Abstract
BACKGROUND
As the survival of children with cardiac disease increases, chronic complications of deep venous thrombosis from cardiac catheterization, particularly post-thrombotic syndrome, may be important to monitor for and treat, if needed. We aimed to determine the prevalence of this syndrome in children who underwent cardiac catheterization.
PROCEDURE
We conducted a cross-sectional study of children <18 years old at least 1 year from first catheterization through the femoral vein. We used the Manco-Johnson instrument, the only tool validated in children, to diagnose post-thrombotic syndrome. We defined the syndrome as a score ≥1. It was considered physically and functionally significant if the score was ≥1 in both physical and functional domains of the instrument. We also conducted ultrasonography to assess for thrombosis and valvular insufficiency.
RESULTS
We enrolled 62 children with a median age of 4 months during catheterization and a median of 5.4 years since catheterization. A total of 40 children had post-thrombotic syndrome (prevalence: 64.5%; 95% confidence interval: 51.3%–76.3%), the majority of which were mild. Presence of cyanotic congenital heart disease, total number of catheterizations, use of antithrombotic agents at any time after the first catheterization, age at first catheterization, or time since first catheterization was not associated with the syndrome. A total of 7 children (prevalence: 11.3%; 95% confidence interval: 3.2%–19.4%) had physically and functionally significant syndrome. None of the children had abnormalities on ultrasonography at the time of enrollment.
CONCLUSIONS
Post-thrombotic syndrome is a common complication after cardiac catheterization. Manifestations are usually mild and unlikely to require treatment.
Keywords: child, deep venous thrombosis, heart disease, pediatrics, ultrasonography
INTRODUCTION
Cardiac catheterization is commonly performed to diagnose and treat cardiac diseases in children [1]. Because catheterization is done through a deep vein, typically the femoral vein, children who undergo the procedure are at risk of deep venous thrombosis (DVT) and its complications. The incidence of DVT within days after catheterization ranges from 0% to 27% [2–7]. While most of the DVT are asymptomatic, these children can still develop chronic complications from the DVT, specifically post-thrombotic syndrome (PTS) [8]. PTS, which is thought to be due to venous hypertension from persistent or recurrent DVT or valvular insufficiency, is the most common chronic complication of DVT with 26% of children and 20–40% of adults developing PTS at least 1 year after the DVT [9]. PTS may present with pain, swelling, dilated superficial collateral veins, stasis dermatitis and ulceration in the affected limb and is associated with poor quality of life in adults [10]. As children with cardiac diseases survive into adulthood, PTS may be an important complication of cardiac catheterization for which children should be monitored for so that we can treat it, if needed. Compression therapy is the mainstay of treatment for PTS [10].
More than a decade ago, van Ommen et al reported from a small number of unselected children with congenital heart disease at least 5 years after cardiac catheterization that half of them had signs or symptoms consistent with PTS [11]. The validity of this finding is unclear because the prevalence seems unusually high based on the known incidence of DVT after cardiac catheterization and the known prevalence of PTS after a radiologically documented DVT [2–7,9]. The investigators diagnosed PTS using the basic clinical-etiologic-anatomic-pathophysiologic (CEAP) classification of chronic lower extremity venous disease, which was developed for adults. There were no validated instruments to diagnose PTS in children at the time of the study. Recently, the Manco-Johnson instrument was validated as a highly accurate and reliable tool for diagnosing PTS in children [9,12]. In this study, we determined the prevalence of PTS in children who underwent cardiac catheterization using the Manco-Johnson instrument.
METHODS
Study Design
We conducted a cross-sectional study of children followed at the pediatric cardiology clinics at Yale-New Haven Children’s Hospital from May 2012 to January 2014. The Human Investigation Committee at Yale approved the study. The committee waived consent for screening purposes and required parental permission for other study-related procedures.
Subjects
Children less than 18 years old who had a cardiac catheterization at least 1 year prior to enrollment were eligible for the study. We excluded children in whom catheterization was not performed through a femoral vein to obtain a relatively homogenous sample of children. Potential subjects were identified from the schedule of clinic visits. It is standard of care for these children to have routine visits regardless of symptomatology. After eligibility was confirmed by reviewing the child’s medical records, we contacted the parents via mail and phone at least one week prior to the visit. All study procedures were done during the scheduled visits to maximize participation.
