Abstract
Recent studies suggest that megakaryocytes (MKs) may play a significant role in skeletal homeostasis, as evident by the occurrence of osteosclerosis in multiple MK related diseases (Thiele, et al., 1999, Lennert, et al., 1975, Chagraoui, et al., 2006). We previously reported a novel interaction whereby MKs enhanced proliferation of osteoblast lineage/osteoprogenitor cells (OBs) by a mechanism requiring direct cell-cell contact. However, the signal transduction pathways and the downstream effector molecules involved in this process have not been characterized. Here we show that MKs contact with OBs, via beta1 integrin, activate the p38/MAPKAPK2/p90RSK kinase cascade in the bone cells, which causes Mdm2 to neutralizes p53/Rb-mediated check point and allows progression through the G1/S. Interestingly, activation of MAPK (ERK1/2) and AKT, collateral pathways that regulate the cell cycle, remained unchanged with MK stimulation of OBs. The MK-to-OB signaling ultimately results in significant increases in the expression of c-fos and cyclin A, necessary for sustaining the OB proliferation. Overall, our findings show that OBs respond to the presence of MKs, in part, via an integrin-mediated signaling mechanism, activating a novel response axis that de-represses cell cycle activity. Understanding the mechanisms by which MKs enhance OB proliferation will facilitate the development of novel anabolic therapies to treat bone loss associated with osteoporosis and other bone-related diseases.
Keywords: Osteoblasts, Megakaryocytes, Mdm2, Cell cycle regulation, Signaling pathways
INTRODUCTION
There are several known mouse models that implicate megakaryocytes (MKs) in regulating skeletal homeostasis. Mice in three mouse models have an increase in bone marrow megakaryopoiesis which results in significant increases in bone volume due to increases in bone formation. Overexpression of thrombopoietin (TPO), the main MK growth factor, causes a dramatic increase in bone marrow MK number, and the mice develop an osteosclerotic bone phenotype with increased bone mineral density (Frey, et al., 1998a, Frey, et al., 1998b, Yan, et al., 1995, Yan, et al., 1996, Villeval, et al., 1997). Mice lacking the transcription factors GATA-1 or NF-E2, which are necessary for normal MK differentiation, develop a marked increase in bone marrow MK number with a concomitant reduction in platelet number and a dramatic increase in trabecular bone volume (Shivdasani, et al., 1995, Shivdasani, et al., 1997, Kacena, et al., 2004, Kacena, et al., 2005).
Platelet-type von Willebrand disease (Pt-vWD) is an inherited genetic disease that affects platelets and a mouse model was created that resembles this human condition. These mice exhibit a marked increase in splenic MKs with splenomegaly, and a high bone mass phenotype with decreased serum measures of bone resorption (Suva, et al., 2008). Of note, when bone marrow (as opposed to splenic) MK number is elevated, bone formation is increased, which also leads to a high bone mass phenotype (Shivdasani, et al., 1995, Shivdasani, et al., 1997, Kacena, et al., 2004). Therefore, these mouse models (TPO, GATA-1, and NF-E2) suggest that in order for anabolic bone formation to occur, MKs must be present in the bone marrow where they can influence proliferation of osteoblast lineage cells or osteoprogenitors, termed OB from here on.
The ability of MKs to stimulate bone formation in vivo is further illustrated in adoptive transfer studies in which irradiated wild-type mice were reconstituted with spleen cells from NF-E2 deficient mice. NF-E2 is a transcription factor required for normal MK development. NF-E2 deficient mice have approximately a 5-fold increase in immature MK number, 5% of the normal numbers of platelets, and 2–3-fold increase in bone mass (Shivdasani, et al., 1995, Kacena, et al., 2004, Kacena, et al., 2005). This same phenomena was also recently reported, whereby spleen cells from GATA-1 deficient mice were transplanted into wild-type mice and a high bone mass phenotype was observed (Cheng, et al., 2013). In each of these models, both the hematologic phenotype and the high bone mass phenotype were adoptively transferred, suggesting a role for hematopoietic cells in this mechanism, most likely MKs (Kacena, et al., 2005). More recently Dominici et al (Dominici, et al., 2009, Olson, et al., 2013) demonstrated that a substantial number of MKs preferentially survive in mice following exposure to potentially lethal doses of radiation. Surviving host MKs migrate to endosteal surfaces in bone where they stimulate a 2-fold increase in OB number thus augmenting the so-called endosteal hematopoietic stem cell niches. Contact between MKs and OBs and/or their precursors have been described (Cheng, et al., 2000, Miao, et al., 2004, Kacena, et al., 2004, Ciovacco, et al., 2009, Ciovacco, et al., 2010, Lemieux, et al., 2010, Dominici, et al., 2009, Kacena, et al., 2012, Cheng, et al., 2013). As an example, Cheng et al. (Cheng, et al., 2000) observed that when isolating bone marrow stromal cells (BMSCs), complexes existed consisting of BMSCs and MKs, demonstrating a physical association between these cells. Furthermore, our group (Kacena, et al., 2004, Ciovacco, et al., 2009, Ciovacco, et al., 2010, Lemieux, et al., 2010, Kacena, et al., 2012, Cheng, et al., 2013) and others (Miao, et al., 2004) have demonstrated that MKs significantly enhance OB proliferation and/or osteoblastogenesis, respectively, in vitro by a mechanism which requires direct MK-OB cell-cell contact. We have also shown the importance of β1 integrin engagement and Pyk2 signaling in MK-mediated OB proliferation (Lemieux, et al., 2010).
Taken together, these observations suggest that MK-OB interactions increase OB proliferation and subsequent bone formation, although how MKs enhance OB proliferation remains to be determined. Of importance, a significantly larger percentage of OBs co-cultured with MKs has been shown to reside in the S phase of cell cycle compared to OBs cultured alone (Kacena, et al., 2004, Kacena, et al., 2012). Because there is an increase in the number of cells in S phase and cell proliferation is a highly regulated process, we further explored cell cycle regulation in MK-stimulated enhancement of OB number and sought to dissect the associated signaling proteins, which govern this process.
MATERIALS AND METHODS
Mice
C57BL/6 mice originally purchased from Jackson Laboratories were used to generate primary cells for these studies. All studies were carried out in compliance with procedures approved by either the Indiana University or Yale University Institutional Animal Care and Use Committees and in accordance with NIH guidelines.
Preparation of fetal liver derived megakaryocytes
Murine MKs were prepared as previously described (Kacena, et al., 2004, Kacena, et al., 2006). In brief, fetuses were dissected from pregnant mice at E13–15. The livers were removed and single cell suspensions made by forcing cells through sequentially smaller gauge needles (18G, 20G, 23G). Cells were washed 2X with DMEM+10% FCS and then seeded (5 fetal livers/100 mm dish) in 100mm culture dishes, in DMEM+10% FCS+1% murine TPO (Villeval, et al., 1997, Kacena, et al., 2006). After 3–5 days, when the cells became confluent, MKs were obtained by separating them from the lymphocytes and other cells using a one-step albumin gradient to obtain a 95% pure MK population (Drachman, et al., 1997). The bottom layer was 3% albumin in PBS (Bovine Albumin, protease free, fatty acid poor, Serologicals Proteins Inc., Kankakee, IL), the middle layer was 1.5% albumin in PBS, and the top layer was media containing the cells to be separated. All of the cells sedimented through the layers at 1 g for approximately 40 min at room temperature. The MK fraction was collected from the bottom of the tube.
