever possible.
3.2 RTA-Specific B-Cell Epitopes and mAbs
Pepscan analysis of mouse, rabbit and human sera has led to the identification of at least six immunodominant regions on RTA (Casteletti et al. 2004; O'Hara et al. 2010). Immune sera from mice and rabbits were subjected to a peptide array consisting of 44 overlapping 12-mers spanning the length of RTA. This analysis revealed six (I–VI) immunodominant linear regions on RTA. Immunodominant regions I and II localized to folding domain 1, regions III and IV to domain 2, while immunodominant regions V and VI were confined (primarily) to folding domain 3. Serum samples from 10 Hodgkin's lymphoma patients who had received treatment with deglycosylated RTA (RTA.dg) immunotoxin therapy were also subjected to a peptide array (Castelletti et al. 2004). RTA-specific antibody levels in the sera of these patients ranged from 0.5 to 10 μg/ml. Pepscan analysis indicated human antibodies reacted with peptides spanning residues 41–90 (immunodominant region II) and 161–175 (immunodominant region IV), revealing some degree of common epitope recognition across species.
3.2.1 Immunodominant Region II
Immunodominant region II is located within folding domain I and spans amino acids Asn78 to Phe108 (O'Hara et al. 2010). A hallmark of this region is a solvent-exposed α-helix (residues Asn97–Phe108) that is structurally conserved among a number of the plant-derived RIPs (Table 2, Fig. 2). Lebeda and colleagues first described this α-helix as being the target of the protective mAb R70 (Lebeda and Olson 1999). Since that report, three other independent studies have isolated protective mAbs directed against either the same or a closely related epitope (Dai et al. 2011; Mantis et al. 2006; Neal et al. 2010; O'Hara et al. 2010). In fact, residues Asn97–Phe108, likely constitute one of the most immunodominant linear regions on RTA (J. O'Hara and N. Mantis, unpublished data).
3.2.2 Immunodominant Region IV
Immunodominant region IV on RTA spans amino acids Ile170–Thr190 (O'Hara et al. 2010). There are at least two linear B-cell epitopes within this region (Fig. 2). Residues Leu161–Ile175, in particular, were identified as being a conserved target of serum Abs from Hodgkin's lymphoma patients who had been treated with RTA.dg immunotoxin (Castelletti et al. 2004). Affinity-purified polyclonal IgG Abs specific for peptide Leu161–Ile175 neutralized ricin in vitro. A murine IgG1 mAb, known as GD12, directed against residues Thr163 to Met174 bound ricin holotoxin with high affinity and neutralized ricin with a 50% inhibitory concentration of 0.25 μg/ml in a Vero cell-based cytotoxicity assay. GD12 was sufficient to protect mice against the effects of intraperitoneal and intragastric ricin challenges, thereby establishing that preexisting serum Abs directed against residues in immunodominant region IV are sufficient to confer both systemic and mucosal immunity to ricin (Neal et al. 2010). The GD12 epitope is situated within α-helix E, which runs through the core of RTA's domain II and which terminates with two residues (Glu177 and Arg180) that are involved in RTA catalytic activity.
A second neutralizing B-cell epitope has been located towards the distal portion of immunodominant Region IV, and is likely an important target of protective Abs in rodents and humans. Human sera from RTA.dg-treated Hodgkin's lymphoma patients reacted with a 30-mer peptide spanning residues 181–201, which completely encompasses α-helix F (Castelletti et al. 2004; Katzin et al. 1991). This same region (190–198) is also considered a “linker” between RTA folding domains II and III (Olson et al. 2004). mAb SyH7 was determined to bind the epitope Glu187–Ser198 and was shown to be sufficient to protect mice against 5 × LD50 of ricin administered by the intraperitoneal route (Katzin et al. 1991; O'Hara et al. 2010). The SyH7 epitope is within an arginine-rich region of RTA that forms a positively charged patch that has been proposed to make contact with rRNA (Katzin et al. 1991; Li et al. 2009). While SyH7 has been shown to partially inhibit RTA's enzymatic activity in an in vitro translation assay, it remains to be determined whether this effect is due to interference with ricin-ribosome interactions or occlusion of RTA's active site (O'Hara et al. 2010).
3.2.3 Immunodominant Regions I, V and VI
Immunodominant Region I spans residues 30–60 and includes a hydrophobic loop (residues 34–43) that was specifically eliminated in a candidate RTA subunit vaccine for fear that it “unfavorably increased overall solvent accessibility of the protein” (Olson et al. 2004). FGA12, a murine IgG1 mAb that binds a linear epitope within this hydrophobic loop, is completely devoid of ricin neutralizing activity, despite the fact that is has an apparent affinity for the holotoxin that is comparable to a number of neutralizing Abs. Thus, the removal of residues 34–43 in a candidate RTA subunit vaccine for the purposes of improving RTA's compactness and solubility may have the added benefit of eliminating a region of RTA that elicits non-protective Abs.
Immunodominant regions V and VI correspond to residues 204–240 and 258–264, respectively. Region V, which contains α-helices G (202–210) and H (211–219), is proposed to be the target of non-neutralizing, and possibly even toxin enhancing Abs. Evidence to support this supposition includes the fact that a mAb known as SB1, which recognizes a linear epitope (223–240) in a strand just beyond α-helix H, fails to neutralize ricin in vitro or protect animals against ricin challenge (O'Hara et al. 2010). In addition, a mAb known as RAC23, which has been proposed to bind in the vicinity of SB1, has been shown to have toxin-enhancing activity, both in vitro and in vivo (Maddaloni et al. 2004). Unfortunately, more mAbs against immunodominant region V, especially within α-helices G and H, are needed before any firm conclusions can be drawn regarding the contribution of this region to eliciting neutralizing (or non-neutralizing) Abs.
In contrast, there is fairly convincing evidence to suggest that region VI does not give rise to toxin neutralizing Abs. Specifically, we have identified mAb known as BD7 that binds an epitope at the very C-terminus of RTA (residues 259–267), effectively blanketing immunodominant region VI. Despite BD7's demonstrably good affinity for RTA, it has no detectable neutralizing or protective activity.
3.3 RTB-Specific B-Cell Epitopes and mAbs
Until just the past several years, there was virtually no information available regarding B-cell epitopes on RTB. In fact until 2006 only a single RTB-specific neutralizing mAb, 75/3B12, had been described in detail (Colombatti et al. 1986). The apparent lack of interest in RTB may be because RTB-specific neutralizing mAbs are relatively rare (Colombatti et al. 1986; Maddaloni et al. 2004; Prigent et al. 2011; Yermakova and Mantis 2011). Indeed, the majority of recently described RTB-specific mAbs have no demonstrable ricin neutralizing activity, even though they bind RTB and ricin holotoxin with affinities that are comparable (or higher) to RTA-specific neutralizing Abs (Colombatti et al. 1986; Maddaloni et al. 2004; O'Hara et al. 2010; Prigent et al. 2011; Yermakova and Mantis 2011). Here, we summarize the neutralizing and non-neutralizing RTB-specific mAbs that have been described to date (Fig. 3).
3.3.1 RTB-Specific Neutralizing Abs
75/3B12 is a murine IgG1 that was shown to block ricin binding to cell surfaces and was capable of neutralizing ricin in vitro (Colombatti et al. 1987; Lemley et al. 1994). 75/3B12 was also protective in vivo, but considerably less effective than R70 when the two mAbs were compared head-to-head (Lemley et al. 1994). It has been proposed that 75/3B12 binds to an epitope (or epitopes) within one or possibly both of RTB's CRDs, based on the observation that 75/3B12 binding toRTB could be competitively inhibited with lactose (Colombatti et al. 1987). Unfortunately, the exact epitope recognized by 75/3B12 was never identified. While 75/3B12 is no longer available to the research community, Prigent and colleagues recently described another mAb (“RB37”) that is similar to 75/3B12 in that its association with RTB was reduced upon addition of lactose (Prigent et al. 2011).
24B11 was identified as an RTB-specific murine IgG1 that was approximately two times more effective at neutralizing ricin than R70 (McGuinness and Mantis 2006). In a mouse model, 24B11 was able to passively protect mice against an intraperitoneal challenge with 5× LD50s of ricin (Yermakova and Mantis 2011). When characterized in vitro, it was observed that 24B11 was fairly effective at interfering with ricin attachment to cell surfaces, although it remains unclear whether this activity fully accounts for 24B11's neutralizing potential. A phage-displayed peptide library was used to identify a consensus sequence recognized by 24B11. Based on this consensus sequence, it was proposed that 24B11 binds an epitope within a small solvent-exposed 6 amino acid loop in subdomain 1α, immediately adjacent to one of RTB's two CRDs (McGuinness and Mantis 2006). Additional lines of evidence are in accordance with this being the epitope recognized by 24B11.
SylH3 is a murine IgG1 that binds RTB, although its reactivity is greatest to ricin holotoxin, possibly because the mAb recognizes an epitope whose conformation is affected by RTB's association with RTA (Yermakova and Mantis 2011). SylH3 protects mice against systemic ricin challenge as well as 24B11 and R70. 24B11 and SylH3 do not bind the same epitope, as the two mAbs do not competitively inhibit each other from binding to RTB. Although SylH3's exact epitope has not been identified, we speculate that it is likely situated in subdomain 2γ, adjacent to RTB's high affinity Gal/GalNac CRD (Yermakova and Mantis 2011). This assumption is based on two observations. First, SylH3 was highly effective at blocking ricin attachment to galactosides, either displayed on cell surfaces or immobilized on plastic. Second, SylH3 was significantly more effective than 24B11 in blocking ricin attachment to terminal galactose residues, which is consistent with SylH3 binding to the high-affinity galactose recognition subdomain of RTB. Definitive identification of the SylH3 epitope will require screening a library of RTB point and deletion mutants and/or solving the structure of SylH3 in complex with ricin holotoxin.
