Abstract
Tumor cells exist in a constantly evolving stromal microenvironment composed of vasculature, immune cells and cancer-associated fibroblasts, all residing within a dynamic extracellular matrix. In this review, we examine the biochemical and biophysical interactions between these various stromal cells and their matrix microenvironment. While the stroma can alter tumor progression via multiple mechanisms, we emphasize the role of homeobox genes in detecting and modulating the mechanical changes in the microenvironment during tumor progression.
Keywords: Tumor microenvironment, extracellular matrix, stiffness, stromal cells, homeobox genes
1. Introduction
Tumor progression is accompanied by increasing tissue stiffness. This is an ancient clinical observation that allows palpation to be used for the detection of many carcinomas within the normal resident tissue. The stiffening phenotype is typically driven by cells of the tumor stroma via increased extracellular matrix (ECM) deposition, as well as enzymatic remodeling and crosslinking. Accumulating evidence shows that increased ECM stiffness promotes malignant behavior via mechanical signaling [1].
As presented in this review, ECM stiffness initiates changes in tumor and stromal cells (via the process of mechanotransduction) that ultimately support cancer progression. The diverse effects of stiffness on stromal cells of the tumor microenvironment (i.e., vascular, immune, fibroblast) suggest that the biomechanical signals within the tumor (e.g., ECM composition and stiffness) are integrated by the activation of pleiotropic regulatory genes or pathways. Homeobox genes are master transcription factors that integrate external signals and regulate biological processes through activation or repression of numerous downstream target genes. By controlling many genes simultaneously, homeobox genes can activate specific transcriptional programs that enable cells to coordinate complex cellular processes, such as cellular differentiation and homeostasis. Not surprisingly, deregulation of homeobox transcription factors is often observed in human cancers. Herein, we discuss the link between matrix stiffness and homeobox gene expression, their usefulness as prognostic markers and their potential as therapeutic targets.
2. Mechanotransduction
Cells of the tumor microenvironment respond to the mechanical changes in their surroundings through mechanotransduction [2]. Prototypical ECM-driven mechanotransduction is initiated by integrin engagement with an ECM ligand (e.g., integrin-α1β1/collagen) and leads to integrin clustering and activation. The intracellular domains of integrin clusters recruit talin and vinculin, which promote focal adhesions formation, followed by activation of focal adhesion kinase (FAK), downstream Rho-guanine nucleotide exchange factors (Rho-GEFs) and the Ras/mitogen-activated protein kinase (MAPK) signaling pathway. Ultimately, these active focal adhesions result in actin reassembly and actomyosin-based cell contractility. This mechanoreciprocity allows cells to respond to external forces and mechanical properties, as a function of ECM composition and stiffness, through changes in cell signaling and contractility that further impact on ECM remodeling (Reviewed in [1]).
Seminal studies revealed that mammary epithelial cells grown in compliant three-dimensional (3D) culture conditions better recapitulate physiological states, in terms of polarity, function, and proliferation, than the same cells grown on conventional tissue culture plastic - which is several orders of magnitude stiffer than recombinant 3D gels [3]. Intriguingly, the malignant phenotype of breast cancer cells can be reverted by growing these cells in 3D gels of a similar stiffness to human breast tissue [4]. These findings led to the hypothesis that, in the absence of genetic changes, cellular phenotype can be regulated by the composition (e.g., collagen, fibronectin, laminin, etc.) and rigidity of the surrounding ECM. Indeed, normal mammary spheres become dysplastic in stiff matrices and when coupled with the activity of a transforming oncogene, cell invasion results in a stiffness-dependent manner [5]. The stiff ECM invasive phenotype can be mimicked in soft gels by expressing an integrin-β1 clustering mutant, which facilitates focal adhesion formation and signaling. Conversely, inhibition of focal adhesion signaling via targeting of FAK, MAPK, Rho or rho-associated, coiled-coil-containing protein kinase (ROCK) can rescue this phenotype [5, 6]. These data have been substantiated in vivo by showing that inhibition of ECM stiffness in the mouse mammary gland causes tumor cells to revert to a more normal epithelial phenotype, characterized by reduced invasion and proliferation [7].