Preparation of neonatal calvarial cells (OBs)
Murine calvarial cells were prepared as previously described (Horowitz, et al., 1994, Ciovacco, et al., 2009). Our technique was a modification of the basic method described by Wong and Cohn (Wong and Cohn, 1975). Briefly, calvaria from mice less than 48 hours-old were pretreated with EDTA in PBS for 30 minutes. The calvaria were then subjected to sequential collagenase digestions. Cells were collected following incubation in collagenase. Fractions 3–5 were used as the initial population. Although heterogeneous, cells collected from later fractions are enriched for osteogenic potential (Towler and St. Arnaud, 2002). These cells were ~95% OB or OB precursors by a variety of criteria (Horowitz, et al., 1994, Simmons, et al., 1982, Jilka and Cohn, 1981) and are referred to as OBs in this manuscript. Freshly prepared OBs were used for all studies and were cultured in α-MEM with 10% FCS. It should be noted that fraction 1 was also cultured as detailed for fractions 3–5. Cells from fraction 1 were used in a series of experiments to determine whether heterogeneous cells containing a higher concentration of fibroblastic cells were also stimulated by MKs. Proliferation was assessed via tritium incorporation as detailed below. Freshly prepared OBs were used for all studies and were cultured in α-MEM with 10% FCS.
Preparation of Bone Marrow Stromal Cells
Tibiae and femurs were dissected from 6–10 week-old C57BL/6 mice, the epiphyses removed, and the marrow flushed with 2–3 mls of ice cold α-MEM with 10% FCS using a 27 gauge needle and syringe. A single cell suspension was prepared and the cells were washed twice prior to use. Five million cells/well were seeded into a 24-well plate and MKs were titrated into wells. To provide osteogenic conditions, cells were cultured in αMEM supplemented with 10% FCS which was further supplemented with ascorbic acid (50μg/ml added on day 0 and at all medium changes) and β-glycerophosphate (5mM added starting on day 7 and all subsequent medium changes). On day 14, cultures were stained for alkaline phosphatase and alkaline phosphatase+ colonies containing ≥25 cells were enumerated. Additionally, BMSCs were also seeded into 96-well plates and MKs were titrated into wells and proliferation assessed via tritium incorporation as detailed below.
Proliferation Assay
To examine the proliferative capacity of cells of the OB lineage neonatal calvarial OBs (primarily used in the studies throughout and referred to as OBs) were seeded at 2500 cells/well (optimal, pretested), cells from fraction 1 of neonatal calvarial digestions containing increased numbers of fibroblasts (termed Fraction 1) were seeded at 1250 cells/well (optimal, pretested), and BMSCs were seeded at 4000 cells/well (optimal, pretested). Cells of the OB lineage were cultured with and without MKs (2500 or 5000 MKs/well, optimal, pretested), cells were seeded in triplicate into 96-well tissue culture plates and incubated for up to 6 days at 37°C in α-MEM supplemented with 10% FCS. Proliferation was measured daily by the incorporation of 3H-thymidine (1 μCi/well; 5–8 Ci/mmol) added during the last 16 hours of culture (Centrella, et al., 1991). To assess OB proliferation alone, MK were removed from wells (4 washes) prior to measuring incorporation of 3H-thymidine (Kacena, et al., 2004).
Cell Cycle Analysis
Calvarial OBs were cultured in the presence or absence of fetal liver-derived MKs. Following culture for 1–3 days, MKs were removed from OBs by forceful washing (4 washes with PBS, results in >90% purity of OB lineage cells (Kacena, et al., 2004)) and OBs alone were analyzed. Cells were stained with equal volumes of staining buffer (0.1mg/ml PI + 0.6% Nonidet P40 in PBS) and 2 mg/ml RNase as described previously (Srour, et al., 1992). The cells were triturated and incubated on ice for 30 minutes. Data were collected on FACS caliber flow cytometer (BDIS) and the percentage of cells in G0/G1 and S/G2+M phases were determined by Modfit.
Apoptosis analysis
Cells were washed once in Dulbecco modified eagle medium (DMEM) followed by the addition of Annexin-V (BioLegend) conjugated with Aallophycocyanin (APC). Cells were incubated on ice for 15 minutes, then washed and resuspended in DMEM followed by the addition of 10μl of 10ng/ml Propidium Iodide. Cells were incubated at room temperature for an additional 10 minutes and data were collected on FACS caliber flow cytometer (BDIS).
Western blot analysis
Western blot was performed by one of two methods. In the first method, OBs were cultured alone or co-cultured with MKs for 5 or 6 days. In some studies soluble tetrapeptide Arg-Gly-Asp-Ser (RGDS; Sigma-Aldrich), an activating β1 integrin antibody (TS2/16; BioLegend, Inc), or IgG1 antibody (BioLegend, Inc.) were titrated into MK-OB co-cultures and OB control cultures. Following respective treatments, cells were lysed in a nonionic detergent buffer (40 mM Tris, 150 mM NaCl, 1% Igepal) supplemented with protease inhibitors and sodium orthovanadate. Protein concentrations were measured using the Bradford Reagent (Biorad Laboratories). Lysates were boiled in laemmli buffer for 10 minutes, fractionated using SDS-PAGE and transferred to PVDF membrane (GE Healthcare). Western blots were blocked for 1 hour at room temperature in 5% non-fat dry milk in PBST. The blots were incubated with primary antibodies for 2 hours at room temperature. Mdm2 (2A10, 4B11) and GAPDH (6C5) antibodies were purchased from Calbiochem. Phosphorylated p90RSK (Ser380), phosphorylated p38 (Thr180/Tyr182), phosphorylated MAPKAPK2 (Thr334) and phosphorylated Elk-1 (Ser383) antibodies were purchased from Cell Signaling. Rb (H-2 or C-15), p53 (FL-393), and Vinculin (N-19) were purchased from Santa Cruz Biotechnology. The blots were then washed in PBST and subsequently incubated with secondary antibody linked to HRP (Biorad Laboratories). Proteins were detected by chemiluminescence (PerkinElmer).
The second method was used for expression of c-fos and cyclin A. In brief, in some studies, OBs were cultured alone or were co-cultured with MKs for 1 day, 3 days or 5 days. Then MKs were removed by washing (4 times with PBS) and in all cultures OB lysates were collected as detailed below. Alternatively, for the short stimulation studies, OBs were cultured until they were ~70% confluent, serum starved overnight (0.1% FCS), and then stimulated the next day for 30, 60 or 120 minutes with MKs, where appropriate. For all cultures, MKs were removed by washing (4 times with PBS). Cell lysates were collected in SDS-PAGE lysis buffer with urea and boiled for approximately 3 minutes. Protein concentrations were measured using the amido black method. The proteins were fractionated via SDS-PAGE and transferred to nitrocellulose. They were then probed for c-fos (SC-52), purchased from Santa Cruz Biotechnology, or cyclin A (#611269), purchased from BD Transduction Laboratories, using the Fujifilm LAS-3000 imager. The HRP-tagged secondary antibodies were purchased from Jackson Immunoresearch. The blots were stripped and reprobed for vinculin (VIN-11-5, Sigma, V 4505) to demonstrate equal protein loading.
Statistics
Unless otherwise stated, all data are presented as the Mean ± SD. Student’s t-test was used to determine significant differences, with p<0.05 (Systat 6.0 for Microsoft Windows, SPSS Inc., Chicago). Within individual experiments, data points are based on a minimum of triplicate representative samples and all experiments were repeated at least once.