Prigent and colleagues recently described two additional RTB-specific protective mAbs, RB34 and RB37 (Prigent et al. 2011). RB34 and RB37 were each sufficient to protect mice from death and weight loss following an intranasal challenge with 5 LD50 of ricin. The epitopes recognized by these two mAbs have not been determined, nor have the mAbs been tested for the ability to compete with 24B11 or SylH3 for ricin binding. Nonetheless, as mentioned above, RB37's ability to bind to RTB was reduced in the presence of lactose, suggesting that the mAb may bind an epitope within one (or both) of RTB's CDRs. Finally, we have recently characterized two additional new neutralizing RTB-specific IgGs (A. Yermakova, D. Vance and N. Mantis, manuscript in preparation). One of these mAbs (“JB4”) was able to passively protect mice against ricin challenge, whereas the other (“B/JF9”) was not. We have tentatively localized the epitope recognized by JB4 to RTB's subdomain 2β.
3.3.2 RTB-Specific Non-Neutralizing mAbs and Their Epitopes
There is evidence to indicate that RTB-immunization predominantly elicits non-neutralizing Abs (Colombatti et al. 1986; Maddaloni et al. 2004; Yermakova and Mantis 2011). For example, we recently observed that a number of RTB-immunized mice succumbed to ricin challenge despite the fact that they had relatively high, RTB-specific serum antibody titers. In addition, analysis of a panel of RTB-specific B-cell hybridomas revealed that >95% of the mAbs secreted by this panel of hybridomas had no demonstrable neutralizing activity. These data suggest that non-neutralizing epitopes constitute a large amount of the surface area of RTB.
In an effort to identify these epitopes, we recently subjected four non-neutralizing mAbs to RTB-specific pepscan analysis. All four mAbs bound to unique peptides corresponding to linear sequences within RTB's subdomains 1β and 2α, two subdomains not involved in galactoside recognition (Fig. 3) (Yermakova and Mantis 2011). Modeling the epitopes on the surface of RTB by PyMol led to the prediction that the antibody-binding sites may be too distant from the Gal/GalNac CRDs to sterically interfere with ricin attachment to cell surfaces. Indeed, this was confirmed experimentally in that none of the non-neutralizing mAbs were effective at blocking ricin attachment to Gal/GalNac in a cell-based or solid-phase binding assay. While these data apparently explain why certain Abs that bind RTB are not capable of neutralizing ricin, the story may be more complex than that. Specifically, we observed that the binding of three mAbs, SA3, CB12 and TFTB-1 to ricin holotoxin (as determined by ELISA, Biacore, and flow cytometry) was abolished when the toxin was prebound to free ligand (i.e., lactose) or when the toxin was associated on the surfaces of cells (Yermakova and Mantis 2011). While the underlying mechanism responsible for the inability of these mAbs to recognize ricin when it has engaged its receptors is yet to be determined, these data nonetheless suggest that a subset of RTB-specific mAbs may fail to neutralize ricin because they are “blind” to the toxin once it has associated with a target cell.
3.4 B-Cell Epitope Identification and Implications for Vaccine Design
While the B-cell epitope maps of RTA and RTB are far from complete, they have already provided insights into the molecular basis of immunogenicity of ricin that have implications for vaccine design (Fig. 4). In the case of RTA, for example, we tentatively determined that immunodominant regions II and IV are responsible for eliciting neutralizing Abs, whereas immundominant regions I, V and VI likely give rise to non-neutralizing mAbs (Table 2) (O'Hara et al. 2010). Based on these findings, we have hypothesized that a subunit antigen lacking regions I, V and VI would be more effective than full length RTA at eliciting neutralizing Abs. In fact, a truncated version of RTA with more or less these exact deletions has already been produced and characterized by the U.S. Army (Carra et al. 2007a; McHugh et al. 2004; McLain et al. 2011a, b). While the truncated RTA subunit vaccine, known as RVEc, has been shown to elicit protective immunity to ricin in mice and rabbits, it has yet to be compared head-to-head with full-length RTA subunit vaccine antigens.
Fig. 4.

Location of neutralizing and non-neutralizing epitopes on ricin holotoxin. The crystal structure of ricin holotoxin (PDB: 2AAI) visualized using PyMOL. Epitopes recognized by neutralizing (blue) and non-neutralizing (rust) mAbs are highlighted on both RTA and RTB. The active site (AS) is highlighted in red. RTB's mannose side chains are colored in cyan, while lactose moieties in RTB's CRDs are shown in white
The tentative B-cell epitope map of RTB also suggests a new avenue for vaccine antigen design. In particular, non-neutralizing Abs tend to bind to internal subdomains of RTB, whereas neutralizing Abs are proposed to recognize RTB's external subdomains that are involved in Gal/GalNAc recognition. Based on these preliminary findings, we have hypothesized that one (or both) of the external subdomains may be sufficient to elicit protective immunity. Moreover, we have also proposed that subdomains 1α and/or 2γ could be useful as carriers for heterologous antigens, especially subunits (or fragments of subunits) from other biothreat toxins (Yermakova and Mantis 2011).
4 Mechanisms of Antibody-Mediated Neutralization of Ricin
Ricin cytotoxicity is a complex, multistep event (Fig. 1). Presumably, derailing the toxin at any one of its intracellular checkpoints would save the cell from destruction. In this section we discuss the mechanisms by which Abs interfere (or are speculated to interfere) with ricin cytotoxicity. We also review evidence that suggests that the primary determinant of antibody-mediated neutralization of ricin is epitope specificity, not antibody affinity or Fc-mediated clearance, as has been observed for other toxins (Abboud et al. 2010; Nowakowski et al. 2002).
4.1 Blocking Ricin Attachment to Cells
Blocking attachment to receptors on cell surfaces is one of the most commonly cited mechanisms by which Abs are proposed to neutralize toxins. In the case of ricin, this is a formidable task considering that it is estimated that there are >107 toxin binding sites on a single cell (Sandvig et al. 1976). Moreover, RTB's two CRDs bind Gal/GalNac residues independently and either one is sufficient to promote toxin internalization (Sphyris et al. 1995; Swimmer et al. 1992). In addition, the two CRDs are separated by approximately 75Å, making it somewhat difficult to envision how a single mAb can occlude both CDRs simultaneously (Rutenber et al. 1987). Nonetheless, at least two RTB-specific neutralizing mAbs, 75/3B12 and SylH3 have been shown to be highly efficient at blocking ricin attachment to cells, presumably by steric hindrance (Colombatti et al. 1987; Yermakova and Mantis 2011). F(ab')2 fragments of 75/3B12 and Fab fragments of 24B11 were sufficient to block ricin binding to host cells, demonstrating that the Fc-portions of neither 75/3B12 (nor 24B11) are required for their inhibitory activity (Colombatti et al. 1987). Ultimately, understanding the mechanism(s) by which 75/3B12 and SylH3 neutralize ricin will require a crystal structure of the Ab-toxin complexes, as has been done for anthrax toxin (Leysath et al. 2009).
Considering that the molecular mass of an IgG molecule is five times greater than the mass of RTB, it is surprising that the association of any mAb with RTB is not sufficient to interfere with toxin attachment, even if the CRDs are not specifically occluded. In both solid-phase and cell-based assays, it has been demonstrated that high-affinity, non-neutralizing mAbs like TFTB-1 only marginally interfere with ricin attachment (McGuinness and Mantis 2006; Yermakova and Mantis 2011). We interpret these data as suggesting that epitope specificity is the critical determinant in dictating whether or not a mAb can block ricin-host cell interactions.
4.2 Interference with Retrograde Transport
The fact that a number of very potent neutralizing mAbs do not interfere with the binding of ricin to cell surfaces, suggests that they must neutralize the toxin at a step downstream of attachment (Maddaloni et al. 2004; Mantis et al. 2006; Neal et al. 2010; O'Hara et al. 2010; Yermakova and Mantis 2011). At this point, however, we can only speculate how this might occur. The retrograde transport of ricin from the plasma membrane to the TGN and the ER is a relatively inefficient process, suggesting that the toxin can easily be put off course (van Deurs et al. 1988). Indeed, it has been shown that simply conjugating gold particles or horseradish peroxidase (HRP) to ricin is sufficient to prevent the toxin from gaining access to the Golgi elements (van Deurs et al. 1986). Thus it would not be surprising if an antibody (or Abs) exerted the same effect upon the toxin. In the case of Shiga toxin, a mAb directed against the toxin's A subunit blocked Shiga toxin transport to the ER, presumably by promoting toxin recycling back to the cell surface (Krautz-Peterson et al. 2008).
Ricin is also proposed to engage with a suite of host proteins during its intra-cellular journey, particularly in the ER, and it seems reasonable that an antibody (or Abs) could interfere with the toxin's ability to interact with one or more of these proteins (Sandvig and van Deurs 2005; Spooner and Lord 2011; Utskarpen et al. 2006). Unfortunately, a direct interaction between ricin and a host protein(s) has yet to be demonstrated, so it is currently not possible to test whether or not specific Abs may neutralize ricin by interrupting such an event.