Study Procedures
After parental permission was obtained, we conducted a standardized interview focusing on the child’s personal history of DVT and other thromboembolic events, and intake of antithrombotic agents, such as aspirin, warfarin or low molecular weight heparin, at any time after the first cardiac catheterization. During catheterization, our standard practice was to administer 100 units/kg of unfractionated heparin intravenously after obtaining venous access. Additional boluses of heparin were given to maintain the activated clotting time at least 200 seconds. Because there were no guidelines for the prevention of DVT after catheterization, we did not have a standardized approach to the use of or choice of antithrombotic agents for these children [13]. Children who were perceived to be at high risk of DVT based on the type of cardiac disease or personal history of DVT were more likely to be on antithrombotic agents.
Independently, 2 members of the research team (MJL, JAT, CGW or EVSF) administered the Manco-Johnson instrument. A random third member of the team arbitrated discordant scores. All members of the research team were blinded to which femoral vein was accessed during catheterization while administering the instrument. The instrument is described in detail elsewhere [9]. In brief, the instrument scores each leg for specific physical and functional findings. A score of 1 is given in the presence of each of the following physical findings: edema, dilated superficial collateral veins, venous stasis dermatitis and venous stasis ulceration. Using the Wong Baker Faces Pain Rating Scale, a score of 0 to 5 is given for each of the following functional findings: chronic pain at rest, chronic pain limiting age-appropriate activities of daily living, and chronic pain limiting exercise. All members of the research team received extensive training on the use of the instrument. This included a standardized online training module and practice sessions prior to the start of the study and additional re-training during the study [12].
Bilateral leg ultrasonography was performed to detect DVT or valvular insufficiency. Certified ultrasonography technicians performed the examination using standard procedures [11,14]. Compression ultrasonography, and color and spectral Doppler were used to image the deep femoral, common femoral, external iliac, common iliac, and inferior caval veins for DVT. As recommended, we used spectral Doppler to investigate insufficiency in the common femoral, deep femoral, femoral and popliteal veins while the child was in reverse Trendelenburg position [14]. Manual compression was applied to the calf musculature to augment blood flow. The pediatric radiologist in the research team (CTS) read and interpreted all images. The technicians and radiologist were blinded to the site of prior femoral venous access and score on the Manco-Johnson instrument.
Using structured case report forms, we collected demographic and medical data from each child’s medical record while blinded to the score on the Manco-Johnson instrument. We confirmed the diagnosis of DVT or any thromboembolic event and its treatment obtained during the interview in the medical records. We reviewed all catheterization reports to collect hemodynamic data and details of the procedure. Although only data from the first catheterization was used for consistency, we reviewed the site of femoral venous access of subsequent catheterizations, including failed attempts to access the vein, and of central venous catheters inserted during hospitalizations. The site of catheterization of the femoral artery was also reviewed.
Outcomes
The primary outcome was the presence of PTS defined as a total score of ≥1 in the Manco-Johnson instrument [9]. Secondary outcomes were the presence of physically and functionally significant PTS, and presence of DVT or valvular insufficiency on ultrasonography [9,11]. We defined physically and functionally significant PTS as a score ≥1 in both physical and functional domains of the instrument [9]. DVT was diagnosed if at least 2 were present on ultrasonography: (1) intravenous echogenic material adherent to the venous wall, (2) non-compressibility of the vein, or (3) abnormal venous Doppler flow pattern [14]. Valvular insufficiency was diagnosed if reflux in the affected vein was >0.5 seconds [11].
Statistical Analysis
Children were categorized based on the presence of PTS. Characteristics of the child on enrollment and during the first catheterization, and hemodynamic data were compared between groups using Mann-Whitney or chi-squared tests, as appropriate. The prevalence of the outcomes was reported as percent with 95% confidence interval (CI). Kappa statistic was used to assess the concordance between the scores provided by the initial 2 examiners on the Manco-Johnson instrument [15]. Logistic regression was used to identify factors associated with PTS. Based on the known risk factors of DVT and the natural history of PTS in children, we determined the association between PTS and the presence of cyanotic congenital heart disease, total number of cardiac catheterizations, use of antithrombotic agents at any time after the first catheterization, age at first catheterization, and time since first catheterization [13,16,17].
Statistical significance was determined at a 2-sided p<0.05. We used Stata 13 (StataCorp, College Station, TX) for all statistical analyses. Convenience sampling was used to enroll as many children as possible during the 20-month study period.
