Abstract
Background
Providing weight support facilitates locomotion in spinal cord injured animals. To control weight support, robotic systems have been developed for treadmill stepping and more recently for overground walking.
New Method
We developed a novel device, the body weight supported ambulatory rodent trainer (i.e. BART). It has a small pneumatic cylinder that moves along a linear track above the rat. When air is supplied to the cylinder, the rats are lifted as they perform overground walking. We tested the BART device in rats that received a moderate spinal cord contusion injury and in normal rats. Locomotor training with the BART device was not performed.
Results
All of the rats learned to walk in the BART device. In the contused rats, significantly greater paw dragging and dorsal stepping occurred in the hindlimbs compared to normal. Providing weight support significantly raised hip position and significantly reduced locomotor deficits. Hindlimb stepping was tightly coupled to forelimb stepping but only when the contused rats stepped without weight support. Three weeks after the contused rats received a complete spinal cord transection, significantly fewer hindlimb steps were performed.
Comparison with Existing Methods
Relative to rodent robotic systems, the BART device is a simpler system for studying overground locomotion. The BART device lacks sophisticated control and sensing capability, but it can be assembled relatively easily and cheaply.
Conclusions
These findings suggest that the BART device is a useful tool for assessing quadrupedal, overground locomotion which is a more natural form of locomotion relative to treadmill locomotion.
Keywords: Contusion, locomotion, transection, kinematics, loading
1. Introduction
Control of limb loading is crucial for generating stepping after spinal cord injury (SCI). For example, treadmill stepping in SCI animals is difficult when the full weight of the body is borne on the legs. Reducing load by manually lifting the body so that only a percentage of body weight is on the hindlimbs facilitates stepping (Lovely et al. 1986; Barbeau and Rossignol 1987). Previously, we developed a robotic body weight support (BWS) treadmill system for a rodent model of SCI (de Leon et al. 2002a, 2002b). The BWS treadmill system supports a desired percentage of the rat's weight while the animal walks bipedally with only its hindlimbs on the treadmill belt. We and others have used the rodent BWS treadmill system for locomotor training and have demonstrated its effectiveness for enhancing locomotor performance in SCI rats (Timoszyk et al. 2002, 2005; Cha et al. 2007; Heng and de Leon 2009) and mice (Fong et al. 2005; Cai et al. 2006).
Despite its usefulness, locomotion in the rodent BWS treadmill system is not a natural form of rodent locomotion. The rodents perform only hindlimb locomotion instead of the quadrupedal pattern of gait. The lack of forelimb movements is problematic given previous findings. Sensory input from both the forelimbs and hindlimbs contributes to the drive of central pattern generators that controls hindlimb stepping (Juvin et al. 2012). A recent study of treadmill training in spinally-hemisected rats reported that hindlimb locomotor recovery was better when the rats were trained with quadrupedal stepping rather than bipedal hindimb stepping (Shah et al. 2013). A major source of sensory stimulation is therefore missing during bipedal stepping. Adding to the artificial nature of the BWS treadmill system is the fact that rats perform stepping on a treadmill rather than overground. Treadmill locomotion is not considered to be a spontaneous behavior and this has implications for locomotor control. Voluntary control of movement is not necessary during treadmill stepping because the moving treadmill belt provides a powerful stimulation to spinal circuits (Forssberg et al. 1980). A recent study reported that cortical control over hindimb movements was achieved by training SCI rats to perform a bipedal, overground locomotor task but treadmill training did not have the same beneficial effect (van den Brand et al. 2012). These findings suggested that in the context of studying the recovery of supraspinal control, overground locomotion was preferred over treadmill locomotion because it encouraged active participation.
Given all these factors, locomotor tests ideally would combine quadrupedal, overground walking with weight support, yet few studies have examined this behavior in rats. Kuerzi and colleagues used shallow water to support the weight of contused rats (Kuerzi et al. 2010). Although walking in shallow water improved, they reported no improvements in overground walking suggesting that shallow water walking did not translate to overground walking. Dominici and colleagues developed a robotic support system to facilitate overground walking in the rat (Dominici et al. 2012). The system is a powerful tool and has been successfully used to improve walking and stair climbing in spinally hemisected rats. However, this system, like other robotic BWS systems (de Leon et al. 2002b; Udoekwere et al. 2014), relies on sophisticated hardware and software in order to control weight support and trunk position.
We introduce a novel device, the body weight supported ambulatory rodent trainer (i.e. BART). In comparison to robotic BWS systems, the BART device has a simpler lifting mechanism that uses air pumped into a small pneumatic cylinder. The cylinder moves along a linear track positioned above the rat. The BWS apparatus lifts the rat as it performs quadrupedal overground locomotion. Here, we evaluated the BART device as an assessment tool for overground locomotion in SCI rats. The BART device was not used for locomotor training. Stepping with and without weight support was tested in rats with moderate spinal cord contusion injuries. We also re-tested the rats after they received a complete spinal cord transection to cut any spared fibers. We show that spinally contused rats and normal rats can successfully execute quadrupedal overground walking in the BART device. The findings have implications not only for the development of tools used to assess locomotor recovery in rodents but also for understanding the role that loading plays in natural forms of locomotion.