4.3 Inhibition of RTA's Enzymatic Activity
Following retrotranslocation, RTA is ultimately delivered into the host cell cytoplasm, where it engages its substrate. Although it is hard to conceive how an antibody could remain associated with RTA throughout this entire “retro” journey, there is nonetheless intriguing data demonstrating that certain neutralizing mAbs interfere with RTA's enzymatic activity. Three RTA-specific neutralizing mAbs, namely R70 (PB10), GD12 and SyH7, partially interfere with RTA's enzymatic activity in an in vitro translation assay (O'Hara et al. 2010). RTA-specific non-neutralizing mAbs, on the other hand, do not. The epitope recognized by neutralizing mAb, SyH7 is situated within a patch of arginine residues that are proposed to serve as the initial contact site between RTA and rRNA, thus offering a possible mechanism by which this mAb may block RNA N-glycosidase activity (Katzin et al. 1991; Li et al. 2009). Alternatively, the neutralizing mAb GD12 binds an epitope on RTA that is spatially close to the toxin's active site (Neal et al. 2010). Thus, GD12 could theoretically function by physically occluding the active site and/or by distorting the enzymatic pocket, which is known to undergo at least one conformational change upon ligand engagement (Wahome et al. 2011). Similarly, R70 binds an epitope within α-helix E whose flexibility is proposed to influence the depurination activity of RTA by controlling the side chain orientation of Glu177 (Dai et al. 2011; Lebeda and Olson 1999; Neal et al. 2010).
4.4 Fc-Mediated Protection and Other Possible Mechanisms In Vivo
There is increasing evidence from other toxins, notably anthrax and botulinum toxins, that Fc-mediated clearance may be an important component of anti-toxin immunity in vivo (Abboud et al. 2010; Nowakowski et al. 2002; Sepulveda et al. 2009). In the case of anthrax toxin, a collection of protective antigen-specific murine IgGs with identical variable regions but different Fc subclasses were tested for the ability to neutralize lethal toxin (LeTx) in vitro and in vivo (Abboud et al. 2010). The studies revealed a clear hierarchy with respect to toxin neutralization efficacy and IgG subclass, despite the fact that the mAbs each bound the toxin with equal affinities. Moreover, the differential capacity of the mAbs to impart protected immunity was Fc receptor dependent, as FcRγ−/− mice were not passively protected by antibody treatment, whereas control animals were.
There is no evidence at present to suggest that FcR-mediated clearance plays a role in immunity to ricin. Simply coating ricin holotoxin with one or more non-neutralizing IgG mAbs of either the IgG1 or IgG2b subclasses, for example, does not confer any detectable level of protection to mice upon toxin challenge (Neal et al. 2010; O'Hara et al. 2010; Yermakova and Mantis 2011). In fact, it has been shown that the half-life of ricin-antibody complexes is greater than ricin alone, presumably due to reduced renal clearance of the toxin (Pimm et al. 1990). Antibody subclass does not appear to influence relative neutralization activity either, as two mAbs with identical epitope specificity but of different subclass were equally effective at neutralizing ricin in vivo (O'Hara et al. 2010). Although the capacity of specific mAbs to neutralize ricin has not been examined in FcRγ−/− mice, it has been demonstrated that Fab fragments of one neutralizing mAb known as RAC18 are sufficient to impart protective immunity to ricin in a mouse model (Seth Pincus, personal communication).
4.5 Interference by Non-Neutralizing Abs
The observation that immunization of mice with ricin toxoid, RTA or RTB elicits a preponderance of non-neutralizing Abs has led to the suggestion that these Abs may interfere with the ability of neutralizing Abs to recognize and/or inactivate ricin. Interfering Abs are generally defined as functionally non-neutralizing Abs that sterically hinder the binding of neutralizing Abs to their respective epitopes. Interfering Abs have been described in a number of viral infections, including chronic infection with hepatitis C virus (HCV) (Zhang et al. 2009). Taking advantage of our collection of RTA-specific mAbs, we recently examined the possibility that non-neutralizing mAbs may interfere with the ability of neutralizing mAbs to bind or inactivate ricin. In fact, even when non-neutralizing mAbs were provided in 10-fold molar excess as compared to neutralizing mAbs, there was no evidence of interference (O'Hara et al. 2010). Thus, at the present time, there is no evidence to support the notion that non-neutralizing mAbs negatively impact overall protective immunity.
4.6 Toxin-Enhancing mAbs
Mononclonal Abs that augment the cytotoxic effects of ricin were first reported by Colombatti et al., (1986). Several years ago one of these mAbs, RAC23, was described in detail and shown to enhance ricin toxicity in a mouse model (Maddaloni et al. 2004). RAC23 (800 μg/kg) was mixed with ricin (30 μg/kg) and administered to mice by intraperitoneal injection. RAC23 enhanced ricin-induced hypoglycemia and accelerated the time to death when compared to an isotype control mAb or a bona fide non-neutralizing antibody. Unfortunately, the underlying mechanism(s) by which RAC23 and similar mAbs stimulate ricin toxicity is unknown. It is possible that RAC23 and related toxin-enhancing Abs augment ricin uptake into cells through toxin aggregation, facilitate disassociation of RTA and RTB or catalyze RTA unfolding in the ER.
4.7 Primary Determinants of Ricin Neutralizing Activity
While it is likely that mAbs neutralize ricin by several different mechanisms, it is clear from the evidence presented above and in a series of papers over the past several years that epitope specificity, not antibody affinity or antibody isotype, is the primary determinant of ricin neutralizing activity (Mantis et al. 2006; McGuinness and Mantis 2006; Neal et al. 2010; O'Hara et al. 2010; Yermakova and Mantis 2011). We postulate that there are limited and defined regions (constituting multiple epitopes) on the surfaces of RTA, RTB and the holotoxin that give rise to neutralizing Abs. These sites likely represent regions of ricin that are involved in toxin attachment, entry, intracellular trafficking, retrograde transport and/or ribosome recognition. In that sense, neutralizing mAbs may be useful as tools to identify the cellular components required to get RTA from the cell plasma membrane to the cell cytosol.
5 Mucosal Immunity to Ricin
It has been recognized for more than a century that ricin is extremely toxic to mucosal tissues, particularly the epithelia lining the respiratory and gastrointestinal tracts. In 1897, for example, Simon Flexner, describing the histological changes in rabbits and guinea pigs associated with parenteral ricin exposure, noted that “Of all the constituents of the intestinal mucosa, the one that feels the most severe effects of the poison is the epithelium” (Flexner 1897). Epithelial damage is also observed when the toxin is administered perorally or by gavage, although relatively high doses (1–10 mg/kg) of ricin are required to provoke a reproducible response (Leek et al. 1989; Mantis et al. 2011; Neal et al. 2011; Sekine et al. 1986; Smallshaw et al. 2007; Yoder et al. 2007). The respiratory tract is orders of magnitude more sensitive to the effects of ricin, as demonstrated by the fact very low amounts of the toxin (1–10 μg/kg) administered by aerosol results in fulminate mucosal inflammation and epithelial destruction (Benson et al. 2011; Brown and White 1997; Lindauer et al. 2009; Roy et al. 2003, 2011).
Investigations aimed at understanding mucosal immunity to ricin have an equally long history. Paul Ehrlich demonstrated that oral administration of sub lethal doses of ricin in so-called “ricin cakes” was sufficient to elicit immunity to subsequent parenteral toxin challenge (Silverstein 2002). In the 1920s, Elizabeth Hazen at Columbia University and subsequently the New York State Department of Public Health, used ricin as a model antigen to probe the relationship between mucosal immunization and local/systemic antibody responses (Hazen 1927). At this point in time, it is now well established that mucosal immunity to ricin can be achieved through immunization with ricin toxoid or RTA-based subunit vaccines, such as RiVax™, and that immunity is antibody-mediated (Neal et al. 2011; Smallshaw et al. 2007; Smallshaw and Vitetta 2011; Yoder et al. 2007). Yet, despite these important advances, surprisingly little is known about the specific mechanisms that govern protective immunity in mucosal compartments. In the respiratory tract, it still remains unresolved as to whether secretory IgA (SIgA) is important (or essential) for complete protection against aerosol challenge. In the gut, it has recently been demonstrated that intestinal immunity can be achieved in the complete absence of SIgA, thus evoking a role of serum IgG in protection (Neal et al. 2011). We summarize the current knowledge of mucosal immunityto ricin in the following sections.
5.1 Intestinal Immunity to Ricin
Delivery of ricin to rodents by gavage elicits dose-dependent lesions in the stomach and proximal small intestine (Sekine et al. 1986; Smallshaw et al. 2007; Yoder et al. 2007). In the small intestine, there is evidence of widespread villus atrophy, crypt elongation, sloughing of the epithelium, and (in some models) infiltration of inflammatory cells, including eosinophils and neutrophils. Ricin is ultimately thought to gain access to the systemic compartment following gavage (Ishiguro et al. 1992). As indicated above, the intestinal epithelium is particularly sensitive to the effects of ricin. When studied in vitro, application of ricin to the apical surfaces of polarized intestinal epithelial cell monolayers results in an arrest of protein synthesis within 3–4 h (Mantis et al. 2006). Moreover, ricin activates cellular stress-activated protein kinase pathways (SAPKs) in intestinal epithelial cells, resulting in the secretion of an array of pro-inflammatory cytokines and chemokines (Jandhyala et al. 2008; Thorpe et al. 1999, 2001; Yamasaki et al. 2004). These observations suggest that the ricin-induced epithelial destruction observed in vivo could be due to the direct cytotoxic effects of ricin on epithelial cells and/or the consequence of a local, acute inflammatory response.