RESULTS
A total of 196 children were eligible for the study. We were able to contact 109 of them of whom 20 did not consent and 27 could not be scheduled for the study. We enrolled 62 of 196 children (31.6%). Of these, 38 children (61.3%) were male, 40 (64.5%) were white, 29 (46.8%) had cyanotic congenital heart disease, and the median number of catheterizations was 2 (interquartile range [IQR]: 1–3) (Table I). The median age during the first cardiac catheterization was 4 months (IQR: 2 months–2.4 years) while the median time from first catheterization to enrollment was 5.4 years (IQR: 2.7–9.0 years). A total of 26 of 62 children (41.9%) were on an antithrombotic agent at any time from first catheterization to enrollment, which was usually aspirin (25 of 26 children, 96.2%). A total of 8 children had previous DVT, of which 3 were diagnosed in the legs after cardiac catheterization (Table II). They were treated with either warfarin or low molecular weight heparin. Compared to those who were enrolled, children who were not enrolled were older during the first catheterization (median: 8 months; IQR: 3 months–3.9 years; p=0.03). The time from first catheterization to screening for potential enrollment in those not enrolled was not statistically different from those who were enrolled (median: 5.9 years; IQR: 2.9–9.5 years; p=0.75).
Table I.
Characteristics of children with and without post-thrombotic syndrome (PTS)
| PTS (n = 40) |
No PTS (n = 22) |
p value | |
|---|---|---|---|
| Demographics | |||
| Age at enrollment (years) | 8.2 (4.4–11.4) | 6.1 (3.1–9.8) | 0.32 |
| Male | 24 (60.0) | 14 (63.6) | 0.78 |
| Race | 0.15 | ||
| White | 26 (65.0) | 14 (63.6) | |
| Black | 3 (7.5) | 5 (22.7) | |
| Others/refused | 11 (27.5) | 3 (13.6) | |
| Medical History | |||
| Cardiac diagnosis | 0.76 | ||
| Right-sided obstruction | 12 (30.0) | 8 (36.4) | |
| Left-sided obstruction | 6 (15.0) | 3 (13.6) | |
| Shunt | 7 (17.5) | 6 (27.3) | |
| Single ventricle | 10 (25.0) | 3 (13.6) | |
| Others | 5 (12.5) | 2 (9.1) | |
| Cyanotic congenital heart disease | 22 (55.0) | 7 (31.8) | 0.08 |
| Number of catheterizations | 2 (1–3) | 1 (1–3) | 0.55 |
| Cardiac surgery | 28 (70.0) | 13 (59.1) | 0.39 |
| History of deep venous thrombosis | 5 (12.5) | 3 (13.6) | 0.90 |
| History of catheterization of the femoral artery | 32 (80.0) | 15 (68.2) | 0.32 |
| Use of antithrombotic agents at any time after catheterization | 18 (45.0) | 8 (36.4) | 0.51 |
| Data from First Cardiac Catheterization | |||
| Age at catheterization (years) | 0.3 (0.2–1.3) | 0.3 (0.1–3.2) | 0.83 |
| Time since catheterization (years) | 5.7 (3.5–9.4) | 4.6 (2.7–8.5) | 0.26 |
| Weight (kg)a | 5.8 (4.6–8.7) | 5.1 (3.9–15) | 0.83 |
| Hemoglobin (g/dL)a | 11.3 (10.0–13.0) | 11.0 (9.7–12.6) | 0.43 |
| Cardiac index (L/min/m2)a | 3.2 (2.6–4.2) | 4.2 (3.7–5.0) | 0.02 |
| Mean central venous pressure (mmHg)a | 7.5 (5–8) | 6 (5–8) | 0.08 |
Data are presented as median (interquartile range) or count (%).
A total of 7 to 15 children had missing data.
Table II.