RESULTS
Megakaryocytes promote osteoblastogenesis and proliferation of several populations of osteoblast lineage cells in vitro
While we have previously demonstrated that MKs stimulate 2 day calvarial OB proliferation (Kacena, et al., 2004, Ciovacco, et al., 2009, Ciovacco, et al., 2010, Lemieux, et al., 2010); here we sought to determine whether MKs could promote osteoblastogenesis from primary bone marrow cells. To accomplish this we isolated bone marrow cells and cultured them under osteogenic conditions (supplemented cultures with ascorbic acid and β-glycerophosphate) in the presence or absence of MKs (10,000 MKs/well). Data from 2 separate studies (n=3/group/study) showed that on day 14, there were significantly (p=0.01) more CFU-F in bone marrow cultures supplemented with MKs (21.5±0.7) as compared to those cultured alone (13.0±1.0), and as would be expected under osteogenic culture conditions (Gronthos, et al., 1994), all of the colonies were also alkaline phosphatase positive. As CFU-F is an indicator of the proliferative and clonogenic capacity of the cells and alkaline phosphatase is an indicator of osteogenic colonies, these data suggest that MKs stimulate a significant increase in progenitor cells giving rise to osteogenic cells (p=0.01). These data suggest that not only do MKs stimulate the proliferation of OBs derived from 2 day calvariae, but they can also promote osteoblastogenesis from bone marrow cells, a more physiologic model of in vivo conditions.
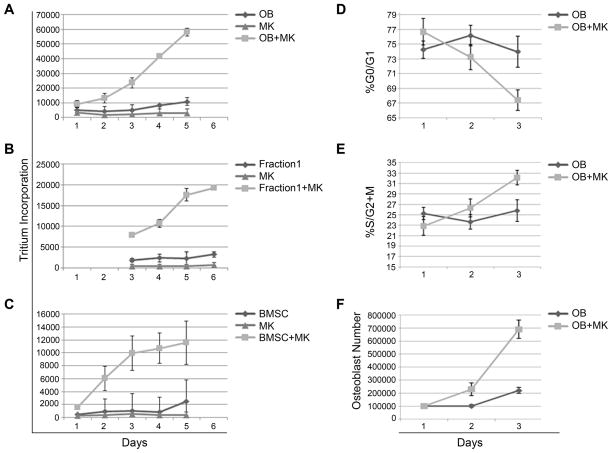
Next we wanted to determine whether MKs can regulate proliferation of several different populations of OB lineage cells. Here we compared the proliferation of 2 day calvarial OBs (as we have previously described, Fractions 3–5) with Fraction 1 cells which contain increased numbers of fibroblastic cells (Kacena, et al., 2004, Ciovacco, et al., 2009, Ciovacco, et al., 2010, Lemieux, et al., 2010). As illustrated in Figures 1A&B, both 2 day calvarial OB and Fraction 1 cell proliferation is markedly elevated when cells are co-cultured with MKs. Although experimental duration varied slightly between the experiments, with both isolated populations, MKs were able to significantly enhance osteogenic cell proliferation by day 3 and the enhancement persisted through the termination of the study (day 5 or 6). It should be noted that proliferation studies were concluded between days 5 and 6 as cells cultured as described here become confluent between days 4–5 for cells co-cultured with MKs. At this time contact inhibition begins, whereby resulting in a reduction in OB proliferation and OBs begin to differentiate. As detailed in Figure 1C, when bone marrow stromal cells (BMSCs) were co-cultured with MKs, osteogenic cell proliferation was markedly enhanced by day 2 of culture. All three populations of cells tested represent heterogeneous osteogenic populations at the earlier stages of the OB lineage (e.g. progenitor or immature OBs primarily), the proliferative OB lineage cells.
Figure 1.
Two day calvarial OBs obtained from Fractions 3–5 (A), Fraction 1 or cells collected from the first collagenase digestion in 2 day calvarial OB preparations (B), and bone marrow stromal cells (BMSCs) were cultured in the presence or absence of MKs and tritium incorporation was determined as a measure of proliferation. By day 3 of co-culture, MKs significantly enhanced proliferation of two day calvarial OBs (A), cells contained in Fraction 1 (B), and BMSCs (C). In Figures 1D–F, two day calvarial OBs were cultured alone or in the presence of MKs. MKs were removed and OB cell cycle status was assessed daily by staining OBs with propidium iodide. Co-culture with MKs induced a higher rate of proliferation and cycling of OBs as demonstrated by the accelerated rate at which OBs entered S/G2+M (D) and exited G0/G1 (E) phases of the cell cycle. This enhanced cycling of OBs co-cultured with MKs resulted in an increased number of cells as compared to OBs cultured alone (F). In data not shown, we also analyzed these cells for the expression of Annexin V. In all cases, less than 2% of the OBs were Annexin V positive, suggesting that apoptosis was not detected.
Megakaryocytes promote cell cycle progression in osteoblast lineage cells
As MKs were able to promote OB lineage number in all three OB lineage populations tested, we then used the well-characterized 2 day calvarial OB lineage cells for further studies. Specifically, for 2 day calvarial OBs stimulated by MKs to increase in number two possible events could occur: a protection of OBs whereby the cells are protected from apoptosis, or an increase in cell cycle progression. We tested whether OBs were undergoing significant apoptosis by analyzing Annexin V positive cells by flow cytometry. We found that less than 2% of OBs were undergoing apoptosis in any of the cultures studied (presence or absence of MKs, data not shown). Next, to investigate the role of MKs in increasing OB number, we analyzed cell cycle progression of OBs cultured in the presence or absence of MKs over 3 days (Figure 1D–F). OB cell cycle status was assessed daily by staining OBs with propidium iodide and analyzing samples using flow cytometry. Of note, we removed MKs prior to flow cytometry. As illustrated in Figure 1E, while 26% of OBs maintained in culture were in the S/G2+M phases of cell cycle on day 3, a marked increase was observed in OBs co-cultured with MKs (33% of OBs were in the S/G2+M phases). Further analysis showed that on day 3, co-culture of MKs resulted in a 165% increase in viable OB number as compared to OBs cultured alone (p=0.0008, Figure 1F). It should be noted that between days 4 and 5 OBs co-cultured with MKs reach confluence and therefore contact inhibition occurs, and in general, OBs begin to differentiate. Therefore, we restricted some of our analyses to cultures in which proliferation was occurring.
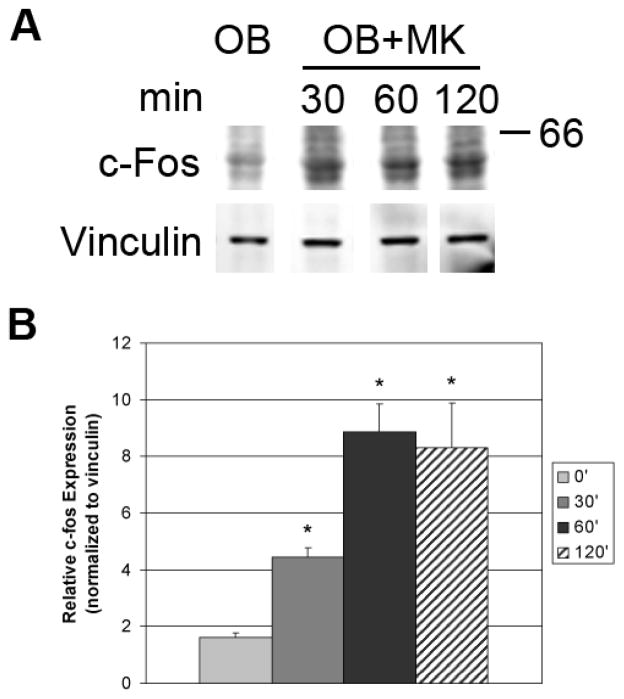
Transcription factors that are involved in S phase movement are necessary to induce expression of numerous genes that are required for proliferation. c-fos and c-Jun comprise a heterodimer referred to as AP-1, which is often assessed as an early marker of proliferation. Upregulation of c-fos is associated with increased transcription of genes involved in cell proliferation. We examined the levels of c-fos in two day calvarial OB lineage cells stimulated with MKs. As illustrated in Figure 2, c-fos levels in OBs were rapidly elevated as early as 30 minutes (> 2-fold increase) and were further elevated with 60 minutes of stimulation (> 4-fold increase) in the presence of MKs. The heightened levels of c-fos were sustained over 120 minutes. Collectively, MKs activated an increase in c-fos and an increase in OBs in S phase which increases OB number as a result of proliferation.