Surprisingly, intestinal immunity to ricin (at least in mice) does not appear to require SIgA. This conclusion is based on the observation that immunization of mice lacking SIgA due to a mutation in the polymeric immunoglobulin receptor (pIgR) with ricin toxoid or RiVAx are impervious to the effects of a ricin challenge by the oral route (Neal et al. 2011). Moreover, passive administration of IgG mAbs against RTA or RTB by intraperitoneal injection to naïve BALB/c mice was sufficient to protect the intestinal mucosa from toxin-induced damage (Neal et al. 2011, 2010). The concentrations of serum IgG required for intestinal immunity were no greater than the concentrations required for systemic immunity, indicating that mucosal protection was not an artifact of overwhelming the system with IgG. In accordance with these results, Vitetta and colleagues reported that mice immunized with RiVax by the intramuscular or intradermal routes (which do not stimulate SIgA Abs in the gut) were protected against a lethal dose of ricin by the oral route (Marconescu et al. 2010; Smallshaw et al. 2007). Collectively, these data strongly suggest that serum Abs play a role in protecting the intestinal epithelium from ricin intoxication in vivo. Sorting out the mechanisms by which this is achieved may have important implications for mucosal vaccine design.
Considering the unique anatomic nature of the gut, it is not unreasonable to speculate that the mechanisms by which Abs neutralize ricin in the intestinal mucosa (or intestinal lumen) are different from those in the systemic compartments. At the present time, however, there is no evidence to suggest that there is a specific population of Abs that function in one compartment but not the other. In fact, mAbs like R70 and GD12 are as effective at mediating systemic immunity, as they are mucosal immunity (Neal et al. 2011, 2010). Moreover, IgA mAbs were no better than comparable IgGs in imparting intestinal immunity in a mouse model. This was surprising considering that only IgA antibodies are capable of intercepting ricin toxin in the intestinal lumen, before the toxin gains access to receptors on epithelial cells.
5.2 Respiratory Immunity to Ricin
The effects of aerosolized ricin on the respiratory tract have been studied in rodents (Brown and White 1997; DaSilva et al. 2003; Doebler et al. 1995; Griffiths et al. 1999), rabbits (McLain et al. 2011b) and rhesus macaques (Wilhelmsen and Pitt 1996), and are reviewed in detail by Roy and colleagues elsewhere in this volume (Roy et al. 2011). Briefly, in rats, for example, ricin inhalation leads to apoptosis of alveolar macrophages within 6 h of exposure, followed by interal-veolar edema, mixed inflammatory cell infiltrates, alveolar flooding and tissue necrosis 12 and 15 h later (Brown and White 1997). Similar gross pathological and histological changes occur in rabbits (McLain et al. 2011b) and non-human primates (Franz and Jaax 1997).
Immunization of mice and rabbits with either of the two lead candidate RTA subunit vaccines (i.e., RiVax and RVEc) has been shown to be sufficient to protect the animals against a subsequent aerosolized challenged with >5 LD50 of ricin (Carra et al. 2007b; McLain et al. 2011b; Smallshaw et al. 2007). Others have shown protection following immunization with ricin toxoid or deglycosylated RTA (Chanh and Hewetson 1995; Griffiths et al. 1997, 1998, 1999; Poli et al. 1996; Yan et al. 1996). While immunized animals described in these studies generally survived challenge, they invariably appeared to suffer from varying degrees of local tissue damage, suggesting that complete protection against mucosal ricin exposure is not achieved by parenteral immunization. Because parenteral immunization (intramuscular or subcutaneous) does not generally elicit SIgA antibody responses in the airways, it is assumed that the protection observed in the aforementioned studies is due entirely to serum IgG. Indeed, Smallshaw et al. noted a good correlation between ricin-specific serum IgG antibody titers and improved lung function following toxin challenge (Smallshaw et al. 2007). Thus, while it is safe to conclude that serum IgG is sufficient to protect mice from death, as well as most of the mucosal damage associated with aerosolized toxin challenge, it remains to be determined whether the addition of SIgA into the equation can further boost immunity and whether eliciting secretory Abs should be an endpoint when evaluating candidate ricin vaccines.
6 Conclusions
The power of antibodies to completely inactivate ricin toxin has been recognized for more than a century. However, the underlying mechanisms by which this is achieved have only begun to be revealed in the past several years, primarily through the detailed characterization of dozens of neutralizing and non-neutralizing mAbs directed against ricin toxin's enzymatic and binding subunits (Table 1). It is now apparent, for example, that epitope specificity is the foremost determinant of antibody neutralizing activity, although affinity is also critically important (O'Hara et al. 2010; Yermakova and Mantis 2011). Taking advantage of a unique collection of neutralizing and non-neutralizing mAbs against defined linear epitopes on RTA and RTB, it has been possible for the first time to compile a rudimentary B-cell epitope map of ricin holotoxin (Fig. 4). This map has revealed distinct “hot” and “cold” regions on the surface of the toxin that correspond to neutralizing and non-neutralizing epitopes, respectively. Theoretically, this information can now be used to engineer mutants of RTA and RTB that are more effective than the native antigens at eliciting protective immunity.
Table 1. Chronological list of ricin-specific mAbs.
| Reference | Aga | Comments and highlights |
|---|---|---|
| Colombatti et al. (1986) | RT | In vitro characterization of neutralizing and toxin-enhancing RTA- and RTB-specific mAbs |
| Colombatti et al. (1987) | RTB | Describe mAb 75/3B12; the first demonstration that an RTB-specific mAb is protective in a mouse model |
| Chanh et al. (1993) | RT | A holotoxin-specific mAb confers partial protection against ricin in a mouse model |
| Lemley et al. (1994) | RT | Identified UNIVAX 70 (aka R70); first demonstration that an RTA-specific mAb is protective in a mouse model |
| Maddaloni et al. (2004) | RT, RTA, RTB | Described neutralizing, non-neutralizing and enhancing RTA- and RTB-specific mAbs and characterized in mouse model |
| Dertzbaugh et al. (2005) | RT, RTA, RTB | In vitro characterization of RTA-and RTB-specific mAbs; examined mAbs for diagnostic use |
| Mantis et al. (2006) | RT | Production and in vitro characterization of IgA mAbs against RTA and RTB |
| McGuinness and Mantis (2006) | RT | Identified an epitope on RTB recognized by a neutralizing mAb |
| Neal et al. (2010) | RT | Demonstrated that an RTA-specific IgG mAb confers systemic and intestinal immunity to ricin |
| Pelat et al. (2009) | RTA | High affinity scFV from Macaca fascicularis |
| O'Hara et al. (2010) | RT | Produced and characterized in vitro and in vivo a collection of RTA-specific mAbs; identified five epitopes on RTA recognized by neutralizing and non-neutralizing mAbs |
| Dai et al. (2011) | RTA | Produced and characterized in vitro and in vivo RTA-specific mAbs similar to R70 |
| Prigent et al. (2011) | RTA, RTB | Produced neutralizing RTA- and RTB-specific mAbs; tested mAb combinations in a mouse intranasal challenge model |
| Yermakova and Mantis (2011) | RT | Produced and characterized in vitro and in vivo a collection of RTB-specific neutralizing and non-neutralizing mAbs; define three epitopes on RTB |
Immunogen used for generation of mAbs
The extreme sensitivity of epithelial cells to the effects of ricin must be taken into account when developing countermeasures against the toxin for the purposes of biodefense (Mantis et al. 2011). In the gastrointestinal tract, SIgA appears not to be required for protection of the epithelium from the effect of mucosal ricin exposure (Neal et al. 2011; Smallshaw et al. 2007). These data are significant because they suggest that a parenteral vaccine capable of eliciting high titer antitoxin antibody titers in serum may be sufficient to impart both systemic and mucosal immunity to ricin. Unfortunately, similar studies have yet to be conducted in the respiratory tract and it remains to be determined whether or not SIgA is required for full protection against aerosol challenge.
In summary, the study of ricin-antibody interactions is not only critical to the ongoing development of countermeasures against the toxin but it also continues to reveal fundamental insights into mechanisms of immunity in both systemic and mucosal compartments.
Acknowledgments
We would like to acknowledge Dr. Robert Brey of Soligenix, Inc. for his invaluable insights and perspectives on ricin immunity and vaccine development. We would like to extend special thanks to Drs. Paul Wahome and David Vance of the Wadsworth Center for their critical review of this chapter. This work was supported by grants AI082210, AI081053 and AI091078 from the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institutes of Health (USA).
Footnotes
The study of ricin has always played a large role in research on immunity. The interaction between ricin and ‘anti-ricin’ can be used to elucidate the complicated interactions between toxins and anti-toxins.
T.H Madsen and L.Walbum, 1904. ‘Toxins and Antitoxines’ Academie Royale des Sciences et Lettres de Danemark 3:1.