2. Materials and Methods
2.1. Experimental Design
Female Sprague Dawley rats weighing approximately 250g were used in the study. One group of rats (n=10) received a moderate spinal cord contusion injury and another group served as normal controls (n=5). Two months later, the rats were acclimated to walking while connected to the BART device. No locomotor training was performed with the BART device. Tests of stepping in the BART device were performed over a 1-month period. After the initial tests, 6 of the contused rats received a complete spinal cord transection. These rats were re-tested in the BART device 3 weeks after the transection.
2.2. Spinal cord contusion and transection surgeries
The surgical procedures have been described in detail previously (Nessler et al. 2006). Briefly, the rats were anaesthetized with isoflurane (VetEquip V-10 Mobile, Pleasanton, CA). The skin over the spine was shaved and cleaned with betadyne solution. The skin was incised and muscle and connective tissue were dissected to expose the T9-T11 vertebrae. A T9 laminectomy was performed and the Infinite Horizon Device (Precision Systems & Instrumentation, Lexington KY) was used to deliver an impact force to the spinal cord that resulted in a moderate contusion injury (average force: 215 ± 5.9 kdyne). The connective tissue and muscle were closed with chromic gut and the skin was closed using staples.
The spinal cord transection was performed as described previously (de Leon and Acosta 2006). The rats were anaesthetized with isoflurane, the skin over the spine was shaved and cleaned with betadyne solution. The skin was incised and muscle and connective tissue were dissected to expose the T9-T11 vertebrae. A laminectomy was made at T10 (i.e. one vertebra caudal to the original laminectomy at T9). The spinal cord was gently lifted with forceps, and completely transected with iridectomy scissors. The two ends of the cord were drawn apart by a distance of approximately 2-3 mm, and gel foam was packed between the two cut ends of the cord.
Immediately following the surgery, rats were placed on a heating pad until they recovered from anesthesia, whereby they were returned to their cages. The animals were monitored twice daily throughout the post-injury survival period for general health. Bladders were manually expressed twice daily for 10-14 days after which the animals developed an automatic bladder voidance reflex. The animals received Baytril (Enroflaxacin 2.5 mg/kg, sub-cutaneously) for 10-14 days after surgery and when necessary, were hydrated with lactated ringers (5mg/100g, sub-cutaneously). All procedures were approved by the Institutional Animal Care and Use Committee at California State University, Los Angeles.
2.3. BART device
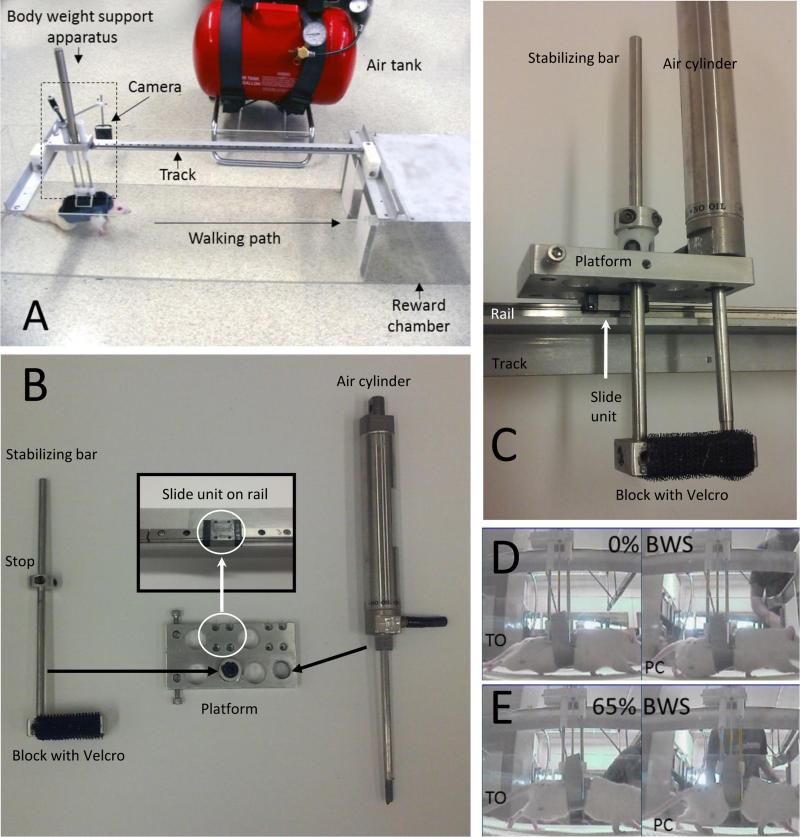
The BART device is shown in Fig. 1A. It consists of a BWS apparatus (see box in Fig. 1A) that moves along a low-friction ~2 ft-long track as the animal walks forward into a reward chamber. The components of the BWS apparatus are shown in Fig. 1B. The BWS apparatus is composed of a pneumatic air cylinder (Airpel Anti-Stiction Air Cylinder M16XDU), a custom-made rectangular, metal platform, a stabilizing bar and a block. Holes were drilled in the metal platform for inserting the air cylinder and stabilizing bar (see black arrows in Fig. 1B). Multiple holes were drilled so that one or two stabilizing bars could be added to the BWS apparatus (i.e. Fig. 1A shows two stabilizing bars whereas Fig. 1C shows only one stabilizing bar) and so the location of the air cylinder could be moved to the front, middle or rear of the platform. Four screw holes in the platform (see white circles and insert in Fig. 1B) are used to attach the BWS apparatus to a sliding unit (IKO, Linear Way L 7 B). The sliding unit slides on rails (IKO, LWL7B F90 S2; see insert Fig. 1B and Fig. 1C) that are secured with screws to the overhead track.