Figure 2.
At ~70% confluence, OBs were serum starved overnight and MKs were added to OBs cultured for indicated times, then removed by washing. OBs were lysed and resolved by SDS-PAGE. Western blotting demonstrates a time-dependent increase in c-fos expression in OBs stimulated with MKs. Blots shown in Figure 2A are representative of 2 separate experiments with triplicate samples which are quantitated in Figure 2B.
Megakaryocytes reduce p53 and Rb protein levels in osteoblasts
Activation of transcription factors is important for proliferation, but equally important is the downregulation of factors that prevent cell cycle movement. Two tumor suppressors, p53 and Rb, are known to play a role in arresting the cell cycle. Here we examined whether p53 and Rb levels were altered in OBs co-cultured with MKs. OBs were cultured alone or were co-cultured with MKs for 5 or 6 days and MKs were removed. Western blot analyses (Figure 3) illustrate that the levels of p53 and Rb were appreciably reduced relative to GAPDH. As Mdm2 has been shown to facilitate p53 and Rb destabilization (Kubbutat, et al., 1997, Sdek, et al., 2005), we next examined whether p53 and Rb levels corresponded with Mdm2 levels. As seen in Figure 3, Mdm2 levels were markedly reduced in OBs co-cultured with MKs as compared to those cultured alone. Mdm2 is a known ubiquitin ligase. When Mdm2 is activated through interactions with other enzymes and higher order complexes it not only can conjugate ubiquitin to substrates, but can also be targeted for destruction. Thus, loss of Mdm2 protein levels with loss of p53 and Rb levels is consistent with the activation of the ubiquitin ligase activity of Mdm2.
Figure 3.
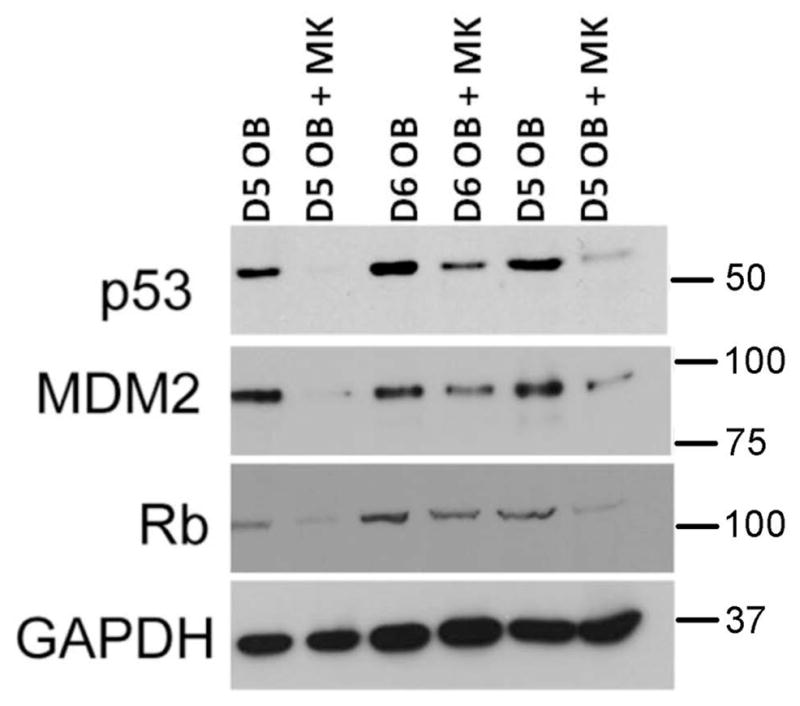
OBs were cultured alone or in the presence of MKs for 5 or 6 days. MKs were removed by washing and OBs were lysed and resolved by SDS-PAGE. Western blotting shows a significant reduction in Mdm2, p53 and Rb protein levels in OBs co-cultured with MKs as compared to OBs cultured alone. It should be noted that the lanes in this blot represent cell lysates from 3 separate experiments (2 at day 5 and 1 at day 6). In all experiments conducted (more than 10 to date) with 4–6 days of culture, co-culture with MKs always results in a marked reduction in Mdm2, p53, and Rb protein levels in OBs.
Role of ƒβ1 Integrin Signaling in OBs on the Expression of p53 and Rb
We have previously shown that MKs stimulate 2 day calvarial OB proliferation by a mechanism that requires direct MK-OB cell-cell contact and the engagement of integrins (Kacena, et al., 2004, Ciovacco, et al., 2009, Ciovacco, et al., 2010, Lemieux, et al., 2010). Specifically, our findings implicated the involvement of fibronectin/RGD-binding integrins including α3β1 and α5β1. In our previous studies we showed that titration of RGDS into OB-MK co-cultures caused a dose-dependent decrease in MK-mediated OB proliferation without affecting OB cultures. Therefore, in these studies we wanted to determine whether addition of RGDS to co-cultures would impact cell cycle regulation as assessed by a modulation in p53 expression. As shown in Figure 4, and as detailed in Figure 3, co-culture of OBs with MKs results in a marked reduction in p53 expression. Of note, titration of RGDS into co-cultures resulted in a dose-dependent increase in p53 expression, although it was unable to return p53 levels to that observed in cultures of OBs alone.
Figure 4.
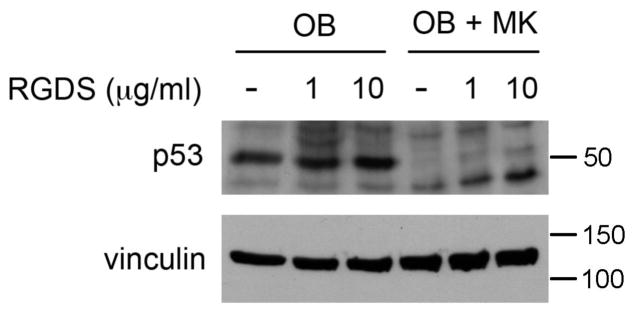
OBs were cultured alone or in the presence of MKs for 5 days. Some cultures were treated with 1 or 10 μl of RGDS (daily), while others remained untreated. MKs were removed by washing and OBs were lysed and resolved by SDS-PAGE. Western blotting shows a significant reduction in p53 protein levels in OBs co-cultured with MKs as compared to OBs cultured alone. Treatment of OBs with RGDS did not alter p53 protein expression whereas treatment of co-cultures with RGDS resulted in a dose-dependent increase in p53 expression.