References
- Abboud N, Chow SK, Saylor C, Janda A, Ravetch JV, Scharff MD, Casadevall A. A requirement for FcgammaR in antibody-mediated bacterial toxin neutralization. J Exp Med. 2010;207:2395–2405. doi: 10.1084/jem.20100995. doi:jem.20100995[pii]10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abboud N, De Jesus M, Nakouzi A, Cordero RJ, Pujato M, Fiser A, Rivera J, Casadevall A. Identification of linear epitopes in Bacillus anthracis protective antigen bound by neutralizing antibodies. J Biol Chem. 2009;284:25077–25086. doi: 10.1074/jbc.M109.022061. doi:M109.022061[pii]10.1074/jbc.M109.022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argent RH, Parrott AM, Day PJ, Roberts LM, Stockley PG, Lord JM, Radford SE. Ribosome-mediated folding of partially unfolded ricin A-chain. J Biol Chem. 2000;275:9263–9269. doi: 10.1074/jbc.275.13.9263. [DOI] [PubMed] [Google Scholar]
- Baenziger JU, Fiete D. Structural determinants of Ricinus communis agglutinin and toxin specificity for oligosaccharides. J Biol Chem. 1979;254:9795–9799. [PubMed] [Google Scholar]
- Barlow DJ, Edwards MS, Thornton JM. Continuous and discontinuous protein antigenic determinants. Nature. 1986;322:747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- Benson JM, Gomez AP, Wolf ML, Tibbetts BM, March TH. The acute toxicity, tissue distribution, and histopathology of inhaled ricin in Sprague Dawley rats and BALB/c mice. Inhal Toxicol. 2011;23:247–256. doi: 10.3109/08958378.2011.565490. [DOI] [PubMed] [Google Scholar]
- Brey RN, Mantis NJ. Vaccines for ricin—a type II ribosome inactivating protein. In: Barrett ADT, Stanberry LR, editors. Vaccines for biodefense and neglected diseases. Elsevier Inc; New York: 2009. [Google Scholar]
- Brissette R, Goldstein NI. The use of phage display peptide libraries for basic and translational research. Methods Mol Biol. 2007;383:203–213. doi: 10.1007/978-1-59745-335-6_13. [DOI] [PubMed] [Google Scholar]
- Brown RF, White DE. Ultrastructure of rat lung following inhalation of ricin aerosol. Int J Exp Path. 1997;78:267–276. doi: 10.1046/j.1365-2613.1997.300363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Volgina A, Korostoff J, DiRienzo JM. Role of intrachain disulfides in the activities of the CdtA and CdtC subunits of the cytolethal distending toxin of Actinobacillus actinomyce-temcomitans. Infect Immun. 2006;74:4990–5002. doi: 10.1128/IAI.00697-06. doi:74/9/4990[pii]10.1128/IAI.00697-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carra JH, McHugh CA, Mulligan S, Machiesky LM, Soares AS, Millard CB. Fragment-based identification of determinants of conformational and spectroscopic change at the ricin active site. BMC Struct Biol. 2007a;7:72. doi: 10.1186/1472-6807-7-72. doi:1472-6807-7-72[pii]10.1186/1472-6807-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carra JH, Wannemacher RW, Tammariello RF, Lindsey CY, Dinterman RE, Schokman RD, Smith LA. Improved formulation of a recombinant ricin A-chain vaccine increases its stability and effective antigenicity. Vaccine. 2007b;25:4149–4158. doi: 10.1016/j.vaccine.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Carter JM, Loomis-Price L. B cell epitope mapping using synthetic peptides. Curr Protoc Immunol. 2004;Chapter 9 doi: 10.1002/0471142735.im0904s60. Unit 9.4. [DOI] [PubMed] [Google Scholar]
- Castelletti D, Fracasso G, Righetti S, Tridente G, Schnell R, Engert A, Colombatti M. A dominant linear B-cell epitope of ricin A-chain is the target of a neutralizing antibody response in Hodgkin's lymphoma patients treated with an anti-CD25 immunotoxin. Clin Exp Immunol. 2004;136:365–372. doi: 10.1111/j.1365-2249.2004.02442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock JA, Monzingo AF, Robertus JD, Lord JM, Roberts LM. Major structural differences between pokeweed antiviral protein and ricin A-chain do not account for their differing ribosome specificity. Eur J Biochem. 1996;235:159–166. doi: 10.1111/j.1432-1033.1996.00159.x. [DOI] [PubMed] [Google Scholar]
- Chanh TC, Hewetson JF. Protection against ricin intoxication in vivo by anti-idiotype vaccination. Vaccine. 1995;13:479–485. doi: 10.1016/0264-410x(94)00020-n. [DOI] [PubMed] [Google Scholar]
- Chanh TC, Romanowski MJ, Hewetson JF. Monoclonal antibody prophylaxis against the in vivo toxicity of ricin in mice. Immunol Invest. 1993;22:63–72. doi: 10.3109/08820139309066194. [DOI] [PubMed] [Google Scholar]
- Colombatti M, Johnson VG, Skopicki HA, Fendley B, Lewis MS, Youle RJ. Identification and characterization of a monoclonal antibody recognizing a galactose-binding domain of the toxin ricin. J Immunol. 1987;138:3339–3344. [PubMed] [Google Scholar]
- Colombatti M, Pezzini A, Colombatti A. Monoclonal antibodies against ricin: effects on toxin function. Hybridoma. 1986;5:9–19. doi: 10.1089/hyb.1986.5.9. [DOI] [PubMed] [Google Scholar]
- Compton JR, Legler PM, Clingan BV, Olson MA, Millard CB. Introduction of a disulfide bond leads to stabilization and crystallization of a ricin immunogen. Proteins. 2011;79:1048–1060. doi: 10.1002/prot.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R, Etzler M. R-type Lectins. In: Varki A, Cummings R, Esko J, Freeze H, Stanley P, Bertozzi C, Hart G, Etzler M, editors. Essentials of glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor; NY: 2009. [PubMed] [Google Scholar]
- Dai J, Zhao L, Yang H, Guo H, Fan K, Wang H, Qian W, Zhang D, Li B, Guo Y. Identification of a novel functional domain of ricin responsible for its potent toxicity. J Biol Chem. 2011;286:12166–12171. doi: 10.1074/jbc.M110.196584. doi:M110.196584[pii]10.1074/jbc.M110.196584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva L, Cote D, Roy C, Martinez M, Duniho S, Pitt ML, Downey T, Dertzbaugh M. Pulmonary gene expression profiling of inhaled ricin. Toxicon. 2003;41:813–822. doi: 10.1016/s0041-0101(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Day PJ, Pinheiro TJ, Roberts LM, Lord JM. Binding of ricin A-chain to negatively charged phospholipid vesicles leads to protein structural changes and destabilizes the lipid bilayer. Biochemistry. 2002;41:2836–2843. doi: 10.1021/bi012012i. [DOI] [PubMed] [Google Scholar]
- Dertzbaugh MT, Rossi CA, Paddle BM, Hale M, Poretski M, Alderton MR. Monoclonal antibodies to ricin: in vitro inhibition of toxicity and utility as diagnostic reagents. Hybridoma (Larchmt) 2005;24:236–243. doi: 10.1089/hyb.2005.24.236. [DOI] [PubMed] [Google Scholar]
- Doebler JA, Wiltshire ND, Mayer TW, Estep JE, Moeller RB, Traub RK, Broomfield CA, Calamaio CA, Thompson WL, Pitt ML. The distribution of [125I]ricin in mice following aerosol inhalation exposure. Toxicology. 1995;98:137–149. doi: 10.1016/0300-483x(94)02978-4. [DOI] [PubMed] [Google Scholar]
- East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002;1572:364–386. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- Ehrlich P. The Collected Papers of Paul Ehrlich. Vol. 2. Pergamaon Press; London: 1957. Experimentelle Untersuchungen uber ImmunitatI. Uber Ricin. [Google Scholar]
- Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxins on eukaryotic ribosomes. J Biol Chem. 1987;262:5908–5912. [PubMed] [Google Scholar]
- Endo Y, Tsurugi K. RNA N-glycosidase activity of ricin A-chain. Mechanism of action of the toxic lectin ricin on eukaryotic ribosomes. J Biol Chem. 1987;262:8128–8130. [PubMed] [Google Scholar]
- Flexner S. The histological changes produced by ricin and abrin intoxications. J Exp Med. 1897;2:197–220. doi: 10.1084/jem.2.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxwell BM, Detre SI, Donovan TA, Thorpe PE. The use of anti-ricin antibodies to protect mice intoxicated with ricin. Toxicology. 1985;34:79–88. doi: 10.1016/0300-483x(85)90080-0. [DOI] [PubMed] [Google Scholar]
- Frankel AE, Fu T, Burbage C, Tagge E, Harris B, Vesely J, Willingham MC. Lectin-deficient ricin toxin intoxicates cells bearing the d-mannose receptor. Carbohydr Res. 1997;300:251–258. doi: 10.1016/s0008-6215(97)00048-7. [DOI] [PubMed] [Google Scholar]
- Franz D, Jaax N. Ricin toxin. In: Zajtchuk R, B R, editors. Textbook of military medicine. 1997. pp. 631–42. [Google Scholar]
- Gage E, Hernandez MO, O'Hara JM, McCarthy EA, Mantis NJ. Role of the mannose receptor (CD206) in immunity to ricin. Toxins (Basel) 2011;3(9):1131–1145. doi: 10.3390/toxins3091131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godal A, Fodstad O, Pihl A. Antibody formation against the cytotoxic proteins abrin and ricin in humans and mice. Int J Cancer. 1983;32:515–521. doi: 10.1002/ijc.2910320420. [DOI] [PubMed] [Google Scholar]
- Griffiths GD, Bailey SC, Hambrook JL, Keyte M, Jayasekera P, Miles J, Williamson E. Liposomally-encapsulated ricin toxoid vaccine delivered intratracheally elicits a good immune response and protects against a lethal pulmonary dose of ricin toxin. Vaccine. 1997;15:1933–1939. doi: 10.1016/s0264-410x(97)00123-0. [DOI] [PubMed] [Google Scholar]