Fig. 1.
(A) The BART device for body weight supported, quadrupedal overground locomotion in rats. Pressurized air from an air tank is supplied to a body weight support apparatus to lift the rats. The BWS apparatus slides along a low-friction rail as the animal walks forward into a chamber for food reward. A camera films the hindlimbs for kinematic analyses. (B) The body weight support apparatus consists of an air cylinder, stabilizing bar, block and platform that is attached to a slide unit on the rail. Screws attach the platform to the slide unit (white arrow and circle). The platform has mutiple holes for holding the stabilizing bar and air cylinder (see black arrows). The assembled body weight support apparatus attached to the rail is shown in (C). (D) Pictures of a spinal cord injured rat walking in the device when no weight support (0% body weight) is provided. (E) Picture of the same rat walking with weight support (65% body weight). BWS, body weight support; PC, paw contact; TO, toe off.
The assembled BWS apparatus is shown in Fig. 1C. A stop on the stabilizing bar prevents the block from dropping to the floor when no air is present in the cyclinder. Velcro on the underside of the block is used to connect to the rat vest. Pressurized air from a 5 gallon air tank (Fig. 1A) is supplied to the pneumatic cylinder via a plastic tube to lift the rat and unload the limbs as the animal walks (compare Fig. 1D and Fig. 1E). Because the volume of the cylinder is small compared to the volume of the pressurized tank, the lifting force provided by the cylinder stays approximately constant as the cylinder piston moves up and down. Specifically, using the ideal gas law, the maximum force variation would be expected to be 100 (1 -Vtank/(Vtank+Vcylinder)) % of the steady state force, which in our case is ~0.07% where Vtank is the volume of the air tank (~19000 cm3) and Vcylinder is the volume of the cylinder (~14 cm3). The air cylinder also added a friction load of 1-2% of the steady state force.
To calculate the coefficient of static friction (μ) for the sliding unit on the rail of the BART device we used an inclined plane and the equations μ = tan θ and tan θ = h/b where θ is the angle between the base of the BART device and horizontal and b is the length of the BART device base. The front end of the BART device was raised until the body weight support apparatus began to slide down the rail and the height, h, that the front end was lifted was measured. The measurements were repeated with different weights (i.e. 250-300g which was the range of rat weights) attached to the body weight support apparatus. The mean coefficient of friction within this range of weight was determined to be 0.068.
2.4. Locomotor tests
The rats were acclimated daily over a 2 week period to stepping while attached to the body weight support apparatus. A Velcro strap was wrapped around the trunk of the rats and the block (see Fig. 1D and E). The strap stabilized the trunk and prevented trunk movement (i.e. tilting laterally) so that the hindlimbs maintained a weight-bearing position under the body during stepping. It was difficult to secure normal rats using the Velcro strap and instead a cloth vest was used (see Fig. 1A). Food rewards were used to motivate the rats to walk into the reward chamber. Stepping in the contused rats was tested with and without weight support (i.e. 0% and 65% BWS respectively). Normal rats were tested at 0% BWS only. For the weight support condition, air pressure was increased to lift the rat so that 65% of its body weight and the weight of device components (i.e. the weight of the strap, block and stabilizing bar) were supported. A rodent electronic scale was used to measure each rat's weight and to confirm the 0% and 65% BWS levels. During testing, the rats performed multiple runs in the BART device. A small video camera (Mini CCTV Camera, Sony) was attached to the platform of the BWS apparatus in order to film the limbs for quantitative movement analyses (see Fig. 1A). Video camera footage was captured online on a computer for subsequent analysis.