Characteristics of children with history of deep venous thrombosis
| Patient | Primary Cardiac Diagnosis |
Site of Thrombosis | Prothrombotic Evaluation |
Treatment of Thrombosis |
Post-Thrombotic Syndrome |
|---|---|---|---|---|---|
| 1 | Pulmonary atresia with ventricular septal defect | Right external iliac vein | Not tested | Warfarin | Absent |
| 2 | Pulmonary atresia with ventricular septal defect | Right femoral vein | Not tested | Warfarin | Present |
| 3 | Aortic hypoplasia | Superior vena cava | Normal levels of protein C and S | Warfarin | Absent |
| 4 | Pulmonary hypertension | Portal vein and portosystemic shunt | Protein C and factor VII deficiency; negative for factor V Leiden and prothrombin mutations | Warfarin | Present |
| 5 | Transposition of the great arteries | Right atrium | Not tested | Low molecular weight heparin | Absent |
| 6 | Pulmonary atresia with ventricular septal defect | Right femoral vein | Not tested | Low molecular weight heparin | Present |
| 7 | Tricuspid atresia | Aortopulmonary shunt and right atrium | Normal levels of protein C, protein S and factor VIII; negative for factor V Leiden and prothrombin mutations |
Low molecular weight heparin | Present |
| 8 | Atrioventricularseptal defect | Cavopulmonary shunt | Not tested | Warfarin | Present |
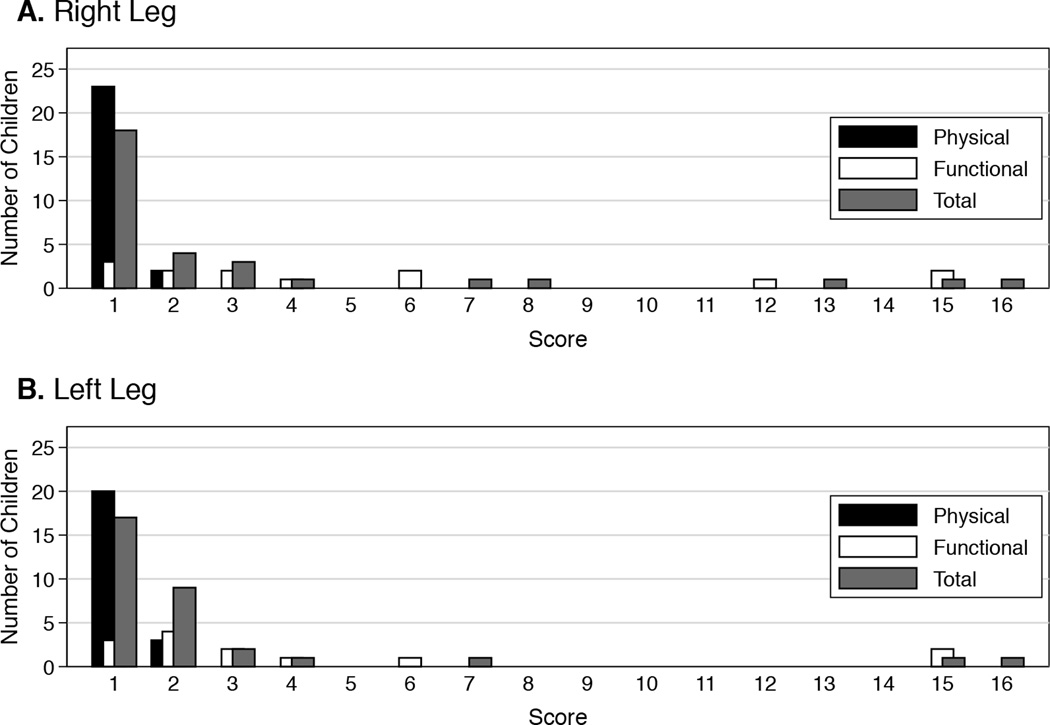
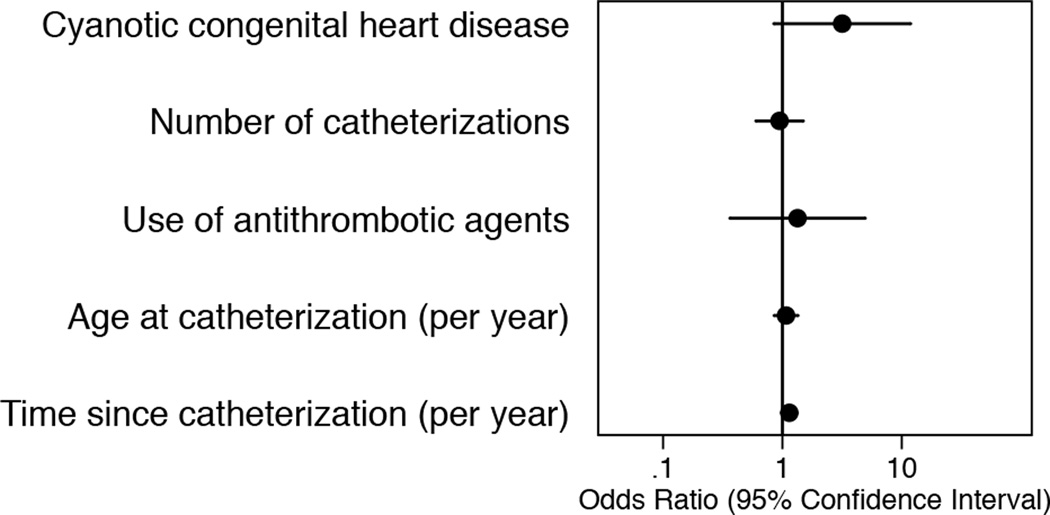
The prevalence of PTS in our study was 64.5% (95% CI: 51.3%–76.3%) (Table I). A total of 40 of 62 children had PTS based on the Manco-Johnson instrument. The median calculated cardiac index during the first catheterization of children with PTS (3.2 L/min/m2; IQR: 2.6–4.2 L/min/m2) was lower than that of children without PTS (4.2 L/min/m2; IQR: 3.7–5.0 L/min/m2; p=0.02). The rest of the characteristics, including history of catheterization of the femoral artery, were not statistically different between those with and without PTS. Of the 62 children enrolled, findings of PTS were unilateral in 17 children (27.4%) and bilateral in 23 children (37.1%) (Table III). PTS was generally mild with a score of 1 in 35 of 63 legs (55.6%) with positive findings (Figure 1). Of the 3 children with DVT in the legs after catheterization, none had findings of PTS in those