Tumor stiffening is an emerging hallmark of carcinomas and the pathways that are regulated by mechanical signaling are frequently associated with tumor suppressor and oncogene function [8]. For instance, focal adhesion activity can enhance G-protein coupled receptor signaling, leading to extracellular-signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and phosphatidylinositol-3-kinase (PI3K)/protein kinase B (PKB, also known as AKT) activation [5, 9]. Additionally, we have recently shown that increased ECM stiffness leads to reduced phosphatase and tensin homolog (PTEN) expression and protein stability [10]. Thus, dynamic changes in the tumor ECM act in concert with oncogene signaling pathways to drive tumor cell proliferation, survival, and invasion.
3. Matrix stiffness and the tumor microenvironment
Tumor cells exist in a stromal microenvironment composed of vasculature, immune cells and cancer-associated fibroblasts (CAFs) residing within a dynamic ECM. It is increasingly evident that mechanical changes in the microenvironment drive tumor progression by impacting both the phenotype of the tumor cells and the surrounding stromal cells. Indeed, increased angiogenesis, leucocyte infiltration, and desmoplasia are all hallmarks of cancer development. Furthermore, these stromal cell types can either promote or preclude tumor progression depending on the context of their microenvironment.
3.1 Vasculature
Angiogenesis, the outgrowth of new vessels, is essential for the delivery of oxygen and nutrients to tumor cells and is also the main conduit for metastatic dissemination (Reviewed in [11, 12]). Furthermore, the vasculature is necessary for the recruitment and infiltration of inflammatory cells into tumors. Not surprisingly, the vasculature has become a major target for the treatment of solid tumors [11]. However, while many tumors regress after initial anti-angiogenic therapy, they often become refractory and relapse with a more aggressive form of disease [13]. While activated tumor vasculature promotes cancer progression, quiescent non-tumor vascular endothelial cells secrete factors, such as thrombospondin 1 (THBS1), that keep micro-metastases dormant within the perivascular niche [14]. Thus, the vasculature can have opposing effects on tumor growth, depending on its surroundings and activation status.
Recent data indicate that the physical microenvironment helps shape vessel growth. Increased extra-vascular collagen stiffness promotes endothelial cell proliferation and angiogenesis [15, 16], and highlight the importance of matrix stiffness in vascular development and orientation [17]. Mechanical signaling favors angiogenesis by promoting vascular endothelial growth factor receptor 2 (VEGFR2) expression in endothelial cells through general transcription factor II-I (TFII-I) and GATA binding protein 2 (GATA2) transcriptional programs [18]. Mechanical signaling in tumor cells can also stimulate angiogenesis by promoting the expression of pro-angiogenic factors. An in vitro model of hepatocellular carcinoma demonstrated that increased matrix stiffness cause up-regulation of VEGF levels in carcinoma cells and this was found to be dependent on the mechanosensing Integrin-β1-PI3K signaling axis. This observation was substantiated through an analysis of tissue microarrays and the discovery that levels of type I collagen and the collagen I crosslinking enzyme lysyl oxidase (LOX) were positively correlated with levels of VEGF and platelet endothelial cell adhesion molecule (PECAM, also known as CD31) in patient hepatocellular carcinomas, thus indicating a link between increased matrix rigidity and angiogenesis [19]. A first study of its kind that performed atomic force microscopy mapping of tumor tissue from the mouse mammary tumor virus driven polyoma middle T—antigen (MMTV-PyMT) mouse model of breast cancer revealed a large heterogeneity in vasculature stiffness, with a stiffer vascular component within the central region of tumors compared to patent vessels in the tumor periphery that were associated with the stiffest extracellular matrix [20]. This data complements the findings that blood and lymphatic vessels within the tumor core are often compressed or collapsed due to tumor solid stress, whereas vessels in the periphery are leaky due to abnormal interstitial fluid pressure [21]. Sustained vessel compression or collapse can in turn lead to hypoxic regions within the tumor, with a myriad of pathological implications including, immunosuppression and inflammation, tumor cell invasion and metastasis, lowered penetration and efficacy of radiation and chemotherapies [22, 23]. Therefore, a shifting paradigm in the treatment of tumor vasculature is emerging, in which vessels are normalized rather than ablated [24].