2.5. Data analysis and statistics
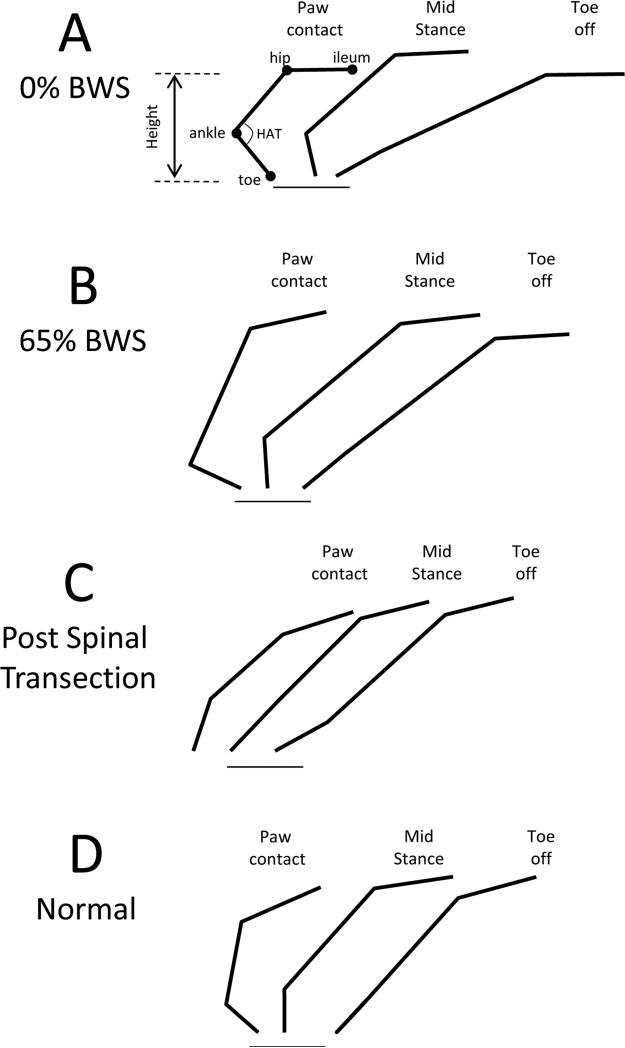
Custom software was used to analyze the hindlimb movements from the video footage (Askari et al. 2013). Briefly, the skin above bony landmarks on the hindlimb was inked (i.e., the iliac crest, greater trochanter, head of the tibia, lateral malleolus, base of the calcaneus, head of the 5th metatarsal (MTP), and the first phalanx of the 5th digit). Video footage of the hindlimb was digitized and calibrated. A text file containing the x and y coordinates of the inked landmarks was generated and exported to MS Excel. Hip height (y coordinate of hip marker – y coordinate of MTP maker) was calculated during three events of the step cycle: toe off (TO), midstance (MS) and paw contact (PC) (see Fig. 3A). PC was identified as the initial contact of the paw with the ground and TO was identified by the first indication of forward movement of the ankle marker. In instances of paw dragging, identification of PC was based on the transition from forward to backward movement of the ankle. MS occurred halfway between PC and TO. The hip-ankle-toe (HAT) angles at PC and TO were calculated using the angle between the skin markers above the greater trochanter, lateral malleolus and MTP joints (see Fig. 3A). The HAT angle excursion was the difference between HAT angle at PC and TO.
Fig. 3.
Stick figure representations of the hindlimbs during the stance phase of stepping in a spinally contused rat at 0% BWS (A), a spinally contused rat at 65% BWS (B), a spinal cord transected rat (C) and a normal rat (D). In (A), the ileum, hip, ankle and toe joints are illustrated in with black circles, the hip-ankle-toe (HAT) angle is shown with a semicircle and the hip height (hip-toe) is shown with the arrow. The horizontal line below the stick figures shows the approximate location of the floor.
Video files recorded during testing were transferred to a computer for analyses. The number of steps performed by the right hindlimb and forelimb were counted. The amount of frames in which the paw dragged during swing was counted. The percentage of paw dragging was calculated by dividing the number of frames with dragging by the total number of frames during swing (i.e. TO-PC) and multiplying by 100. During stance, the number of frames in which the dorsal surface of the toes touched the ground was counted. The percentage of dorsal stepping was calculated by dividing the number of dorsal stepping frames by the total number of frames during stance (i.e. PC-TO) and multiplying by 100. Hindlimb-forelimb coordination was measured by dividing the number of hindlimb steps by forelimb steps and multiplying by 100. Weight bearing steps performed on either the dorsal or plantar surface of the hindpaw were counted. This analysis is based on the Plantar Surface Index used for moderate-severe spinally contused rats (Kuerzi et al. 2010) but here dorsal steps in the hindlimbs were also included. In addition, the phase shift between the right forelimb and right hindlimb was calculated by dividing the time interval between forelimb paw contact and hindlimb paw contact by the forelimb cycle period (as previously described (Górska et al. 1999)). Because of an error in video recording (i.e. forelimb paw contact was out of frame), the phase shift was not calculated in the normal rats.
All measurements from each rat were obtained from three runs on the BART device so that a minimum of 15 step cycles were analyzed. A repeated measures ANOVA was used to test statistical significance when comparing mean values between 0% and 65% BWS conditions. A repeated measures ANOVA was also used to test statistical significance before and after spinal cord transection. A one-way ANOVA was used for comparing mean values between the normal rats and spinally contused rats. Statistically significant differences were defined as p values less than 0.05.
3. Results
3.1. Use of BART device in Normal and spinally contused rats
We tested the ability of normal rats and spinally contused rats to perform quadrupedal locomotion in the BART device (Fig. 1A). The components of the BART device are shown in Fig. 1B and C. A sling wrapped around the trunk was sufficient to secure the spinally contused rats to the weight support apparatus (Fig. 1D,E), but in some normal rats it was necessary to use a cloth vest (Fig. 1A). The sling prevented the trunk from tilting laterally and this helped to keep the hindlimbs and hindpaws positioned under the body (see Fig. 1D and E). To lift the rats, pressurized air from a 5 gallon air tank was supplied to the air cylinder (see Fig. 1A) until 65% of the body weight was supported. Lifting the rats altered trunk mechanics (i.e. the back arched) and applied pressure to the abdomen (see Fig. 1E). After 1-2 weeks of acclimation, the rats learned to walk while attached to the weight support apparatus. The weight support apparatus was attached to a platform that slid on a track rail above the rats (see Fig. 1A,C). The coefficient of friction for the body weight support apparatus sliding on the rail was 0.068 and we observed that the rats easily overcame the friction when moving forward. Because the track was linear the rats only moved in the forward direction and lateral deviation was not possible.