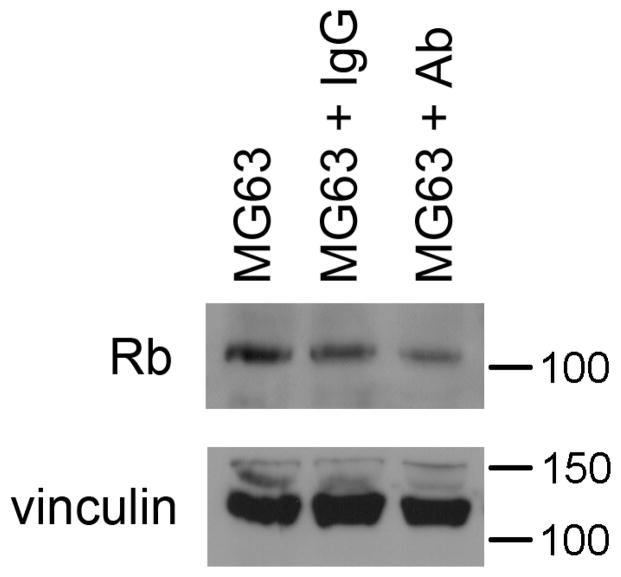
Next we further assessed the role of β1 integrin signaling and the subsequent downstream alterations in cell cycle regulators in OBs. To accomplish this we cultured a human OB-like cell line, MG63 cells (ATCC), with the β1 integrin-activating antibody (TS2/16). As illustrated in Figure 5, we were able to mimic the same trend seen when OBs were stimulated with MKs (which activates β1 integrin signaling), in the MG63 cells, where we saw a reduction in Rb. It should be noted that MG63 cells do not express p53. Together with the RGDS data, the activating antibody data suggest that β1 integrin signaling is upstream of cell cycle regulators p53 and Rb.
Figure 5.
Human OB-like cells (MG63 cell line) were treated with 10 μg/ml of TS2/16, a β1 integrin activating antibody or 10 μg/ml IgG1 control. OBs were lysed and resolved by SDS-PAGE. Western blotting shows a significant reduction in Rb protein expression in OBs treated with TS2/16 as compared to OBs treated with IgG1 or untreated OBs. MG63 cells do not express p53 (data not shown).
Activation of the p38/MAPKAPK2/p90RSK pathway in megakaryocyte-stimulated osteoblasts
For Mdm2 to function as a ubiquitinating enzyme, it must receive activation signals from kinases. Mdm2 is phosphorylated by AKT, p90RSK, and MAPKAPK2 (Mayo and Donner, 2001, Jackson, et al., 2006, Weber, et al., 2005). Dependent on the stimulation, AKT or MAPKAPK2 can facilitate Mdm2 translocation from the cytoplasm to the nucleus (Mayo and Donner, 2001, Weber, et al., 2005). On the other hand, nuclear to cytoplasmic export of Mdm2 is regulated by p90RSK (Jackson, et al., 2006). These kinases are also important for the Mdm2 mediated ubquitination of p53 and the ultimate destruction of p53. To further analyze the signaling transduction pathways that are activated when OBs are co-cultured with MKs for 5 days, we examined by Western blot active kinases known to regulate Mdm2 (Figure 6). Activation of p38 was observed. Downstream kinases that are activated by p38, p90RSK and MAPKAPK2, were both activated when OBs were co-cultured with MKs. We also analyzed Elk-1 as a positive control to verify the activation of these kinases. Phosphorylated ERK1/2 and AKT levels remained unchanged when MKs were co-cultured with OBs (data not shown). Thus, the activation of MAPKAPK2 and p90RSK pathways show a new signaling pathway in OBs that is activated specifically by MKs and also results in the destruction of p53, Rb, and Mdm2 for cell cycle progression.
Figure 6.

OBs were cultured alone or in the presence of MKs for 5 days. MKs were removed by washing and OBs were lysed and resolved by SDS-PAGE. Western blotting shows the intracellular signaling proteins activated in OBs co-cultured with MKs for 5 days. Phosphorylated p38, MAPKAPK2 (MK2), p90RSK, and Elk1 were all activated. ERK1/2 and AKT remained unaltered, data not shown.
Cyclin A levels are increased in megakaryocyte-stimulated osteoblasts
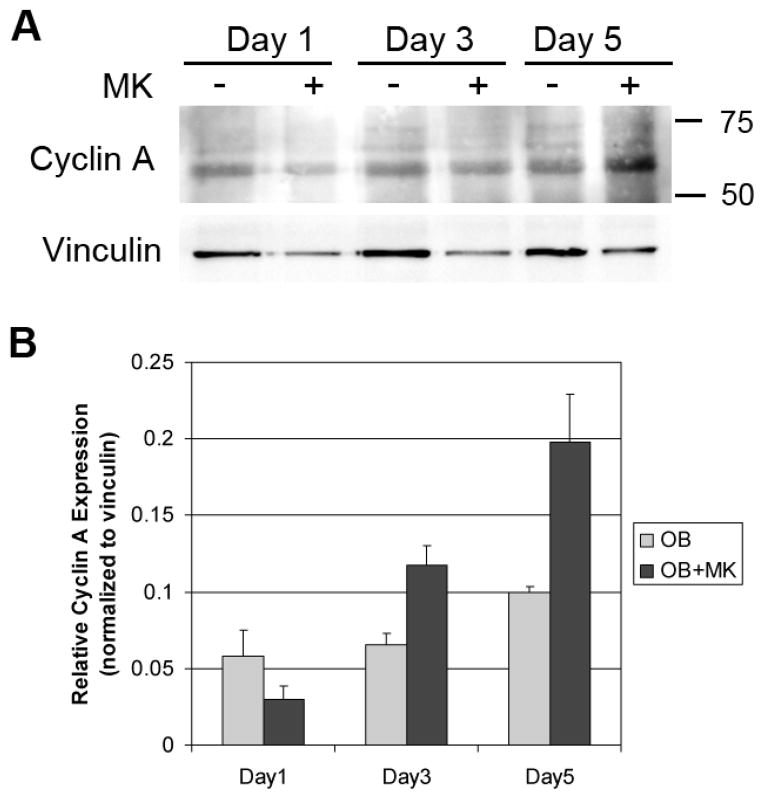
Mdm2 has been implicated in the regulation of cyclin A expression for progression through S phase of the cell cycle (Frum, et al., 2009). It is known that degradation of hyperphosphorylated Rb can activate E2F, which in turn can enhance transcription of multiple cell cycle genes including cyclin A. Because cyclin A is also known to be required for progression through S phase and considering the link of Mdm2 and cyclin A, we examined cyclin A levels in OBs cultured with and without MKs. The data in Figure 7 show that OBs cultured alone had an increase in cyclin A levels over time, and co-culture of OBs with MKs resulted in a more robust increase in cyclin A expression over time.
Figure 7.
OBs were cultured alone or in the presence of MKs for 1, 3, or 5 days. MKs were removed by washing and OBs were lysed and resolved by SDS-PAGE. Western blotting demonstrates a time-dependent increase in cyclin A expression in OBs treated with MKs. Blots shown in Figure 7A are representative of 2 separate experiments with triplicate samples which are quantitated in Figure 7B.
DISCUSSION
Delineating the functional role of megakaryocytic cells in regulating OB homeostasis has important implications for correcting pathophysiological conditions such as osteoporosis. Our group as well as others have been working to understand how MKs regulate bone mass. We previously reported that MKs, co-cultured with OBs, were able to stimulate OB proliferation (Kacena, et al., 2004, Ciovacco, et al., 2009, Ciovacco, et al., 2010, Lemieux, et al., 2010, Kacena, et al., 2012, Cheng, et al., 2013). Of note, Miao et al (Miao, et al., 2004) demonstrated the bone marrow stromal cell aggregates containing MKs expressed higher levels of alkaline phosphatase than did single cell suspensions. Further, their studies suggested that for maximal osteogenic differentiation, bone marrow stromal cells require direct contact with MKs. Here we show that in single cell primary bone marrow suspensions that osteoblastogenesis is promoted when MKs are added to the cultures, confirming the physiologic importance of MKs in regulating OBs. As stated earlier, our previous studies demonstrated the importance of direct cell-cell contact and β1 integrin signaling in MK-mediated proliferation of 2 day calvarial OBs (Kacena, et al., 2004, Ciovacco, et al., 2009, Ciovacco, et al., 2010, Lemieux, et al., 2010, Kacena, et al., 2012, Cheng, et al., 2013). The identification of several key components that are specific to MK-mediated OB proliferation have yet to be determined. In this study we showed that co-culture with MKs induced a higher rate of proliferation and cycling of OBs as demonstrated by the accelerated rate at which OBs exited G0/G1 phases of cell cycle. The greater number of cells progressing through the cell cycle resulted in a significant increase in OB number by day 3 of co-culture in the MK-stimulated OB cultures as compared to OBs cultured alone.