3.2 Immune Cells
Immune cells (Reviewed in [25, 26]) are a heterogeneous population of cells whose representation within a tumor can vary greatly between cancer types. Both tumor promoting/pro-angiogenic (e.g., M2 macrophages, myeloid derived suppressor cells (MDSCs) and regulatory T cells (TREG)) and tumor suppressive/anti-angiogenic (e.g., M1 macrophages, CD8+ and CD4+ T cells) immune cells exist within tumors and the balance between these modulators will determine whether tumors will escape or be eliminated by the immune system. This balance is complex and involves integration of cytokine signals from all cells of the tumor microenvironment.
Hypoxic conditions arising in tumors under high solid stress with enhanced stiffness are associated with increased immune cell infiltration but also evasion of anti-tumor immunity. Hypoxia induced transcription factor alpha (HIF1α), a master regulator of hypoxic transcription programs, has been shown to induce numerous cytokines that promote immune infiltration. In solid tumors, HIF1α preferentially recruits pro-tumor M2-type macrophages and elicits a suppressive effect on anti-tumor T cells while promoting their maturation into anti-inflammatory TREG cells [27]. Infiltrating macrophages in hypoxic environments will further contribute to HIF1α induced VEGF expression and angiogenesis in concert with tumor cells [28]. A stiffened ECM may also impair T cell proliferation and differentiation through an inhibition of IL-2 production, which is required for these T cell processes and subsequent anti-tumor actions [29]. At the invasive front of tumors, a stiffer ECM contributes to a more patent vasculature that permits enhanced immune infiltration [20]. It was found that the deposition of stiffened and linearized collagen provides tracks for macrophage migration. Macrophages are then able to establish paracrine signaling with tumor cells to guide their invasion and access to the blood vessels for dissemination [30]. Thus, mechanical pressure in the tumor microenvironment intensifies pro-tumor inflammation and chronic inflammation can promote a fibrotic response and desmoplasia in tissues.
3.3 Cancer-associated fibroblasts
While the source of ECM deposition can derive from numerous cell types in the microenvironment including the tumor cells themselves, CAFs are largely considered the major regulators of collagen deposition and organization (Reviewed in [31]). Matrix reorganization by CAFs is thought to increase tissue tension and promote cancer progression. CAFs not only produce large amounts of collagen, but also modify collagen ultrastructure and mechanical properties through the secretion of proteases and collagen crosslinking enzymes, such as LOX [32]. LOX activity is directly related to ECM stiffness of the tumor microenvironment and its inhibition severely diminished breast cancer aggression and metastasis [7]. Moreover, stromal fibroblast derived LOX expression was able to drive ECM stiffening and metastasis of mouse mammary carcinomas [33]. CAFs can also secrete many ECM components and proteases that modify ECM composition and orientation to favor tumor progression. Activated pancreatic stromal fibroblasts dramatically increase production and linear organization of collagen and fibronectin, leading to a stiffened ECM and tumor cell invasion through integrin-β1/FAK mediated mechanosignaling [34]. These data demonstrate that CAF-mediated organization and stiffening of the matrix promote aggressive tumor behaviors. However, caution must be taken in perceiving CAFs as only pro-tumorigenic, as paradoxically, ablation of CAFs in pancreatic ductal adenocarcinomas (PDAC) leads to more invasive tumors and decreased survival [35].
4. Matrix stiffness and homeobox gene expression
These diverse and context-dependent effects of ECM stiffness on tumor and stromal cells suggest that biomechanical signals may be coordinated by specific, pleiotropic regulatory proteins. Homeobox genes are master transcriptional regulators, capable of integrating multiple signals and inducing phenotypic switches through their control of numerous downstream target genes. For example, many homeobox genes regulate the production of cell cycle proteins, cytokines and matrix components. Thus, homeobox genes are likely to mediate or reinforce the pro-tumorigenic effects (e.g., increased angiogenesis, inflammation and ECM deposition) observed in response to a stiffened tumor matrix.