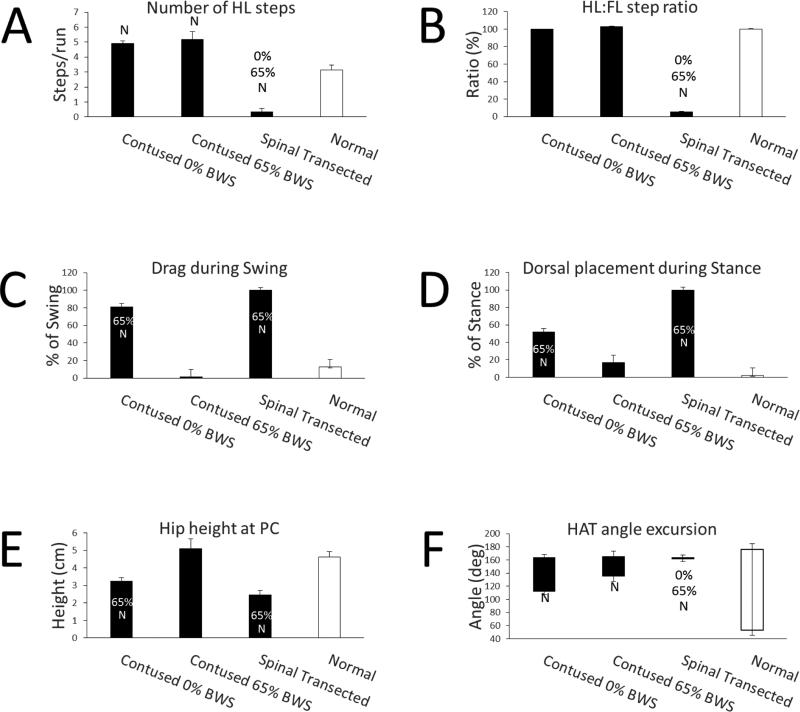
In the absence of any weight support (0% BWS), spinally contused rats performed 4.7 ± 0.4 hindlimb steps to perform one run which consisted of walking from one end of the BART device into the reward chamber (see Contused 0% BWS in Fig. 2A). Normal rats performed the same task with significantly fewer steps, 3.2 ± 0.5 steps/run (see Normal in Fig. 2A). Deficits in hindlimb stepping were apparent in the spinally contused rats. When no weight support was provided, paw drag occurred during approximately 80% of the swing phase and dorsal contact of the paw occurred during approximately 50% of the stance phase (see Contused 0% BWS in Fig. 2C and D). The occurrence of paw drag and dorsal placement at 0% BWS was significantly greater than normal (compare Contused 0% BWS to Normal in Fig. 2C and D).
Fig. 2.
The number of hindlimb steps per run (A), ratio of hindlimb to forelimb steps (B), drag during swing (C), dorsal paw placement during stance (D), hip height at paw contact (E) and the hip-ankle-toe (HAT) angle excursion (F). The black bars are data from six spinally contused rats stepping at 0% and 65% body weight support and from the same rats 3 weeks after a spinal cord transection. The white bars are data from normal rats. Average ± SE are shown. 0%, 65% and N denote statistically different than 0% BWS, 65% BWS and normal respectively (p<0.05). BWS, body weight support.
We next used the weight support apparatus to lift the spinally contused rats while they stepped in the BART device. Providing weight support raised the hip position during stance (compare hip position in Fig. 3A and B). Hip height at paw contact was significantly greater at 65% BWS than 0% BWS (compare Contused 0% BWS to Contused 65% BWS in Fig. 1E). At 65% BWS, hip height was not significantly different than normal (compare Contused 65% BWS with Normal in Fig. 2E). Providing weight support to the spinally contused rats did not significantly change the number of hindlimb steps (compare Contused 0% BWS with Contused 65% BWS in Fig. 2A). However, fewer locomotor deficits occurred when weight support was provided. At 65% BWS, the occurrence of drag and dorsal placement was 2% and 17% respectively. These values were significantly lower than at 0% BWS and were similar to normal values (compare Contused 65% BWS to Contused 0% BWS and Normal in Fig. 2C and D). Providing weight support did not significantly change the excursion of the hip-ankle-toe angle (compare Contused 0% BWS with Contused 65% BWS in Fig. 2F). Hip-ankle-toe angle excursion was significantly less in the spinally contused rats than in normal rats (compare Contused 0% BWS and Contused 65% BWS with Normal in Fig. 2F).