- Griffiths GD, Bailey SC, Hambrook JL, Keyte MP. Local and systemic responses against ricin toxin promoted by toxoid or peptide vaccines alone or in liposomal formulations. Vaccine. 1998;16:530–535. doi: 10.1016/s0264-410x(97)80007-2. [DOI] [PubMed] [Google Scholar]
- Griffiths GD, Lindsay CD, Allenby AC, Bailey SC, Scawin JW, Rice P, Upshall DG. Protection against inhalation toxicity of ricin and abrin by immunisation. Hum Exp Toxicol. 1995;14:155–164. doi: 10.1177/096032719501400201. [DOI] [PubMed] [Google Scholar]
- Griffiths GD, Phillips GJ, Bailey SC. Comparison of the quality of protection elicited by toxoid and peptide liposomal vaccine formulations against ricin as assessed by markers of inflammation. Vaccine. 1999;17:2562–2568. doi: 10.1016/s0264-410x(99)00054-7. [DOI] [PubMed] [Google Scholar]
- Hazen EL. General and local immunity to ricin. J Immunol. 1927;13:171–218. [Google Scholar]
- Hewetson JF, Rivera VR, Creasia DA, Lemley PV, Rippy MK, Poli MA. Protection of mice from inhaled ricin by vaccination with ricin or by passive treatment with heterologous antibody. Vaccine. 1993;11:743–746. doi: 10.1016/0264-410x(93)90259-z. [DOI] [PubMed] [Google Scholar]
- Houston LL. Protection of mice from ricin poisoning by treatment with antibodies directed against ricin. J Toxicol–Clin Toxicol. 1982;19:385–389. doi: 10.3109/15563658208992492. [DOI] [PubMed] [Google Scholar]
- Inoue K, Sobhany M, Transue TR, Oguma K, Pedersen LC, Negishi M. Structural analysis by X-ray crystallography and calorimetry of a haemagglutinin component (HA1) of the progenitor toxin from Clostridium botulinum. Microbiology. 2003;149:3361–3370. doi: 10.1099/mic.0.26586-0. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Matori Y, Tanabe S, Kawase Y, Sekine I, Sakakibara R. Biochemical studies on oral toxicity of ricin. V. The role of lectin activity in the intestinal absorption of ricin. Chem Pharm Bull. 1992;40:1216–1220. doi: 10.1248/cpb.40.1216. [DOI] [PubMed] [Google Scholar]
- Jandhyala DM, Ahluwalia A, Obrig T, Thorpe CM. ZAK: a MAP3Kinase that transduces Shiga toxin- and ricin-induced proinflammatory cytokine expression. Cell Microbiol. 2008 doi: 10.1111/j.1462-5822.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- Katzin BJ, Collins EJ, Robertus JD. Structure of ricin A-chain at 2.5 A. Proteins. 1991;10:251–259. doi: 10.1002/prot.340100309. [DOI] [PubMed] [Google Scholar]
- Kelly-Cirino CD, Mantis NJ. Neutralizing monoclonal antibodies directed against defined linear epitopes on domain 4 of anthrax protective antigen. Infect Immun. 2009;77:4859–4867. doi: 10.1128/IAI.00117-09. doi:IAI.00117-09[pii]10.1128/IAI.00117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krautz-Peterson G, Chapman-Bonofiglio S, Boisvert K, Feng H, Herman IM, Tzipori S, Sheoran AS. Intracellular neutralization of shiga toxin 2 by an a subunit-specific human monoclonal antibody. Infect Immun. 2008;76:1931–1939. doi: 10.1128/IAI.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Tejero M, Galan JE. CdtA, CdtB, and CdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect Immun. 2001;69:4358–4365. doi: 10.1128/IAI.69.7.4358-4365.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Largent BL, Walton KM, Hoppe CA, Lee YC, Schnaar RL. Carbohydrate-specific adhesion of alveolar macrophages to mannose-derivatized surfaces. J Biol Chem. 1984;259:1764–1769. [PubMed] [Google Scholar]
- Lebeda FJ, Olson MA. Prediction of a conserved, neutralizing epitope in ribosome-inactivating proteins. Int J Biol Macromol. 1999;24:19–26. doi: 10.1016/s0141-8130(98)00059-2. [DOI] [PubMed] [Google Scholar]
- Leek MD, Griffiths GD, Green MA. Intestinal pathology following intramuscular ricin poisoning. J Pathol. 1989;159:329–334. doi: 10.1002/path.1711590411. [DOI] [PubMed] [Google Scholar]
- Lemley PV, Amanatides P, Wright DC. Identification and characterization of a monoclonal antibody that neutralizes ricin toxicity in vitro and in vivo. Hybridoma. 1994;13:417–421. doi: 10.1089/hyb.1994.13.417. [DOI] [PubMed] [Google Scholar]
- Lemley PV, Wright DC. Mice are actively immunized after passive monoclonal antibody prophylaxis and ricin toxin challenge. Immunology. 1992;76:511–513. [PMC free article] [PubMed] [Google Scholar]
- Lewis MS, Youle RJ. Ricin subunit association. Thermodynamics and the role of the disulfide bond in toxicity. J Biol Chem. 1986;261:11571–11577. [PubMed] [Google Scholar]
- Leysath CE, Monzingo AF, Maynard JA, Barnett J, Georgiou G, Iverson BL, Robertus JD. Crystal structure of the engineered neutralizing antibody M18 complexed to domain 4 of the anthrax protective antigen. J Mol Biol. 2009;387:680–693. doi: 10.1016/j.jmb.2009.02.003. doi:S0022-836(09)00146-6[pii]10.1016/j.jmb.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Chiou JC, Remacha M, Ballesta JP, Tumer NE. A two-step binding model proposed for the electrostatic interactions of ricin a chain with ribosomes. Biochemistry. 2009;48:3853–3863. doi: 10.1021/bi802371h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer ML, Wong J, Iwakura Y, Magun BE. Pulmonary inflammation triggered by ricin toxin requires macrophages and IL-1 signaling. J Immunol. 2009;183:1419–1426. doi: 10.4049/jimmunol.0901119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord JM, Roberts LM, Robertus JD. Ricin: structure, mode of action, and some current applications. Faseb J. 1994;8:201–208. [PubMed] [Google Scholar]
- Maddaloni M, Cooke C, Wilkinson R, Stout AV, Eng L, Pincus SH. Immunological characteristics associated with the protective efficacy of antibodies to ricin. J Immunol. 2004;172:6221–6228. doi: 10.4049/jimmunol.172.10.6221. [DOI] [PubMed] [Google Scholar]
- Magnusson S, Berg T. Endocytosis of ricin by rat liver cells in vivo and in vitro is mainly mediated by mannose receptors on sinusoidal endothelial cells. Biochem J. 1993;291(Pt 3):749–755. doi: 10.1042/bj2910749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson S, Berg T, Turpin E, Frenoy JP. Interactions of ricin with sinusoidal endothelial rat liver cells. Different involvement of two distinct carbohydrate-specific mechanisms in surface binding and internalization. Biochem J. 1991;277(Pt 3):855–861. doi: 10.1042/bj2770855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson S, Kjeken R, Berg T. Characterization of two distinct pathways of endocytosis of ricin by rat liver endothelial cells. Exp Cell Res. 1993;205:118–125. doi: 10.1006/excr.1993.1065. [DOI] [PubMed] [Google Scholar]
- Mantis NJ, McGuinness CR, Sonuyi O, Edwards G, Farrant SA. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect Immun. 2006;74:3455–3462. doi: 10.1128/IAI.02088-05. doi:74/6/3455[pii]10.1128/IAI.02088-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantis NJ, Morici LA, Roy CJ. Mucosal vaccines for biodefense. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconescu PS, Smallshaw JE, Pop LM, Ruback SL, Vitetta ES. Intradermal administration of RiVax protects mice from mucosal and systemic ricin intoxication. Vaccine. 2010;28:5315–5322. doi: 10.1016/j.vaccine.2010.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer PU, Cook JP, Wahlman J, Pinheiro TT, Moore KA, Lord JM, Johnson AE, Roberts LM. Ricin A chain insertion into endoplasmic reticulum membranes is triggered by a temperature increase to 37 {degrees}C. J Biol Chem. 2009;284:10232–10242. doi: 10.1074/jbc.M808387200. doi:M808387200[pii]10.1074/jbc.M808387200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness CR, Mantis NJ. Characterization of a novel high-affinity monoclonal immunoglobulin G antibody against the ricin B subunit. Infect Immun. 2006;74:3463–3470. doi: 10.1128/IAI.00324-06. doi:74/6/3463[pii]10.1128/IAI.00324-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh CA, Tammariello RF, Millard CB, Carra JH. Improved stability of a protein vaccine through elimination of a partially unfolded state. Protein Sci. 2004;13:2736–2743. doi: 10.1110/ps.04897904ps.04897904[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLain DE, Horn TL, Detrisac CJ, Lindsey CY, Smith LA. Int J Toxicol. 2011a. Progress in biological threat agent vaccine development: A repeat-dose toxicity study of a recombinant ricin toxin a-chain (rRTA) 1-33/44-198 Vaccine (RVEc) in male and female new zealand white rabbits. doi:1091581810396730 [pii]10.1177/1091581810396730. [DOI] [PubMed] [Google Scholar]
- McLain DE, Lewis BS, Chapman JL, Wannemacher RW, Lindsey CY, Smith LA. Protective effect of two recombinant ricin subunit vaccines in the New Zealand white rabbit subjected to a lethal aerosolized ricin challenge: survival, immunological response and histopathological findings. Toxicol Sci. 2011b doi: 10.1093/toxsci/kfr274. doi:kfr274 [pii]10.1093/toxsci/kfr274. [DOI] [PubMed] [Google Scholar]