Akin to tissue matrix stiffening in cancer, aberrant homeobox gene expression is emerging as a critical driver of tumor progression. The observations that plating breast tumor cells on a compliant ECM (vs. tissue culture plastic) could up-regulate homeobox A1 (HoxA1) and HoxB7 expression in vitro [36] and that collagen I/integrin-β1 signaling reduced caudal-related homeobox (Cdx2) expression in colorectal tumor cells [37], suggested that matrix stiffness could potentially regulate homeobox genes in cancer. However, only recently was a direct link between matrix stiffness and homeobox gene expression demonstrated in vivo, where increased ECM tension in breast tumors diminished HoxA9 expression as a consequence of microRNA 18a (miR-18a) up-regulation [10] (Figure 1). This discovery provides compelling evidence for a tangible connection between ECM tension, homeobox gene expression and cancer progression.
Figure 1. Matrix stiffness reduces HOXA9 expression.
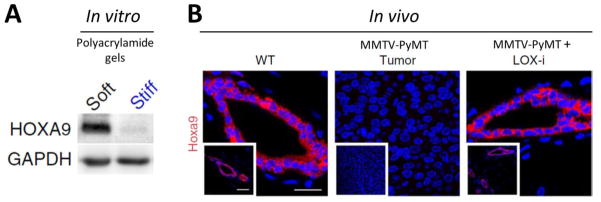
Recent work from Mouw et al. [10] shows that (a) culturing human mammary epithelial cells on stiff polyacrylamide gels decreases HOXA9 expression in vitro and (b) treatment of MMTV-PyMT mice with a LOX inhibitor (LOX-i) reduces tissue fibrosis and matrix stiffening in vivo, restores HOXA9 expression to wild-type (WT) levels and impedes tumor progression.
5. The prognostic value of homeobox gene expression and their role in the tumor microenvironment
There are many reports of altered homeobox gene expression in cancers (Reviewed in [38–40]) however the direct consequences of this aberrant expression are an ongoing area of study. Although typically considered as developmental genes, essential for cell fate-decisions in undifferentiated cells, many homeobox genes play important roles in maintaining cellular differentiation and homeostasis in adult tissues.
Recent efforts have focused on demonstrating statistical correlations between homeobox promoter methylation and/or gene expression levels and patient prognosis. A notable example is HoxA9, whose loss of expression is correlated with poor patient prognosis, increased matrix stiffness and more aggressive breast cancer subtypes [10, 41]. Interestingly, in contrast, HoxA9 over-expression in ovarian cancer and glioblastomas is predictive of poor prognosis [42, 43]. This difference may reflect the levels of intracellular HoxA9, the tumor cell type of origin (presence of different cell type specific co-factors), the underlying oncogenic driver of transformation, differences in tissue stiffness and matrix composition (normal breast, which is rich in collagen, is softer than brain, whose ECM is largely proteoglycan and hyaluronic acid [1]), stromal cell diversity or any combination of these factors. Increased HoxA9 expression in ovarian cancer cells was shown to up-regulate transforming growth factor β2 (TGF-β2) and stimulate CAF secretion of pro-tumorigenic and pro-angiogenic factors (stromal cell-derived factor 1 (SDF-1, also known as CXCL12), interleukin 6 (IL-6) and VEGF) and promote immunosuppressive skewing of the immune component through increased infiltration of M2 macrophages [42, 44]. Future studies on the mechano-responsiveness and tumor suppressive effects of HoxA9 in the stromal components of other tumors (e.g., breast) are needed to address the contribution of stromal cells to cancer outcome.