3.2. Hindlimb-Forelimb Coordination
The ratio of hindlimb to forelimb steps was not significantly different between the spinally contused rats and Normal rats (compare Contused 0%, Contused 65% with Normal in Fig. 2B). On average, the contused rats performed one hindlimb step for each forelimb step. However, variability in hindlimb-forelimb coordination was observed in individual rats and these data are shown in Table 1. At 0% BWS, a 1:1 hindlimb to forelimb step ratio was observed in all rats (see HL:FL Ratio, 0% in Table 1). At 65% BWS, the ratio changed in five out of six rats (see HL:FL Ratio for Rats 41, 42, 49,52,53, 65% in Table 1). The mean absolute change in hindlimb to forelimb step ratio (0.17±0.04) was significant.
Table 1.
Coordination between the right HL and right FL for each spinally contused rat stepping at 0% and 65% BWS.
| HL:FL Ratio of Steps (HL steps/FL steps) | Phase Shift Right FL to Right HL | |||
|---|---|---|---|---|
| Rat | 0% BWS | 65% BWS | 0% BWS (mean ± SD) | 65% BWS (mean ± SD) |
| 41 | 1 | 1.2 | 68 ± 8.5% | 48 ± 14% |
| 42 | 1 | 0.8 | 67 ± 8.6% | 56 ± 18% |
| 49 | 1 | 1.2 | 66 ± 0.9% | 56 ± 14% |
| 52 | 1 | 0.83 | 82 ± 3.7% | 72 ± 10% |
| 53 | 1 | 1.3 | 35 ± 15% | 58 ± 8.4% |
| 62 | 1 | 1 | 62 ± 2.8% | 61 ± 18% |
We also examined phase shift between hindlimb paw contact and forelimb paw contact (see Phase Shift, Table 1). No significant difference was observed in the mean phase shifts at 0% and 65% BWS. However, there was greater variability in phase shift when rats stepped at 65% BWS (compare SD at 0% and 65%, Phase Shift in Table 1). Variability in phase shift (measured by standard deviation) was significantly greater at 65% compared to 0% BWS.
3.3. Use of BART device after spinal cord transection
We performed a complete spinal cord transection in the spinally contused rats and re-tested stepping in the BART device 3 weeks later. After spinal transection, hindlimb stepping was poor. The rats performed fewer hindlimb steps and relied on their forelimbs to move in the BART device. For example, the number of hindlimb steps/run and the hindlimb to forelimb ratio were significantly decreased after spinal transection (compare Spinal Transected with Contused 0% and 65% BWS in Fig. 2A and B). Dragging of the hindlimb and dorsal paw placement occurred through the entire step cycle (see Spinal Transected, Fig. 2C and D). Drag and dorsal placement were significantly increased after transection and significantly greater than normal (compare Spinal Transected with Contused 0% BWS, 65% BWS and Normal in Fig. 2C and D). The hip-ankle-toe angle excursion after spinal transection was significantly smaller than in the contused rats and normal rats (compare Spinal Transected with Contused 0% BWS, 65% BWS and Normal in Fig. 2F; also see Fig. 3C).
4. Discussion
4.1. Devices that promote active participation during locomotion in SCI
Compared to treadmill locomotion, overground locomotion is considered a more goal-oriented task and thus engages the supraspinal structures. Findings from a recent study support this conclusion (van den Brand et al. 2012). When combined with pharmacological treatment and spinal stimulation, overground locomotor training was superior to treadmill training in terms of improving functional recovery and enhancing cortical control of hindlimb stepping (van den Brand et al. 2012). It has been pointed out, however, that an upright posture during bipedal hindimb stepping may have played a significant role in recovery given recent comparisons of stepping during horizontal and upright postures in rats (Sławioska et al. 2012). In addition, there is growing evidence that the use of the forelimbs contributes to the recovery of hindlimb stepping in SCI rats (Juvin et al. 2012; Shah et al. 2013) Taken together, these findings indicate that devices used to facilitate SCI rodent locomotion should be designed to incorporate natural gait characteristics (i.e. posture, forelimb use) during weight-supported overground locomotion.
The present findings suggest that the BART device can be used successfully for studying quadrupedal, overground locomotion in spinally contused rats. The advantage of the device is that it consists primarily of low-cost, commercially-available components. The only custom-made component was the platform of the weight support apparatus. Because an air cylinder is used, there are no electronic parts such as computer hardware or software components used to control weight support. Another advantage is that quantitative measurements of stepping were obtained. By incorporating a small video camera with the BART device, we were able to perform basic kinematic analyses of the limb movements. In particular, hindlimb joint angles and the phase shift between the right forelimb and right hindlimb were calculated. A second camera on the opposite side could easily be incorporated to also examine homologous and diagonal phase shifts.
Compared to robotic systems, the BART device lacks sophisticated control over forces applied to the trunk (Dominici et al. 2012; Udoekwere et al. 2014). Robotic systems have flexible control over vertical, front to back and lateral trunk movements (Udoekwere et al. 2014). In contrast, the BART device only provides a vertical force offset and any other trunk movements are prevented by the sling. This did not appear to hinder walking, but the restriction of trunk movements would likely limit the speeds at which the rats could walk while in the BART device (e.g. galloping). Unlike robotic systems (Dominici et al. 2012) , the BART device only allows locomotion in the forward direction and lateral deviation is not possible. Stepping in the BART device is also constrained to a short path which meant that only 4-5 steps were performed per run. The initial thought was that it would be easier to acclimate the rats to the BART device if a short track was used. Based on the relative ease in training the rats, we believe a longer track could be used thereby increasing the number of steps.