As cell cycle progression is a tightly regulated process, we next investigated whether the observed enhancement in OB proliferation was due to the disruption of key cell cycle regulators. We found that Rb and p53 were both decreased when MKs were co-cultured with OBs (Figure 3). Of interest, as we previously published, MK-mediated enhancement of OB proliferation involves β1 integrin signaling (Kacena, et al., 2004, Ciovacco, et al., 2009, Ciovacco, et al., 2010, Lemieux, et al., 2010, Kacena, et al., 2012, Cheng, et al., 2013). Here we demonstrate that β1 integrin signaling also alters the downstream cell cycle regulators, p53 and Rb (Figures 4 and 5). The loss of these cell cycle regulators correlated with an increase in OB cell number and the percentage of OBs in S phase was markedly increased (Figure 1). This suggests that MK-mediated OB proliferation was promoted by decreasing the levels of these two key regulators of cell cycle.
Rb and p53 are well-known tumor suppressors that control cell cycle arrest (Hartwell and Kastan, 1994, Weinberg, 1995, Hollingsworth, et al., 1993a, Hollingsworth, et al., 1993b). Both Rb and p53 proteins have been shown to regulate cell cycle at the G0/G1 phases (Goodrich and Lee, 1992, Goodrich, et al., 1991, Hinds, et al., 1992, Gjetting, et al., 1995, Diller, et al., 1990, Mercer, et al., 1990)(Goodrich and Lee, 1992, Goodrich, et al., 1991, Hinds, et al., 1992, Gjetting, et al., 1995, Diller, et al., 1990, Mercer, et al., 1990)(merged). Specifically, overexpression of Rb causes G1 cell cycle arrest while reduction in Rb has been shown to induce re-entry of quiescent cells into the cell cycle (Huang, et al., 1988, Sage, et al., 2003). In brief, Rb binds to and inactivates E2F transcription factors (Chellappan, et al., 1991, Trimarchi and Lees, 2002). However, during G1 phase of the cell cycle, Rb becomes hyperphosphorylated and this phosphorylation releases E2Fs from Rb. E2Fs can then induce the transcription of cellular genes essential for S phase entry and cell division such as cyclin A and cyclin E (Berman, et al., 2008). Mdm2 interacts with Rb and suppresses its function by inhibiting the formation of Rb-E2F complexes, allowing E2F to induce transcription of critical cell cycle genes (Xiao, et al., 1995, Sdek, et al., 2004, Ying and Xiao, 2006, Bremner, et al., 1997, Hsieh, et al., 1999). Mdm2 also promotes the degradation of Rb through both ubiquitin-dependent and ubiquitin-independent pathways (Ying and Xiao, 2006). In addition, Mdm2 can form a complex with TBP and TAF250, which has been shown to augment the cyclin A promoter (Leveillard and Wasylyk, 1997). Thus Mdm2 plays an important role in regulating the Rb-E2F axis for entry into the cell cycle.
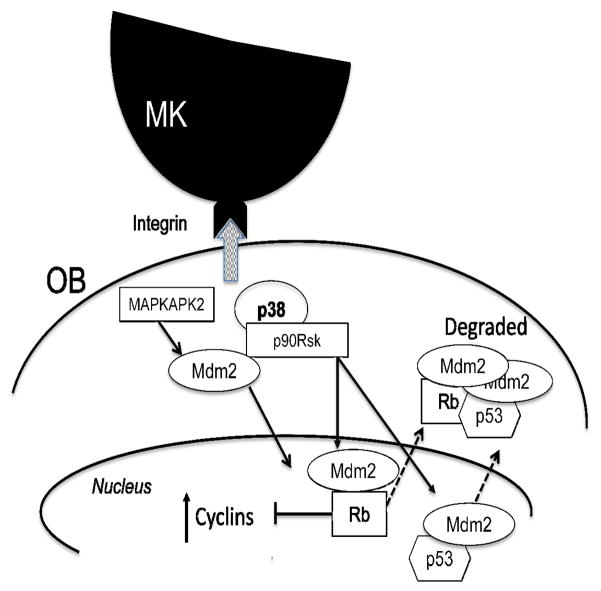
Because Mdm2 is a protein that is regulated by numerous kinases, here we examined the kinases that were specifically activated in OBs stimulated by MKs. As depicted in Figure 6, several signaling proteins were upregulated in OBs in response to co-culture with MKs. Of note, p38, MAPKAPK2, and p90RSK were all activated specifically by MK co-culture. While ERK1/2 and AKT were activated, they were not specifically activated by MK co-culture. Although the PI3K/AKT pathway mediates nuclear translocation by AKT directly phosphorylating Mdm2 in the nuclear localization sequence; MAPKAPK2 has also been shown to phosphorylate the same sites in Mdm2 as AKT. For nuclear export, the p38/ERK/p90RSK pathway is responsible for Mdm2/p53 cytoplasmic translocation from the nucleus and the destruction of the complex. The corresponding loss of Rb, p53, and Mdm2 and the activation of MAPKAPK2 and p90RSK most likely leads to E2F activation and an increase in cyclin A in response to integrin activation. Thereby providing a temporal association of MAPKAPK2/p90RSK/Mdm2/cyclin A as an integrated signaling pathway that is regulating MK-mediated OB proliferation (Lengner, et al., 2006, Momand, et al., 1992, Chen, et al., 1995, Xirodimas, et al., 2004, Honda, et al., 1997, Haupt, et al., 1997, Kubbutat, et al., 1997, Li, et al., 2003, Freedman and Levine, 1998, Geyer, et al., 2000). Figure 8 illustrates upon which phases of cell cycle the key signaling proteins examined in this study act in OBs stimulated by MKs. Overall, these studies have begun to identify signaling pathways by which MKs regulate OB proliferation and may provide valuable novel targets for activation to induce anabolic bone formation to treat bone loss diseases such as osteoporosis.
Figure 8.
Schematic depicting MK-OB interaction, signaling pathways, and their impact on cell cycle progression.
Acknowledgments
Grant information:
NIH: RR025760; RR025761; AR055269; AR060332; AR052682; HL55716; CA109262; CA082709
This work was sponsored in part by the Department of Orthopaedic Surgery at Indiana University School of Medicine, the Medical Student Affairs Summer Research Program in Academic Medicine, Indiana University School of Medicine funded in part by NIH grant HL110854 (DAS) a Biomedical Research Grant and Pilot Funding for Research Use of Core Facilities Award both from Indiana University School of Medicine (MAK), by the Indiana - Clinical and Translational Sciences Institute funded in part by NIH grants RR025760 and RR025761 (MAK), and the following NIH grants: R01 AR060332 (MAK), R01 AR060863 (MAK), R01 AR052682 (FMP), R01 HL55716 (EFS), and R01 CA109262 (LDM). Finally, we would like to thank the operators of the Indiana University Melvin and Bren Simon Cancer Center Flow Cytometry Resource Facility for their technical help and support (partially funded by P30 CA082709) as well as support from the the Indiana University Center of Excellence in Molecular Hematology (funded by NIH P30 DK090948).