Nevertheless, these reports exemplify the utility of a homeobox gene as independent predictors of survival. A major limitation of most studies is that homeobox gene expression is assessed in isolated tumor cells or throughout the whole tumor, making no distinction as to the cellular origin of gene expression. Given its pivotal role in epithelial-mesenchymal transition (EMT), not surprisingly, high expression of the zinc finger E-box-binding homeobox 1 (Zeb1) gene correlates with poor prognosis in PDAC patients [45]. Interestingly, although Zeb1 was increased in both the epithelial and stromal compartments, only stromal Zeb1 was statistically correlated with patient prognosis [45]. This finding highlights the importance of identifying the cellular origin of homeobox gene expression within the tumor microenvironment, as the regulation and outcome of homeobox gene expression is often cell type specific. It is interesting to speculate whether homeobox genes may also be useful biomarkers of tumor resistance to therapy or whether patterns of homeobox gene expression in a tumor could be used to better predict tumor grade/invasiveness.
Likewise, as homeobox genes are master transcriptional regulators, future work should be aimed at uncovering the role of homeobox genes in modulating mechanoreciprocity. Specifically, do stiffness-induced changes in homeobox gene expression feed-forward to further changes in ECM tension and increased mechanosignaling through regulation of downstream target genes in tumor and stromal cells? This feedback is certainly suggested by the fact that several homeobox genes (e.g., Zeb1 [46], Zeb2 [47], HoxB9 [48], ladybird homeobox 1 (Lbx1) [49], paired related homeobox 1 (Prrx1) [50], sine oculis-related homeobox 1 (Six1) [51]), have been demonstrated to be sufficient for EMT induction in tumor cells, a process dependent upon ECM remodeling. Both Lbx1 and Six1 are examples of homeobox genes that are over-expressed in breast cancer and have been shown to induce EMT, augment tumor cell expression of the ECM component fibronectin and promote tumor cell invasiveness [49, 51].
6. Can homeobox genes be exploited for cancer therapy?
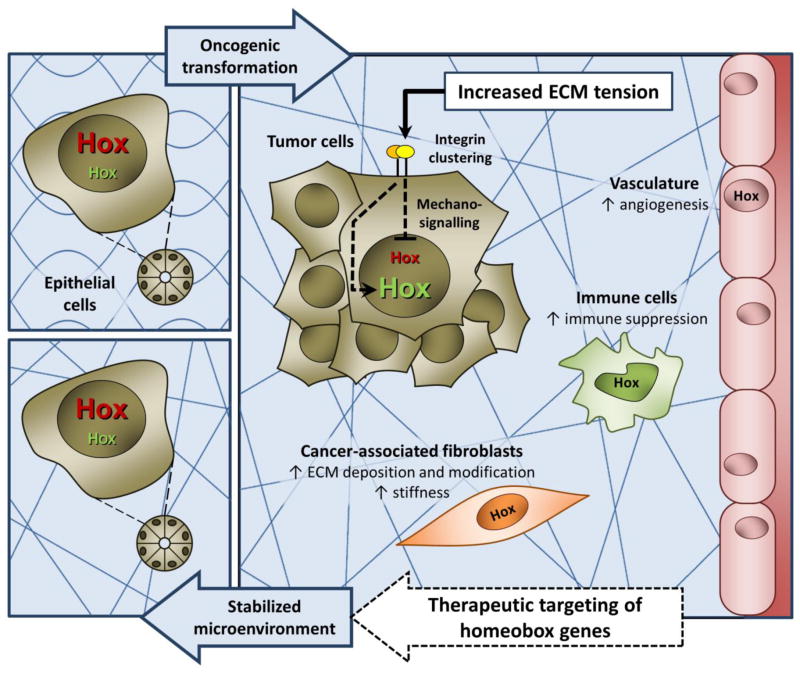
The human genome is estimated to contain 235 functional homeobox genes [52], representing a wealth of possible therapeutic targets. In practice, therapeutic targeting of over-expressed homeobox genes may be achieved using siRNAs. On the other side of the spectrum, homeobox gene down-regulation in cancer is often the result of promoter methylation or silencing by miRNAs and long non-coding RNAs (e.g., HOTAIR). Thus, anti-miRs or DNA methyltransferase inhibitors may be useful therapeutic strategies for repressed genes. An alternative method is restoration of the repressed homeobox gene using a targeted gene transfer approach. The consequence of re-introducing homeobox genes into cells that normally express these genes under homeostatic conditions would be expected to be minimal and to cause fewer adverse side effects for patients receiving therapy. Thus, research aimed at restoring normal levels of homeobox gene expression in tumor and stromal cells (Figure 2) may yield safer and more effective therapeutic strategies to stabilize the tumor microenvironment and impede tumor progression.