We transected the spinal cord after the initial BART tests and found hindlimb stepping had significantly worsened when the rats were re-tested. While we realize that interpreting recovery when a spinal transection is used after an initial SCI is complex, these findings are consistent with the conclusion that active participation was necessary for hindlimb stepping in the BART device. In contrast, spinally transected rats can perform hindlimb stepping on a treadmill. The moving treadmill belt generates sensory feedback that stimulates hindlimb stepping. In addition, spinally transected rats are typically held in an upright posture (i.e. bipedal hindlimb stepping) and this also facilitates hindlimb movements on the treadmill.
4.2. The importance of weight support during locomotion after SCI
It is well known that that body weight support (BWS) improves hindlimb treadmill stepping in SCI animals. The findings of the present study suggest that the performance of overground locomotion in SCI rats was also improved by providing weight support. With weight support, less toe dragging and knee dragging was observed. A similar effect was reported by Dominici and colleagues using a robotic-controlled weight support system (Dominici et al. 2012). In that study, weight support reduced dragging and stumbling during staircase climbing. Kuerzi and colleagues likewise found improved plantar stepping when SCI rats walked in shallow water (Kuerzi et al. 2010). We agree with Kuerzi and colleagues who speculated that the importance of weight support was to “unmask” the inherent ability of the spinal circuits to generate stepping. Taken together, these findings indicated that reducing weight support has a beneficial effect on the ability of SCI rats to perform a range of locomotor tasks.
Interestingly, we found forelimb and hindlimb stepping was not tightly coupled when weight support was provided. This suggested loading on the limbs was critical for coordinating forelimb and hindlimb movements. Load-related sensory information has a powerful effect on spinally-generated locomotion (Norton and Mushahwar 2010). Recent findings have provided evidence that that forelimb movements provided drive via propriospinal connections to the circuits controlling the hindlimbs (Shah et al. 2013). Taken together, these findings suggested that hindlimb-forelimb coupling in the contused rat involved an integration of load-related sensory information from the hindlimbs with forelimb sensory information.
It is also important to recognize the role that stabilizing the trunk played in improving stepping. Moderate spinally contused rats have poor trunk control and this contributes to their inability to perform hindlimb stepping. BBB scores of moderate spinally contused rats (using the infinite horizon device) reflect a lack of weight support, sweeping of the hindlimbs and inability to coordinate the forelimbs and hindlimbs (Nessler et al. 2006). In contrast, contused rats performed weight bearing hindlimb steps and exhibited consistent hindlimb-forelimb coordination in the BART device. Stabilizing the trunk in the lateral direction helped to position the hindlimbs. Specifically, the hindpaws were in a weight bearing position under the body. This likely generated important sensory feedback used to coordinate the hindlimb-forelimb movements. This result was consistent with recent reports that robotic-assisted trunk control improved quadrupedal treadmill stepping in spinally transected rats (Udoekwere et al. 2014) and overground walking in spinally hemisected rats (Dominici et al. 2012).
4.3. Conclusion
We have developed a novel device that provides weight support while an animal performs quadrupedal, overground locomotion. Unlike robotic BWS systems, the BART device uses a pneumatic cylinder to lift the rat. Stepping in the BART device is a more natural form of gait compared to hindlimb stepping on a treadmill and this may be useful for training that aims to restore voluntary control of locomotion in SCI rats.
Highlights.
A novel device, i.e. the body weight supported ambulatory rodent trainer (BART), was developed and used to facilitate overground, weight supported stepping in spinally contused rats and normal rats.
Compared to robotic systems, the BART device is simpler and uses a pneumatic air cylinder to lift the rats while they step
When weight support was provided by the BART device, stepping quality improved in the contused rats evident by greater plantar surface stepping and a raising of the hindquarters
Stepping was poor, however, when the contused rats were re-tested three weeks after a complete spinal cord transection was performed
The findings suggest that the BART device can be used to examine the recovery of stepping in SCI rats and that successful stepping in the device required voluntary control
Acknowledgements
The authors would like to thank Mr. Koyiro Minakata for his excellent technical contributions to developing the BART device. This study was supported by NIH 1R15NS082711 awarded to RD.