- Montfort W, Villafranca JE, Monzingo AF, Ernst SR, Katzin B, Rutenber E, Xuong NH, Hamlin R, Robertus JD. The three-dimensional structure of ricin at 2.8 A. J Biol Chem. 1987;262:5398–5403. [PubMed] [Google Scholar]
- Monzingo AF, Robertus JD. X-ray analysis of substrate analogs in the ricin A-chain active site. J Mol Biol. 1992;227:1136–1145. doi: 10.1016/0022-2836(92)90526-p. [DOI] [PubMed] [Google Scholar]
- Mullaney BP, Pallavicini MG, Marks JD. Epitope mapping of neutralizing botulinum neurotoxin A antibodies by phage display. Infect Immun. 2001;69:6511–6514. doi: 10.1128/IAI.69.10.6511-6514.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal LM, McCarthy EA, Morris CR, Mantis NJ. Vaccine-induced intestinal immunity to ricin toxin in the absence of secretory IgA. Vaccine. 2011;29:681–689. doi: 10.1016/j.vaccine.2010.11.030. doi:S0264-410X(10)01639-7[pii]10.1016/j.vaccine.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal LM, O'Hara J, Brey RN, 3rd, Mantis NJ. A monoclonal immunoglobulin G antibody directed against an immunodominant linear epitope on the ricin A chain confers systemic and mucosal immunity to ricin. Infect Immun. 2010;78:552–561. doi: 10.1128/IAI.00796-09. doi:IAI.00796-09[pii]10.1128/IAI.00796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesic D, Hsu Y, Stebbins CE. Assembly and function of a bacterial genotoxin. Nature. 2004;429:429–433. doi: 10.1038/nature02532. [DOI] [PubMed] [Google Scholar]
- Nesic D, Stebbins CE. Mechanisms of assembly and cellular interactions for the bacterial genotoxin CDT. PLoS Pathog. 2005;1:e28. doi: 10.1371/journal.ppat.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton DL, Wales R, Richardson PT, Walbridge S, Saxena SK, Ackerman EJ, Roberts LM, Lord JM, Youle RJ. Cell surface and intracellular functions for ricin galactose binding. J Biol Chem. 1992;267:11917–11922. [PubMed] [Google Scholar]
- Nguyen ML, Crowe SR, Kurella S, Teryzan S, Cao B, Ballard JD, James JA, Farris AD. Sequential B-cell epitopes of Bacillus anthracis lethal factor bind lethal toxin-neutralizing antibodies. Infect Immun. 2009a;77:162–169. doi: 10.1128/IAI.00788-08. doi:IAI.00788-08[pii]10.1128/IAI.00788-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen ML, Terzyan S, Ballard JD, James JA, Farris AD. The major neutralizing antibody responses to recombinant anthrax lethal and edema factors are directed to non-cross-reactive epitopes. Infect Immun. 2009b;77:4714–4723. doi: 10.1128/IAI.00749-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski A, Wang C, Powers DB, Amersdorfer P, Smith TJ, Montgomery VA, Sheridan R, Blake R, Smith LA, Marks JD. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc Natl Acad Sci U S A. 2002;99:11346–11350. doi: 10.1073/pnas.172229899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hara JM, Neal LM, McCarthy EA, Kasten-Jolly JA, Brey RN, 3rd, Mantis NJ. Folding domains within the ricin toxin A subunit as targets of protective antibodies. Vaccine. 2010 doi: 10.1016/j.vaccine.2010.08.020. doi:S0264-410X (10)01129-1 [pii]10.1016/j.vaccine.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004;44:361–370. doi: 10.1016/j.toxicon.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Olsnes S, Pappenheimer AM, Jr, Meren R. Lectins from Abrus precatorius and Ricinus communis II. Hybrid toxins and their interaction with chain-specific antibodies. J Immunol. 1974;113:842–847. [PubMed] [Google Scholar]
- Olsnes S, Saltvedt E. Conformation-dependent antigenic determinants in the toxic lectin ricin. J Immunol. 1975;114:1743–1748. [PubMed] [Google Scholar]
- Olson MA, Carra JH, Roxas-Duncan V, Wannemacher RW, Smith LA, Millard CB. Finding a new vaccine in the ricin protein fold. Protein Eng Des Sel. 2004;17:391–397. doi: 10.1093/protein/gzh043. [DOI] [PubMed] [Google Scholar]
- Pelat T, Hust M, Hale M, Lefranc MP, Dubel S, Thullier P. Isolation of a human-like antibody fragment (scFv) that neutralizes ricin biological activity. BMC Biotechnol. 2009;9:60. doi: 10.1186/1472-6750-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimm MV, Gunn B, Lord JM, Baldwin RW. The influence of anti-(ricin toxin A chain) monoclonal antibodies on the pharmacokinetics of ricin toxin A chain and recombinant ricin A chain in mice. Cancer Immunol Immunother. 1990;32:235–240. doi: 10.1007/BF01741706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli MA, Rivera VR, Pitt ML, Vogel P. Aerosolized specific antibody protects mice from lung injury associated with aerosolized ricin exposure. Toxicon. 1996;34:1037–1044. doi: 10.1016/0041-0101(96)00047-5. [DOI] [PubMed] [Google Scholar]
- Prigent J, Panigai L, Lamourette P, Sauvaire D, Devilliers K, Plaisance M, Volland H, Creminon C, Simon S. Neutralising antibodies against ricin toxin. PLoS One. 2011;6:e20166. doi: 10.1371/journal.pone.0020166. doi:10.1371/journal.pone.0020166PONE-D-11-01765 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapak A, Falnes PO, Olsnes S. Retrograde transport of mutant ricin to the endoplasmic reticulum with subsequent translocation to cytosol. Proc Natl Acad Sci U S A. 1997;94:3783–3788. doi: 10.1073/pnas.94.8.3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready MP, Kim Y, Robertus JD. Site-directed mutagenesis of ricin A-chain and implications for the mechanism of action. Proteins. 1991;10:270–278. doi: 10.1002/prot.340100311. [DOI] [PubMed] [Google Scholar]
- Roche JK, Stone MK, Gross LK, Lindner M, Seaner R, Pincus SH, Obrig TG. Post-exposure targeting of specific epitopes on ricin toxin abrogates toxin-induced hypoglycemia, hepatic injury, and lethality in a mouse model. Lab Invest. 2008;88:1178–1191. doi: 10.1038/labinvest.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CJ, Hale M, Hartings JM, Pitt L, Duniho S. Impact of inhalation exposure modality and particle size on the respiratory deposition of ricin in BALB/c mice. Inhalation Toxicol. 2003;15:619–638. doi: 10.1080/08958370390205092. [DOI] [PubMed] [Google Scholar]
- Roy CJ, Song K, Sivasubramani SK, Gardner DJ, Pincus SH. Animal models of ricin toxicosis. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutenber E, Katzin BJ, Ernst S, Collins EJ, Mlsna D, Ready MP, Robertus JD. Crystallographic refinement of ricin to 2.5 A. Proteins. 1991;10:240–250. doi: 10.1002/prot.340100308. [DOI] [PubMed] [Google Scholar]
- Rutenber E, Ready M, Robertus JD. Structure and evolution of ricin B chain. Nature. 1987;326:624–626. doi: 10.1038/326624a0. [DOI] [PubMed] [Google Scholar]
- Rutenber E, Robertus JD. Structure of ricin B-chain at 2.5 A resolution. Proteins. 1991;10:260–269. doi: 10.1002/prot.340100310. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Olsnes S, Pihl A. Kinetics of binding of the toxic lectins abrin and ricin to surface receptors of human cells. J Biol Chem. 1976;251:3977–3984. [PubMed] [Google Scholar]
- Sandvig K, Torgersen ML, Engedal N, Skotland T, Iversen TG. Protein toxins from plants and bacteria: probes for intracellular transport and tools in medicine. FEBS Lett. 2010;584:2626–2634. doi: 10.1016/j.febslet.2010.04.008. doi:S0014-5793(10)00277-2[pii]10.1016/j.febslet.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Sandvig K, van Deurs B. Delivery into cells: lessons learned from plant and bacterial toxins. Gene Ther. 2005;12:865–872. doi: 10.1038/sj.gt.3302525. [DOI] [PubMed] [Google Scholar]
- Scotcher MC, Johnson EA, Stanker LH. Characterization of the epitope region of F1–2 and F1–5, two monoclonal antibodies to Botulinum neurotoxin type A. Hybridoma (Larchmt) 2009a;28:315–325. doi: 10.1089/hyb.2009.0022. [DOI] [PubMed] [Google Scholar]
- Scotcher MC, McGarvey JA, Johnson EA, Stanker LH. Epitope characterization and variable region sequence of f1–40, a high-affinity monoclonal antibody to botulinum neurotoxin type a (Hall strain) PLoS One. 2009b;4:e4924. doi: 10.1371/journal.pone.0004924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine I, Kawase Y, Nishimori I, Mitarai M, Harada H, Ishiguro M, Kikutani M. Pathological study on mucosal changes in small intestine of rat by oral administration of ricin. I Microscopical observation. Acta Pathologica Japonica. 1986;36:1205–1212. doi: 10.1111/j.1440-1827.1986.tb02840.x. [DOI] [PubMed] [Google Scholar]
- Sepulveda J, Mukherjee J, Tzipori S, Simpson LL, Shoemaker CB. Efficient serum clearance of botulinum neurotoxin using a pool of small antitoxin binding agents. Infect Immun. 2009 doi: 10.1128/IAI.01084-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd VL, Lee YC, Schlesinger PH, Stahl PD. L-Fucose-terminated glycoconjugates are recognized by pinocytosis receptors on macrophages. Proc Natl Acad Sci U S A. 1981;78:1019–1022. doi: 10.1073/pnas.78.2.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein A. Paul Ehrlich's receptor immunology: the magnificent obsession. Academic Press; San Diego: 2002. [Google Scholar]