Footnotes
The authors have no conflicts of interest to disclose.
The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Berman SD, Yuan TL, Miller ES, Lee EY, Caron A, Lees JA. The retinoblastoma protein tumor suppressor is important for appropriate osteoblast differentiation and bone development. Mol Cancer Res. 2008;6:1440–1451. doi: 10.1158/1541-7786.MCR-08-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner R, Du DC, Connolly-Wilson MJ, Bridge P, Ahmad KF, Mostachfi H, Rushlow D, Dunn JM, Gallie BL. Deletion of RB exons 24 and 25 causes low-penetrance retinoblastoma. Am J Hum Genet. 1997;61:556–570. doi: 10.1086/515499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centrella M, McCarthy TL, Canalis E. Glucocorticoid regulation of transforming growth factor beta 1 activity and binding in osteoblast-enriched cultures from fetal rat bone. Mol Cell Biol. 1991;11:4490–4496. doi: 10.1128/mcb.11.9.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagraoui H, Wendling F, Vainchenker W. Pathogenesis of myelofibrosis with myeloid metaplasia: Insight from mouse models. Best Pract Res Clin Haematol. 2006;19:399–412. doi: 10.1016/j.beha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991;65:1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Trimarchi JM, Lees JA. Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol. 2002;3:11–20. doi: 10.1038/nrm714. [DOI] [PubMed] [Google Scholar]
- Chen J, Lin J, Levine AJ. Regulation of transcription functions of the p53 tumor suppressor by the mdm-2 oncogene. Mol Med. 1995;1:142–152. [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Qasba P, Vanguri P, Thiede MA. Human mesenchymal stem cells support megakaryocyte and pro-platelet formation from CD34(+) hematopoietic progenitor cells. J Cell Physiol. 2000;184:58–69. doi: 10.1002/(SICI)1097-4652(200007)184:1<58::AID-JCP6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Hooker RA, Nguyen K, Gerard-O’Riley R, Waning DL, Chitteti BR, Meijome TE, Chua HL, Plett AP, Orschell CM, Srour EF, Mayo LD, Pavalko FM, Bruzzaniti A, Kacena MA. Pyk2 regulates megakaryocyte-induced increases in osteoblast number and bone formation. J Bone Miner Res. 2013;28:1434–1445. doi: 10.1002/jbmr.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciovacco WA, Goldberg CG, Taylor AF, Lemieux JM, Horowitz MC, Donahue HJ, Kacena MA. The role of gap junctions in megakaryocyte-mediated osteoblast proliferation and differentiation. Bone. 2009;44:80–86. doi: 10.1016/j.bone.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciovacco WA, Cheng YH, Horowitz MC, Kacena MA. Immature and mature megakaryocytes enhance osteoblast proliferation and inhibit osteoclast formation. J Cell Biochem. 2010;109:774–781. doi: 10.1002/jcb.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diller L, Kassel J, Nelson CE, Gryka MA, Litwak G, Gebhardt M, Bressac B, Ozturk M, Baker SJ, Vogelstein B. P53 Functions as a Cell Cycle Control Protein in Osteosarcomas. Mol Cell Biol. 1990;10:5772–5781. doi: 10.1128/mcb.10.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Rasini V, Bussolari R, Chen X, Hofmann TJ, Spano C, Bernabei D, Veronesi E, Bertoni F, Paolucci P, Conte P, Horwitz EM. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood. 2009;114:2333–2343. doi: 10.1182/blood-2008-10-183459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drachman JG, Sabath DF, Fox NE, Kaushansky K. Thrombopoietin signal transduction in purified murine megakaryocytes. Blood. 1997;89:483–492. [PubMed] [Google Scholar]
- Freedman DA, Levine AJ. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol Cell Biol. 1998;18:7288–7293. doi: 10.1128/mcb.18.12.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey BM, Rafii S, Crystal RG, Moore MA. Adenovirus long-term expression of thrombopoietin in vivo: a new model for myeloproliferative syndrome and osteomyelofibrosis. Schweiz Med Wochenschr. 1998a;128:1587–1592. [PubMed] [Google Scholar]
- Frey BM, Rafii S, Teterson M, Eaton D, Crystal RG, Moore MA. Adenovector-mediated expression of human thrombopoietin cDNA in immune-compromised mice: insights into the pathophysiology of osteomyelofibrosis. J Immunol. 1998b;160:691–699. [PubMed] [Google Scholar]
- Frum R, Ramamoorthy M, Mohanraj L, Deb S, Deb SP. MDM2 controls the timely expression of cyclin A to regulate the cell cycle. Mol Cancer Res. 2009;7:1253–1267. doi: 10.1158/1541-7786.MCR-08-0334. [DOI] [PubMed] [Google Scholar]
- Geyer RK, Yu ZK, Maki CG. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat Cell Biol. 2000;2:569–573. doi: 10.1038/35023507. [DOI] [PubMed] [Google Scholar]
- Gjetting T, Lukas J, Bartek J, Strauss M. Regulated expression of the retinoblastoma susceptibility gene in mammary carcinoma cells restores cyclin D1 expression and G1-phase control. Biol Chem Hoppe Seyler. 1995;376:441–446. [PubMed] [Google Scholar]
- Goodrich DW, Wang NP, Qian YW, Lee EY, Lee WH. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991;67:293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- Goodrich DW, Lee WH. Abrogation by c-myc of G1 phase arrest induced by RB protein but not by p53. Nature. 1992;360:177–179. doi: 10.1038/360177a0. [DOI] [PubMed] [Google Scholar]
- Hartwell LH, Kastan MB. Cell cycle control and cancer. Science. 1994;266:1821–1828. doi: 10.1126/science.7997877. [DOI] [PubMed] [Google Scholar]
- Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992;70:993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hollingsworth RE, Jr, Chen PL, Lee WH. Integration of cell cycle control with transcriptional regulation by the retinoblastoma protein. Curr Opin Cell Biol. 1993a;5:194–200. doi: 10.1016/0955-0674(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Hollingsworth RE, Jr, Hensey CE, Lee WH. Retinoblastoma protein and the cell cycle. Curr Opin Genet Dev. 1993b;3:55–62. doi: 10.1016/s0959-437x(05)80341-7. [DOI] [PubMed] [Google Scholar]
- Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- Horowitz MC, Fields A, DeMeo D, Qian HY, Bothwell AL, Trepman E. Expression and regulation of Ly-6 differentiation antigens by murine osteoblasts. Endocrinology. 1994;135:1032–1043. doi: 10.1210/endo.135.3.7520861. [DOI] [PubMed] [Google Scholar]
- Hsieh JK, Chan FS, O’Connor DJ, Mittnacht S, Zhong S, Lu X. RB regulates the stability and the apoptotic function of p53 via MDM2. Mol Cell. 1999;3:181–193. doi: 10.1016/s1097-2765(00)80309-3. [DOI] [PubMed] [Google Scholar]
- Huang HJ, Yee JK, Shew JY, Chen PL, Bookstein R, Friedmann T, Lee EY, Lee WH. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988;242:1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Jackson MW, Patt LE, LaRusch GA, Donner DB, Stark GR, Mayo LD. Hdm2 nuclear export, regulated by insulin-like growth factor-I/MAPK/p90Rsk signaling, mediates the transformation of human cells. J Biol Chem. 2006;281:16814–16820. doi: 10.1074/jbc.M511617200. [DOI] [PubMed] [Google Scholar]