legs. A total of 46 children had unilateral venous access, inclusive of cardiac catheterizations, failed attempts to access the femoral vein and insertion of central venous catheters. Of these, 14 children had dilated superficial collateral veins in the leg that was not accessed. Ten of them (71.4%) self-identified as white. A total of 9 children had pain in the leg that was not accessed. None of them had a history of catheterization of the femoral artery of the affected leg. The concordance between the scores on the Manco-Johnson instrument provided by the 2 examiners was almost perfect with kappa of 0.88 (p<0.001) [15]. The presence of dilated superficial collateral veins accounted for all 7 legs in which the scores were discordant. None of the putative prevalence factors were associated with PTS (Figure 2).
Table III.
Summary of findings from the Manco-Johnson instrument
| Right Leg | Left Leg | |||
|---|---|---|---|---|
| Count (%) (n=62) |
Median (IQR)a (n=40) |
Count (%) (n=62) |
Median (IQR)a (n=40) |
|
| Physical domain | ||||
| Edema | 6 (9.7) | 10 (16.1) | ||
| Dilated superficial collateral veins | 21 (33.9) | 16 (25.8) | ||
| Venous stasis dermatitis | 0 (0) | 0 (0) | ||
| Venous stasis ulcers | 0 (0) | 0 (0) | ||
| Total | 25 (40.3) | 1 (1-1) | 23 (37.1) | 1 (1-1) |
| Functional domain | ||||
| Chronic pain at rest | 7 (11.3) | 4 (1–5) | 7 (11.3) | 3 (1–5) |
| Chronic pain limiting activities of daily living | 6 (9.7) | 3.5 (2–5) | 4 (6.5) | 4 (2–5) |
| Chronic pain limiting aerobic exercise | 8 (12.9) | 3.5 (2.5–4.5) | 7 (11.3) | 3 (2–5) |
| Total | 13 (21.0) | 3 (2–6) | 13 (21.0) | 2 (2–4) |
| Post-thrombotic syndrome (PTS)b | 31 (50.0) | 1 (1–3) | 32 (51.6) | 1 (1–2) |
| Physically and functionally significant PTSc | 7 (11.3) | 7 (2–13) | 4 (6.5) | 4.5 (2–11.5) |
Medians and interquartile ranges (IQR) were calculated only from children with positive findings.
PTS was defined as a score ≥1 in either physical or functional domain.
Physically and functionally significant PTS was defined as a score ≥1in both physical and functional domains.
Figure 1.
Distribution of scores from the Manco-Johnson instrument for the (A) right and (B) left legs of children with post-thrombotic syndrome (n=40)
Figure 2.