Abbreviations
- BWS
Body Weight Support
- BART
Body weight supported Ambulatory Rodent Trainer
- SCI
spinal cord injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Askari S, Chao T, de Leon RD, Won DS. The effect of timing electrical stimulation to robotic-assisted stepping on neuromuscular activity and associated kinematics. J Rehabil Res Dev. 2013;50(6):875–92. doi: 10.1682/JRRD.2012.06.0111. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987 May 26;412(1):84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012 Jun 1;336(6085):1182–5. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- Cai LL, Fong AJ, Otoshi CK, Liang Y, Burdick JW, Roy RR, et al. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J Neurosci Off J Soc Neurosci. 2006 Oct 11;26(41):10564–8. doi: 10.1523/JNEUROSCI.2266-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Heng C, Reinkensmeyer DJ, Roy RR, Edgerton VR, De Leon RD. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J Neurotrauma. 2007 Jun;24(6):1000–12. doi: 10.1089/neu.2006.0233. [DOI] [PubMed] [Google Scholar]
- Dominici N, Keller U, Vallery H, Friedli L, van den Brand R, Starkey ML, et al. Versatile robotic interface to evaluate, enable and train locomotion and balance after neuromotor disorders. Nat Med. 2012 Jul;18(7):1142–7. doi: 10.1038/nm.2845. [DOI] [PubMed] [Google Scholar]
- Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, et al. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci Off J Soc Neurosci. 2005 Dec 14;25(50):11738–47. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J, Rossignol S. The locomotion of the low spinal cat. II. Interlimb coordination. Acta Physiol Scand. 1980 Mar;108(3):283–95. doi: 10.1111/j.1748-1716.1980.tb06534.x. [DOI] [PubMed] [Google Scholar]
- Górska T, Zmysłowski W, Majczyoski H. Overground locomotion in intact rats: interlimb coordination, support patterns and support phases duration. Acta Neurobiol Exp (Warsz) 1999;59(2):131–44. doi: 10.55782/ane-1999-1304. [DOI] [PubMed] [Google Scholar]
- Heng C, de Leon RD. Treadmill training enhances the recovery of normal stepping patterns in spinal cord contused rats. Exp Neurol. 2009 Mar;216(1):139–47. doi: 10.1016/j.expneurol.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvin L, Le Gal J-P, Simmers J, Morin D. Cervicolumbar coordination in mammalian quadrupedal locomotion: role of spinal thoracic circuitry and limb sensory inputs. J Neurosci Off J Soc Neurosci. 2012 Jan 18;32(3):953–65. doi: 10.1523/JNEUROSCI.4640-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerzi J, Brown EH, Shum-Siu A, Siu A, Burke D, Morehouse J, et al. Task-specificity vs. ceiling effect: step-training in shallow water after spinal cord injury. Exp Neurol. 2010 Jul;224(1):178–87. doi: 10.1016/j.expneurol.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leon RD, Acosta CN. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J Neurotrauma. 2006 Jul;23(7):1147–63. doi: 10.1089/neu.2006.23.1147. [DOI] [PubMed] [Google Scholar]
- De Leon RD, Kubasak MD, Phelps PE, Timoszyk WK, Reinkensmeyer DJ, Roy RR, et al. Using robotics to teach the spinal cord to walk. Brain Res Brain Res Rev. 2002a Oct;40(1-3):267–73. doi: 10.1016/s0165-0173(02)00209-6. [DOI] [PubMed] [Google Scholar]
- De Leon RD, Reinkensmeyer DJ, Timoszyk WK, London NJ, Roy RR, Edgerton VR. Use of robotics in assessing the adaptive capacity of the rat lumbar spinal cord. Prog Brain Res. 2002b;137:141–9. doi: 10.1016/s0079-6123(02)37013-4. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp Neurol. 1986 May;92(2):421–35. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Nessler JA, De Leon RD, Sharp K, Kwak E, Minakata K, Reinkensmeyer DJ. Robotic gait analysis of bipedal treadmill stepping by spinal contused rats: characterization of intrinsic recovery and comparison with BBB. J Neurotrauma. 2006 Jun;23(6):882–96. doi: 10.1089/neu.2006.23.882. [DOI] [PubMed] [Google Scholar]
- Norton JA, Mushahwar VK. Afferent inputs to mid- and lower-lumbar spinal segments are necessary for stepping in spinal cats. Ann N Y Acad Sci. 2010 Jun;1198:10–20. doi: 10.1111/j.1749-6632.2010.05540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PK, Garcia-Alias G, Choe J, Gad P, Gerasimenko Y, Tillakaratne N, et al. Use of quadrupedal step training to re-engage spinal interneuronal networks and improve locomotor function after spinal cord injury. Brain J Neurol. 2013 Nov;136(Pt 11):3362–77. doi: 10.1093/brain/awt265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sławioska U, Majczyoski H, Dai Y, Jordan LM. The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J Physiol. 2012 Apr 1;590(Pt 7):1721–36. doi: 10.1113/jphysiol.2011.224931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timoszyk WK, De Leon RD, London N, Roy RR, Edgerton VR, Reinkensmeyer DJ. The rat lumbosacral spinal cord adapts to robotic loading applied during stance. J Neurophysiol. 2002 Dec;88(6):3108–17. doi: 10.1152/jn.01050.2001. [DOI] [PubMed] [Google Scholar]
- Timoszyk WK, Nessler JA, Acosta C, Roy RR, Edgerton VR, Reinkensmeyer DJ, et al. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 2005 Jul 19;1050(1-2):180–9. doi: 10.1016/j.brainres.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Udoekwere UI, Oza CS, Giszter SF. A pelvic implant orthosis in rodents, for spinal cord injury rehabilitation, and for brain machine interface research: construction, surgical implantation and validation. J Neurosci Methods. 2014 Jan 30;222:199–206. doi: 10.1016/j.jneumeth.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]