- Simeral LS, Kapmeyer W, MacConnell WP, Kaplan NO. On the role of the covalent carbohydrate in the action of ricin. J Biol Chem. 1980;255:11098–11101. [PubMed] [Google Scholar]
- Simmons BM, Stahl PD, Russell JH. Mannose receptor-mediated uptake of ricin toxin and ricin A chain by macrophages. Multiple intracellular pathways for a chain translocation. J Biol Chem. 1986;261:7912–7920. [PubMed] [Google Scholar]
- Skanland SS, Walchli S, Utskarpen A, Wandinger-Ness A, Sandvig K. Phosphoinositide-regulated retrograde transport of ricin: crosstalk between hVps34 and sorting nexins. Traffic. 2007;8:297–309. doi: 10.1111/j.1600-0854.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- Skilleter DN, Paine AJ, Stirpe F. A comparison of the accumulation of ricin by hepatic parenchymal and non-parenchymal cells and its inhibition of protein synthesis. Biochim Biophys Acta. 1981;677:495–500. doi: 10.1016/0304-4165(81)90264-6. [DOI] [PubMed] [Google Scholar]
- Slominska-Wojewodzka M, Gregers TF, Walchli S, Sandvig K. EDEM is involved in retrotranslocation of ricin from the endoplasmic reticulum to the cytosol. Mol Biol Cell. 2006;17:1664–1675. doi: 10.1091/mbc.E05-10-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallshaw JE, Richardson JA, Pincus S, Schindler J, Vitetta ES. Preclinical toxicity and efficacy testing of RiVax, a recombinant protein vaccine against ricin. Vaccine. 2005;23:4775–4784. doi: 10.1016/j.vaccine.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Smallshaw JE, Richardson JA, Vitetta ES. RiVax, a recombinant ricin subunit vaccine, protects mice against ricin delivered by gavage or aerosol. Vaccine. 2007;25:7459–7469. doi: 10.1016/j.vaccine.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallshaw JE, Vitetta ES. Ricin Vaccine Development. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_156. [DOI] [PubMed] [Google Scholar]
- Smith GP, Petrenko VA. Phage Display. Chem Rev. 1997;97:391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- Sokolowska I, Walchli S, Wegrzyn G, Sandvig K, Slominska-Wojewodzka M. A single point mutation in ricin A-chain increases toxin degradation and inhibits EDEM1-dependent ER retrotranslocation. Biochem J. 2011;436:371–385. doi: 10.1042/BJ20101493. doi:BJ20101493[pii]10.1042/BJ20101493. [DOI] [PubMed] [Google Scholar]
- Sphyris N, Lord JM, Wales R, Roberts LM. Mutational analysis of the Ricinus lectin B-chains. Galactose-binding ability of the 2 gamma subdomain of Ricinus communis agglutinin B-chain. J Biol Chem. 1995;270:20292–20297. doi: 10.1074/jbc.270.35.20292. [DOI] [PubMed] [Google Scholar]
- Spooner RA, Hart PJ, Cook JP, Pietroni P, Rogon C, Hohfeld J, Roberts LM, Lord JM. Cytosolic chaperones influence the fate of a toxin dislocated from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2008;105:17408–17413. doi: 10.1073/pnas.0809013105. doi:0809013105[pii]10.1073/pnas.0809013105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner RA, Lord JM. How ricin and shiga toxin reach the cytosol of target cells: Retrotranslocation from the endoplasmic reticulum. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_154. [DOI] [PubMed] [Google Scholar]
- Spooner RA, Watson PD, Marsden CJ, Smith DC, Moore KA, Cook JP, Lord JM, Roberts LM. Protein disulphide-isomerase reduces ricin to its A and B chains in the endoplasmic reticulum. Biochem J. 2004;383:285–293. doi: 10.1042/BJ20040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swimmer C, Lehar SM, McCafferty J, Chiswell DJ, Blattler WA, Guild BC. Phage display of ricin B chain and its single binding domains: system for screening galactose-binding mutants. Proc Natl Acad Sci U S A. 1992;89:3756–3760. doi: 10.1073/pnas.89.9.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PR, Gordon S, Martinez-Pomares L. The mannose receptor: linking homeostasis and immunity through sugar recognition. Trends Immunol. 2005;26:104–110. doi: 10.1016/j.it.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Thorpe CM, Hurley BP, Lincicome LL, Jacewicz MS, Keusch GT, Acheson DW. Shiga toxins stimulate secretion of interleukin-8 from intestinal epithelial cells. Infect Immun. 1999;67:5985–5993. doi: 10.1128/iai.67.11.5985-5993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe CM, Smith WE, Hurley BP, Acheson DW. Shiga toxins induce, superinduce, and stabilize a variety of C-X-C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. Infect Immun. 2001;69:6140–6147. doi: 10.1128/IAI.69.10.6140-6147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe PE, Detre SI, Foxwell BM, Brown AN, Skilleter DN, Wilson G, Forrester JA, Stirpe F. Modification of the carbohydrate in ricin with metaperiodate-cyanoborohydride mixtures. Effects on toxicity and in vivo distribution. Eur J Biochem. 1985;147:197–206. doi: 10.1111/j.1432-1033.1985.tb08737.x. [DOI] [PubMed] [Google Scholar]
- Utskarpen A, Slagsvold HH, Iversen TG, Walchli S, Sandvig K. Transport of ricin from endosomes to the Golgi apparatus is regulated by Rab6A and Rab6A'. Traffic. 2006;7:663–672. doi: 10.1111/j.1600-0854.2006.00418.x. [DOI] [PubMed] [Google Scholar]
- van Deurs B, Sandvig K, Petersen OW, Olsnes S, Simons K, Griffiths G. Estimation of the amount of internalized ricin that reaches the trans-Golgi network. J Cell Biol. 1988;106:253–267. doi: 10.1083/jcb.106.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deurs B, Tonnessen TI, Petersen OW, Sandvig K, Olsnes S. Routing of internalized ricin and ricin conjugates to the Golgi complex. J Cell Biol. 1986;102:37–47. doi: 10.1083/jcb.102.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita R, Zarebski L, Greenbaum JA, Emami H, Hoof I, Salimi N, Damle R, Sette A, Peters B. The immune epitope database 2.0. Nucleic Acids Res. 2010;38:D854–62. doi: 10.1093/nar/gkp1004. doi:gkp1004[pii]10.1093/nar/gkp1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitetta ES, Smallshaw JE, Coleman E, Jafri H, Foster C, Munford R, Schindler J. A pilot clinical trial of a recombinant ricin vaccine in normal humans. Proc Natl Acad Sci U S A. 2006;103:2268–2273. doi: 10.1073/pnas.0510893103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahome PG, Robertus JD, Mantis NJ. Small-molecule inhibitors of ricin and shiga toxins. Curr Top Microbiol Immunol. 2011 doi: 10.1007/82_2011_177. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen CL, Pitt ML. Lesions of acute inhaled lethal ricin intoxication in rhesus monkeys. Vet Pathol. 1996;33:296–302. doi: 10.1177/030098589603300306. [DOI] [PubMed] [Google Scholar]
- Wu YN, Gadina M, Tao-Cheng JH, Youle RJ. Retinoic acid disrupts the Golgi apparatus and increases the cytosolic routing of specific protein toxins. J Cell Biol. 1994;125:743–753. doi: 10.1083/jcb.125.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki C, Nishikawa K, Zeng XT, Katayama Y, Natori Y, Komatsu N, Oda T. Induction of cytokines by toxins that have an identical RNA N-glycosidase activity: Shiga toxin, ricin, and modeccin. Biochim Biophys Acta. 2004;1671:44–50. doi: 10.1016/j.bbagen.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Yan C, Rill WL, Malli R, Hewetson J, Naseem H, Tammariello R, Kende M. Intranasal stimulation of long-lasting immunity against aerosol ricin challenge with ricin toxoid vaccine encapsulated in polymeric microspheres. Vaccine. 1996;14:1031–1038. doi: 10.1016/0264-410x(96)00063-1. [DOI] [PubMed] [Google Scholar]
- Yermakova A, Mantis NJ. Protective immunity to ricin toxin conferred by antibodies against the toxin's binding subunit (RTB) Vaccine. 2011 doi: 10.1016/j.vaccine.2011.08.075. doi:S0264-410X(11)01334-X [pii]10.1016/j.vaccine.2011.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder JM, Aslam RU, Mantis NJ. Evidence for widespread epithelial damage and coincident production of monocyte chemotactic protein 1 in a murine model of intestinal ricin intoxication. Infect Immun. 2007;75:1745–1750. doi: 10.1128/IAI.01528-06. doi:IAI.01528-06[pii]10.1128/IAI.01528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentz C, Frenoy JP, Bourrillon R. Binding of galactose and lactose to ricin. Equilibrium studies Biochim Biophys Acta. 1978;536:18–26. doi: 10.1016/0005-2795(78)90047-8. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhong L, Struble EB, Watanabe H, Kachko A, Mihalik K, Virata-Theimer ML, Alter HJ, Feinstone S, Major M. Depletion of interfering antibodies in chronic hepatitis C patients and vaccinated chimpanzees reveals broad cross-genotype neutralizing activity. Proc Natl Acad Sci U S A. 2009;106:7537–7541. doi: 10.1073/pnas.0902749106. [DOI] [PMC free article] [PubMed] [Google Scholar]