Association between putative prevalence factors and post-thrombotic syndrome
A total of 7 of 62 children (prevalence: 11.3%; 95% CI: 3.2%–19.4%) had physically and functionally significant PTS (Table III). The findings were unilateral in 3 children and bilateral in 4 children.
A total of 58 of the 62 children (93.5%) completed ultrasonography. One subject left before having the ultrasonography and 3 children would not cooperate for the procedure. All were negative for DVT and valvular insufficiency.
DISCUSSION
In this cross-sectional study of children at least 1 year after cardiac catheterization through the femoral vein, we report that the prevalence of PTS, using the highly accurate and reliable Manco-Johnson instrument, was 64.5%. The majority were mild. The prevalence of physically and functionally significant PTS was lower at 11.3%. We did not find any DVT or valvular insufficiency on ultrasonography. It is important to note that this study was conducted by non-hematologists who follow children regularly after cardiac catheterization. Because it is not routine to image the veins after catheterization, these physicians would need to recognize the signs and symptoms of PTS in children without a documented history of DVT in order to treat it, if needed [10].
Similar to the prevalence of 50% reported by van Ommen et al using the CEAP classification, we found an unusually high prevalence of PTS at 64.5% in a comparable group of children after cardiac catheterization [11]. In contrast, Brandao et al reported a lower prevalence of 28.7% using the Manco-Johnson instrument in a slightly different group of children with congenital heart disease and their siblings [18]. Assuming 27% of children develop DVT after cardiac catheterization and 26% of those with DVT develop PTS, we anticipated that the prevalence of PTS would only be about 7% [2–7,9]. The prevalence of physically and functionally significant PTS in our study of 11.3% was more consistent with the expected prevalence. The difference in the instruments used to diagnose PTS is unlikely to be the source of the discrepancies [18].
The high prevalence of PTS in our study may be worrisome because it seems to question the validity of the Manco-Johnson instrument when used by non-hematologists. This may partly explain the discrepancies in the prevalence of PTS among studies [11,18]. The concordance between our examiners was almost perfect except when assessing for the presence of dilated superficial collateral veins. Similar to van Ommen et al, we noted dilated superficial collateral veins even in legs without any documentation of femoral venous access, particularly in those who self-identified as white [11]. Although these children may have had unprovoked DVT in those legs, this is uncommon in children [16]. Alternatively, access to these veins, such as from failed attempts or insertion of central venous catheters during hospitalizations, may not have been adequately documented in the medical records. The functional domain of the instrument was also problematic. It was difficult for some parents, particularly those of younger children, to determine the presence and degree of chronic pain in the legs of their children even in the context of age-appropriate activities [18]. Some parents reported pain in legs in which the femoral vein was not accessed. Although arterial insufficiency from thrombosis secondary to catheterization may explain these findings, we did not find evidence that a history of catheterization of the femoral artery was associated with PTS [19]. Despite our difficulties with the instrument, it is important to note that the majority of children had mild PTS, which is consistent with previous studies [11,18]. It will be worthwhile to follow these children for possible progression of PTS [17]. However, it appears unlikely that any interventions will be needed.
It would be ideal to have a more objective test, such as imaging, to diagnose PTS. We used ultrasonography to investigate for DVT and valvular insufficiency. In contrast to van Ommen et al, we did not find evidence of either abnormality [11]. Vascular imaging is poorly correlated with clinical findings of PTS [9]. Although ultrasonography is the imaging of choice, its accuracy is fair in identifying and quantifying valvular insufficiency [20]. A better understanding of the pathophysiology of PTS may lead to better and more objective tools to diagnose PTS [10]. In the meantime, we suggest using the more restrictive score of ≥1 in each of the domains in the Manco-Johnson instrument, which is the current definition of physically and functionally significant PTS, to define PTS [9]. In adults, PTS is diagnosed in the presence of symptoms and findings in the physical examination [10].
Our study has a number of strengths. We enrolled children as early as 1 year after catheterization when PTS may start to manifest and until 17 years old to capture the breadth of the pediatric population [9,17]. We used the Manco-Johnson instrument, which is the only tool validated for diagnosing PTS in children and which was also validated in children without any history of DVT [9,12]. We supplemented the physical examinations with ultrasonography. The examiners and radiologist were blinded to which femoral vein was accessed during catheterization to minimize assessment bias. We had multiple examiners conduct the instrument to maximize the reliability of the assessments.