Figure 2. Homeobox genes as therapeutic targets to modulate mechanical changes in the tumor microenvironment.
As tumors progress they become increasingly stiff. Tumor and stromal cells sense mechanical changes in the microenvironment which leads, via mechanotransduction, to activation or repression of homeobox gene expression (represented as green and red ‘Hox’, respectively). Restoring normal levels of homeobox gene expression in tumor and stromal cells is a potential therapeutic strategy to stabilize the tumor microenvironment and impede tumor progression (black ‘Hox’ represent any homeobox gene with altered expression).
Although targeting nuclear transcription factors in specific tissues is technically challenging, the therapeutic potential of targeting homeobox genes that display aberrant expression in cancer was recently demonstrated by multiple researchers, using various strategies. For example, topical application of methylcellulose pellets containing Hox gene expression plasmids [53] has been used to successfully delay tumor progression in a murine model of skin cancer [I. Cuevas et al., Plos One (2015) in press]. Furthermore, intraductal delivery of nanoparticles containing HoxA1-targeted small interfering RNA (siRNA) was employed during the early stage of mammary tumorigenesis to prevent progression [54]. Loss of epithelial cell polarity and matrix reorganization during the initial stages of breast cancer result in the ectopic expression of HoxA1 in tumor cells. Silencing of HoxA1 in a model of basal breast cancer was sufficient to reduce tumor incidence, epithelial hyperplasia and prevent the loss of estrogen and progesterone receptor expression [54]. These approaches have the advantages of being both minimally invasive and site directed.
Another homeobox gene-targeted therapeutic approach capable of impeding tumor growth and progression in vivo is the HXR9 synthetic peptide [55, 56]. This peptide functions as a competitive inhibitor of Hox/pre-B-cell leukemia homeobox (Pbx) protein binding. Disruption of the Hox and Pbx transcription co-factor complex induces tumor cell apoptosis [55, 56]. Similarly, an amino acid substitution approach that disrupts the Six1/eyes absent 2 (Eya2) transcriptional complex prevents Six1-induced EMT and metastasis of intravenously delivered breast cancer cells [57]. Thus, development of small molecule inhibitors capable of abolishing specific homeobox/transcription cofactor interactions may represent an effective therapeutic strategy in future.
As stiffness impacts the entire tumor microenvironment, including the epithelium, endothelium, immune cells and fibroblasts, then it may be advantageous to target homeobox genes that impact more than one cellular compartment of the tumor. For example, anti-angiogenic HoxD10 and HoxA5, are expressed in normal quiescent breast epithelium and endothelium, however their expression is lost during breast cancer progression [58, 59]. Restoring these homeobox genes in both tumor and endothelial cells would be expected to have a more comprehensive effect on stabilizing the tumor microenvironment than either one alone. In order to exploit homeobox genes as cancer therapeutics, forthcoming research should focus on identifying upstream regulators of homeobox gene expression and on understanding how restoring normal homeobox gene expression in tumor and stromal cells can prevent tumorigenesis through downstream target gene activation.
7. Conclusion
Current advances in the fields of tissue mechanics and tumor microenvironment regulation of cancer progression have highlighted the importance of biomechanical interactions between stromal cells and their matrix. Given their roles in maintaining cellular differentiation and tissue homeostasis, their deregulated expression in cancer, and the recent discovery of altered HoxA9 expression in response to matrix stiffness, homeobox genes are emerging as attractive targets for therapeutic intervention.
Highlights.
The effects of mechanosignaling on tumor microenvironment cell types are reviewed.
Links between ECM stiffness, homeobox genes and cancer progression are emphasized.