- Jilka RL, Cohn DV. Role of phosphodiesterase in the parathormone-stimulated adenosine 3′,5′-monophosphate response in bone cell populations enriched in osteoclasts and osteoblasts. Endocrinology. 1981;109:743–747. doi: 10.1210/endo-109-3-743. [DOI] [PubMed] [Google Scholar]
- Kacena MA, Shivdasani RA, Wilson K, Xi Y, Troiano N, Nazarian A, Gundberg CM, Bouxsein ML, Lorenzo JA, Horowitz MC. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J Bone Miner Res. 2004;19:652–660. doi: 10.1359/JBMR.0301254. [DOI] [PubMed] [Google Scholar]
- Kacena MA, Gundberg CM, Nelson T, Horowitz MC. Loss of the transcription factor p45 NF-E2 results in a developmental arrest of megakaryocyte differentiation and the onset of a high bone mass phenotype. Bone. 2005;36:215–223. doi: 10.1016/j.bone.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Kacena MA, Nelson T, Clough ME, Lee SK, Lorenzo JA, Gundberg CM, Horowitz MC. Megakaryocyte-mediated inhibition of osteoclast development. Bone. 2006;39:991–999. doi: 10.1016/j.bone.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Kacena MA, Eleniste PP, Cheng Y-H, Huang S, Shivanna M, Meijome TE, Mayo LD, Bruzzaniti A. Megakaryocytes regulate the expression of Pyk2 isoforms and the caspase-mediated cleavage of actin in osteoblasts. J Biol Chem. 2012;287:17257–17268. doi: 10.1074/jbc.M111.309880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- Lemieux JM, Horowitz MC, Kacena MA. Involvement of integrins alpha(3)beta(1) and alpha(5)beta(1) and glycoprotein IIb in megakaryocyte-induced osteoblast proliferation. J Cell Biochem. 2010;109:927–932. doi: 10.1002/jcb.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengner CJ, Steinman HA, Gagnon J, Smith TW, Henderson JE, Kream BE, Stein GS, Lian JB, Jones SN. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol. 2006;172:909–921. doi: 10.1083/jcb.200508130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveillard T, Wasylyk B. The MDM2 C-terminal region binds to TAFII250 and is required for MDM2 regulation of the cyclin A promoter. J Biol Chem. 1997;272:30651–30661. doi: 10.1074/jbc.272.49.30651. [DOI] [PubMed] [Google Scholar]
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Lennert K, Nagai K, Schwarze EW. Patho-anatomical features of the bone marrow. Clin Haematol. 1975;4:331–351. [PubMed] [Google Scholar]
- Li M, Brooks CL, Wu-Baer F, Chen D, Baer R, Gu W. Mono-versus polyubiquitination: differential control of p53 fate by Mdm2. Science. 2003;302:1972–1975. doi: 10.1126/science.1091362. [DOI] [PubMed] [Google Scholar]
- Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer WE, Shields MT, Amin M, Sauve GJ, Appella E, Romano JW, Ullrich SJ. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci U S A. 1990;87:6166–6170. doi: 10.1073/pnas.87.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao D, Murant S, Scutt N, Genever P, Scutt A. Megakaryocyte-bone marrow stromal cell aggregates demonstrate increased colony formation and alkaline phosphatase expression in vitro. Tissue Eng. 2004;10:807–817. doi: 10.1089/1076327041348473. [DOI] [PubMed] [Google Scholar]
- Olson TS, Caselli A, Otsuru S, Hofmann TJ, Williams R, Paolucci P, Dominici M, Horwitz EM. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood. 2013;121:5238–5249. doi: 10.1182/blood-2012-10-463414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, Jacks T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424:223–228. doi: 10.1038/nature01764. [DOI] [PubMed] [Google Scholar]
- Sdek P, Ying H, Zheng H, Margulis A, Tang X, Tian K, Xiao ZX. The central acidic domain of MDM2 is critical in inhibition of retinoblastoma-mediated suppression of E2F and cell growth. J Biol Chem. 2004;279:53317–53322. doi: 10.1074/jbc.M406062200. [DOI] [PubMed] [Google Scholar]
- Sdek P, Ying H, Chang DL, Qiu W, Zheng H, Touitou R, Allday MJ, Xiao ZX. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol Cell. 2005;20:699–708. doi: 10.1016/j.molcel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte growth and platelet development. EMBO J. 1997;16:3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DJ, Kent GN, Jilka RL, Scott DM, Fallon M, Cohn DV. Formation of bone by isolated, cultured osteoblasts in millipore diffusion chambers. Calcif Tissue Int. 1982;34:291–294. doi: 10.1007/BF02411253. [DOI] [PubMed] [Google Scholar]
- Srour EF, Brandt JE, Leemhuis T, Ballas CB, Hoffman R. Relationship between cytokine-dependent cell cycle progression and MHC class II antigen expression by human CD34+ HLA-DR-bone marrow cells. J Immunol. 1992;148:815–820. [PubMed] [Google Scholar]
- Suva LJ, Hartman E, Dilley JD, Russell S, Akel NS, Skinner RA, Hogue WR, Budde U, Varughese KI, Kanaji T, Ware J. Platelet dysfunction and a high bone mass phenotype in a murine model of platelet-type von Willebrand disease. Am J Pathol. 2008;172:430–439. doi: 10.2353/ajpath.2008.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele J, Kvasnicka HM, Fischer R. Histochemistry and morphometry on bone marrow biopsies in chronic myeloproliferative disorders - aids to diagnosis and classification. Ann Hematol. 1999;78:495–506. doi: 10.1007/s002770050546. [DOI] [PubMed] [Google Scholar]
- Towler DA, St Arnaud R. Use of Cultured Osteoblastic Cells to Identify and Characterize Transcriptional Regulatory Complexes. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. San Diego, CA: Academic Press; 2002. pp. 1503–1527. [Google Scholar]
- Villeval JL, Cohen-Solal K, Tulliez M, Giraudier S, Guichard J, Burstein SA, Cramer EM, Vainchenker W, Wendling F. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997;90:4369–4383. [PubMed] [Google Scholar]
- Weber HO, Ludwig RL, Morrison D, Kotlyarov A, Gaestel M, Vousden KH. HDM2 phosphorylation by MAPKAP kinase 2. Oncogene. 2005;24:1965–1972. doi: 10.1038/sj.onc.1208389. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Wong GL, Cohn DV. Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci U S A. 1975;72:3167–3171. doi: 10.1073/pnas.72.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xirodimas DP, Saville MK, Bourdon JC, Hay RT, Lane DP. Mdm2-mediated NEDD8 conjugation of p53 inhibits its transcriptional activity. Cell. 2004;118:83–97. doi: 10.1016/j.cell.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Xiao ZX, Chen J, Levine AJ, Modjtahedi N, Xing J, Sellers WR, Livingston DM. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995;375:694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- Yan XQ, Lacey D, Fletcher F, Hartley C, McElroy P, Sun Y, Xia M, Mu S, Saris C, Hill D, Hawley RG, McNiece IK. Chronic exposure to retroviral vector encoded MGDF (mpl-ligand) induces lineage-specific growth and differentiation of megakaryocytes in mice. Blood. 1995;86:4025–4033. [PubMed] [Google Scholar]
- Yan XQ, Lacey D, Hill D, Chen Y, Fletcher F, Hawley RG, McNiece IK. A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88:402–409. [PubMed] [Google Scholar]
- Ying H, Xiao ZX. Targeting retinoblastoma protein for degradation by proteasomes. Cell Cycle. 2006;5:506–508. doi: 10.4161/cc.5.5.2515. [DOI] [PubMed] [Google Scholar]