Certain limitations should also be considered. Although our sample size was more than double that of van Ommen et al, it is still small [11]. Only about a third of eligible children were enrolled. The difference in the characteristics of children who were and were not enrolled may affect the generalizability of our results. We also lacked statistical power to effectively assess for prevalence factors for PTS. We were not able to do a formal sample size calculation for the multivariable analysis because of the uncertainty in the published prevalence of PTS [11]. Our study design was retrospective and relied on medical records as a major source of data. Potentially important factors that may be associated with PTS, such as the dose of heparin during catheterization, number of attempts to access the vein and hemodynamic data, were not consistently documented [21]. Ultrasonography was obtained only on study enrollment. We could not ascertain the presence of prior DVT or arterial thrombosis because ultrasonography was not done after catheterization. Valvular insufficiency may have also been present prior to enrollment [17]. A prospective cohort study with serial examinations would be able to address most of these concerns. Our study was done in one center. Clinical practice during catheterization may be different in other centers [5]. The involvement of a hematologist would also have been desirable. This would have allowed us to compare the performance of the Manco-Johnson instrument when conducted by physicians with and without training in hematology.
In conclusion, the prevalence of PTS in children with cardiac disease at least 1 year after cardiac catheterization through the femoral vein was 64.5%. Physically and functionally significant PTS was less common with a prevalence of 11.3%. None of these clinical findings were supported by abnormalities on ultrasonography. Although PTS appears to be common, the majority of the cases were mild and unlikely to require treatment. It will be worthwhile to follow these children to monitor for possible progression of PTS.
Acknowledgments
This work was supported in part by awards from the Friends of Yale-New Haven Children’s Hospital, American Heart Association (Award Number 14CRP20490002), and Clinical and Translational Science Award (Grants Numbers UL1 TR000142 and KL2 TR000140) from the National Center for Research Resources and the National Center for Advancing Translational Science of the National Institutes of Health to Dr. Faustino.
Abbreviation key
- DVT
deep venous thrombosis
- PTS
post-thrombotic syndrome
- CEAP classification
clinical-etiologic-anatomic-pathophysiologic classification
- CI
confidence interval
- IQR
interquartile range
Footnotes
CONFLICT OF INTEREST STATEMENT
The other authors have no real or perceived conflicts of interests.
REFERENCES
- 1.Armsby L, Beekman RH, 3rd, Benson L, Fagan T, Hagler DJ, Hijazi ZM, Holzer R, Ing F, Kreutzer J, Lang P, Levi DS, Latson L, Moore P, Mullins C, Ruiz C, Vincent R. SCAI expert consensus statement for advanced training programs in pediatric and congenital interventional cardiac catheterization. Catheter Cardiovasc Interv. 2014;84(5):779–784. doi: 10.1002/ccd.25550. [DOI] [PubMed] [Google Scholar]
- 2.Moore RA, McNicholas KW, Naidech H, Flicker S, Gallagher JD. Clinically silent venous thrombosis following internal and external jugular central venous cannulation in pediatric cardiac patients. Anesthesiology. 1985;62(5):640–643. doi: 10.1097/00000542-198505000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Laurin S, Lundstrom NR. Venous thrombosis after cardiac catheterization in infants. Acta Radiol. 1987;28(3):241–246. [PubMed] [Google Scholar]
- 4.Ruud E, Natvig S, Holmstrom H, Wesenberg F. Low prevalence of femoral venous thrombosis after cardiac catheterizations in children: A prospective study. Cardiol Young. 2002;12(6):513–518. doi: 10.1017/s1047951102000938. [DOI] [PubMed] [Google Scholar]
- 5.Hanslik A, Kitzmuller E, Thom K, Haumer M, Mlekusch W, Salzer-Muhar U, Michel-Behnke I, Male C. Incidence of thrombotic and bleeding complications during cardiac catheterization in children: Comparison of high-dose vs. low-dose heparin protocols. J Thromb Haemost. 2011;9(12):2353–2360. doi: 10.1111/j.1538-7836.2011.04539.x. [DOI] [PubMed] [Google Scholar]