Recent advances in therapeutic targeting of homeobox genes in cancer are discussed
Acknowledgments
We apologize to all colleagues whose work cannot be cited owing to space limitations. This work was supported by AACR Fellowship 14-40-01-NORT (JMN), NRSA Fellowship F32CA174319 (JMB), DOD BCRP Grants W81XWH-05-1-0330 and W81XWH-13-1-0216 (VMW), NIH NCI Grants R01 CA138818, U54 CA143836, U54 CA163155, R01 CA085492, R01 GM066047, U01 CA151925 and U01 ES019458, R33 CA183685 (VMW), AACR PANCAN A121989 (VMW), and Susan G. Komen Grant KG110560PP (VMW).
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nature reviews Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiological reviews. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 3.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. The Journal of cell biology. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Samuel MS, Lopez JI, McGhee EJ, Croft DR, Strachan D, Timpson P, Munro J, Schroder E, Zhou J, Brunton VG, Barker N, Clevers H, Sansom OJ, Anderson KI, Weaver VM, Olson MF. Actomyosin-mediated cellular tension drives increased tissue stiffness and beta-catenin activation to induce epidermal hyperplasia and tumor growth. Cancer cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO reports. 2014;15:1243–1253. doi: 10.15252/embr.201439246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. The Journal of cell biology. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, Hwang ES, Chen YY, Weaver VM. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nature medicine. 2014;20:360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nature reviews. Cancer. 2010;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 12.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature medicine. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 13.Lu KV, Chang JP, Parachoniak CA, Pandika MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ, Cheresh DA, Park M, Bergers G. VEGF inhibits tumor cell invasion and mesenchymal transition through a MET/VEGFR2 complex. Cancer cell. 2012;22:21–35. doi: 10.1016/j.ccr.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY, Chen EI, Lyden D, Bissell MJ. The perivascular niche regulates breast tumour dormancy. Nature cell biology. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee PF, Bai Y, Smith RL, Bayless KJ, Yeh AT. Angiogenic responses are enhanced in mechanically and microscopically characterized, microbial transglutaminase crosslinked collagen matrices with increased stiffness. Acta biomaterialia. 2013;9:7178–7190. doi: 10.1016/j.actbio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Agarwal S. Mechanical signals activate vascular endothelial growth factor receptor-2 to upregulate endothelial cell proliferation during inflammation. Journal of immunology. 2010;185:1215–1221. doi: 10.4049/jimmunol.0903660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Underwood CJ, Edgar LT, Hoying JB, Weiss JA. Cell-generated traction forces and the resulting matrix deformation modulate microvascular alignment and growth during angiogenesis. American journal of physiology. Heart and circulatory physiology. 2014;307:H152–164. doi: 10.1152/ajpheart.00995.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Y, Xie X, Wang Z, Hu C, Zheng Q, Wang Y, Chen R, Xue T, Chen J, Gao D, Wu W, Ren Z, Cui J. Increasing matrix stiffness upregulates vascular endothelial growth factor expression in hepatocellular carcinoma cells mediated by integrin beta1. Biochemical and biophysical research communications. 2014;444:427–432. doi: 10.1016/j.bbrc.2014.01.079. [DOI] [PubMed] [Google Scholar]