- 6.Miga DE, McKellar LF, Denslow S, Wiles HB, Case CL, Gillette PC. Incidence of femoral vein occlusion after catheter ablation in children: Evaluation with magnetic resonance angiography. Pediatr Cardiol. 1997;18(3):204–207. doi: 10.1007/s002469900151. [DOI] [PubMed] [Google Scholar]
- 7.Nowak-Gottl U, Dubbers A, Kececioglu D, Koch HG, Kotthoff S, Runde J, Vielhaber H. Factor V Leiden, protein C, and lipoprotein (a) in catheter-related thrombosis in childhood: A prospective study. J Pediatr. 1997;131(4):608–612. doi: 10.1016/s0022-3476(97)70071-4. [DOI] [PubMed] [Google Scholar]
- 8.Kuhle S, Spavor M, Massicotte P, Halton J, Cherrick I, Dix D, Mahoney D, Bauman M, Desai S, Mitchell LG. Prevalence of post-thrombotic syndrome following asymptomatic thrombosis in survivors of acute lymphoblastic leukemia. J Thromb Haemost. 2008;6(4):589–594. doi: 10.1111/j.1538-7836.2008.02901.x. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg NA, Donadini MP, Kahn SR, Crowther M, Kenet G, Nowak-Gottl U, Manco-Johnson MJ. Post-thrombotic syndrome in children: A systematic review of frequency of occurrence, validity of outcome measures, and prognostic factors. Haematologica. 2010;95(11):1952–1959. doi: 10.3324/haematol.2010.026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn SR. The post thrombotic syndrome. Thromb Res. 2011;127(Suppl 3):S89–S92. doi: 10.1016/S0049-3848(11)70024-X. [DOI] [PubMed] [Google Scholar]
- 11.van Ommen CH, Ottenkamp J, Lam J, Brennickmeier M, Heijmans HS, Buller HR, Peters M. The risk of postthrombotic syndrome in children with congenital heart disease. J Pediatr. 2002;141(4):582–586. doi: 10.1067/mpd.2002.127276. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg NA, Pounder E, Knapp-Clevenger R, Manco-Johnson MJ. Validation of upper extremity post-thrombotic syndrome outcome measurement in children. J Pediatr. 2010;157(5):852–855. doi: 10.1016/j.jpeds.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monagle P, Chan AK, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Gottl U, Vesely SK. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e737S–e801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Institute of Ultrasound in Medicine. AIUM practice guideline for the performance of peripheral venous ultrasound examinations. J Ultrasound Med. 2011;30(1):143–150. doi: 10.7863/jum.2011.30.1.143. [DOI] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 16.Monagle P, Adams M, Mahoney M, Ali K, Barnard D, Bernstein M, Brisson L, David M, Desai S, Scully MF, Halton J, Israels S, Jardine L, Leaker M, McCusker P, Silva M, Wu J, Anderson R, Andrew M, Massicotte MP. Outcome of pediatric thromboembolic disease: A report from the Canadian Childhood Thrombophilia Registry. Pediatr Res. 2000;47(6):763–766. doi: 10.1203/00006450-200006000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Creary S, Heiny M, Croop J, Fallon R, Vik T, Hulbert M, Knoderer H, Kumar M, Sharathkumar A. Clinical course of postthrombotic syndrome in children with history of venous thromboembolism. Blood Coagul Fibrinolysis. 2012;23(1):39–44. doi: 10.1097/MBC.0b013e32834bdb1c. [DOI] [PubMed] [Google Scholar]
- 18.Brandao LR, Manlhiot C, Williams S, McCrindle BC. Characterization of post-thrombotic syndrome in children with congenital heart disease: A comparison of instruments. J Thromb Haemost. 2011;9(Suppl 2):765. [Google Scholar]
- 19.Avila ML, Shah PS, Brandao LR. Different unfractionated heparin doses for preventing arterial thrombosis in children undergoing cardiac catheterization. Cochrane Database Syst Rev. 2014;3:CD010196. doi: 10.1002/14651858.CD010196.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Meissner MH, Moneta G, Burnand K, Gloviczki P, Lohr JM, Lurie F, Mattos MA, McLafferty RB, Mozes G, Rutherford RB, Padberg F, Sumner DS. The hemodynamics and diagnosis of venous disease. J Vasc Surg. 2007;46(Suppl S):4S–24S. doi: 10.1016/j.jvs.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 21.Alten JA, Borasino S, Gurley WQ, Law MA, Toms R, Dabal RJ. Ultrasound-guided femoral vein catheterization in neonates with cardiac disease. Pediatr Crit Care Med. 2012;13(6):654–659. doi: 10.1097/PCC.0b013e318250af0c. [DOI] [PubMed] [Google Scholar]