- 20.Lopez JI, Kang I, You WK, McDonald DM, Weaver VM. In situ force mapping of mammary gland transformation. Integrative biology : quantitative biosciences from nano to macro. 2011;3:910–921. doi: 10.1039/c1ib00043h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, Popovic Z, Huang P, Bawendi MG, Boucher Y, Jain RK. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nature communications. 2013;4:2516. doi: 10.1038/ncomms3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stylianopoulos T, Martin JD, Chauhan VP, Jain SR, Diop-Frimpong B, Bardeesy N, Smith BL, Ferrone CR, Hornicek FJ, Boucher Y, Munn LL, Jain RK. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:15101–15108. doi: 10.1073/pnas.1213353109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stylianopoulos T, Martin JD, Snuderl M, Mpekris F, Jain SR, Jain RK. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer research. 2013;73:3833–3841. doi: 10.1158/0008-5472.CAN-12-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiological reviews. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkholder B, Huang RY, Burgess R, Luo S, Jones VS, Zhang W, Lv ZQ, Gao CY, Wang BL, Zhang YM, Huang RP. Tumor-induced perturbations of cytokines and immune cell networks. Biochimica et biophysica acta. 2014;1845:182–201. doi: 10.1016/j.bbcan.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature reviews. Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 27.Mamlouk S, Wielockx B. Hypoxia-inducible factors as key regulators of tumor inflammation. International journal of cancer. Journal international du cancer. 2013;132:2721–2729. doi: 10.1002/ijc.27901. [DOI] [PubMed] [Google Scholar]
- 28.Palazon A, Goldrath AW, Nizet V, Johnson RS. HIF Transcription Factors, Inflammation, and Immunity. Immunity. 2014;41:518–528. doi: 10.1016/j.immuni.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Connor RS, Hao X, Shen K, Bashour K, Akimova T, Hancock WW, Kam LC, Milone MC. Substrate rigidity regulates human T cell activation and proliferation. Journal of immunology. 2012;189:1330–1339. doi: 10.4049/jimmunol.1102757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patsialou A, Wyckoff J, Wang Y, Goswami S, Stanley ER, Condeelis JS. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer research. 2009;69:9498–9506. doi: 10.1158/0008-5472.CAN-09-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madar S, Goldstein I, Rotter V. ‘Cancer associated fibroblasts’--more than meets the eye. Trends in molecular medicine. 2013;19:447–453. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Akiri G, Sabo E, Dafni H, Vadasz Z, Kartvelishvily Y, Gan N, Kessler O, Cohen T, Resnick M, Neeman M, Neufeld G. Lysyl oxidase-related protein-1 promotes tumor fibrosis and tumor progression in vivo. Cancer research. 2003;63:1657–1666. [PubMed] [Google Scholar]
- 33.Pickup MW, Laklai H, Acerbi I, Owens P, Gorska AE, Chytil A, Aakre M, Weaver VM, Moses HL. Stromally derived lysyl oxidase promotes metastasis of transforming growth factor-beta-deficient mouse mammary carcinomas. Cancer research. 2013;73:5336–5346. doi: 10.1158/0008-5472.CAN-13-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC cancer. 2011;11:245. doi: 10.1186/1471-2407-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV, De Jesus-Acosta A, Sharma P, Heidari P, Mahmood U, Chin L, Moses HL, Weaver VM, Maitra A, Allison JP, LeBleu VS, Kalluri R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srebrow A, Friedmann Y, Ravanpay A, Daniel CW, Bissell MJ. Expression of Hoxa-1 and Hoxb-7 is regulated by extracellular matrix-dependent signals in mammary epithelial cells. Journal of cellular biochemistry. 1998;69:377–391. doi: 10.1002/(sici)1097-4644(19980615)69:4<377::aid-jcb1>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 37.Brabletz T, Spaderna S, Kolb J, Hlubek F, Faller G, Bruns CJ, Jung A, Nentwich J, Duluc I, Domon-Dell C, Kirchner T, Freund JN. Down-regulation of the homeodomain factor Cdx2 in colorectal cancer by collagen type I: an active role for the tumor environment in malignant tumor progression. Cancer research. 2004;64:6973–6977. doi: 10.1158/0008-5472.CAN-04-1132. [DOI] [PubMed] [Google Scholar]
- 38.Abate-Shen C. Deregulated homeobox gene expression in cancer: cause or consequence? Nature reviews. Cancer. 2002;2:777–785. doi: 10.1038/nrc907. [DOI] [PubMed] [Google Scholar]
- 39.Bhatlekar S, Fields JZ, Boman BM. HOX genes and their role in the development of human cancers. Journal of molecular medicine. 2014;92:811–823. doi: 10.1007/s00109-014-1181-y. [DOI] [PubMed] [Google Scholar]
- 40.Shah N, Sukumar S. The Hox genes and their roles in oncogenesis. Nature reviews. Cancer. 2010;10:361–371. doi: 10.1038/nrc2826. [DOI] [PubMed] [Google Scholar]
- 41.Gilbert PM, Mouw JK, Unger MA, Lakins JN, Gbegnon MK, Clemmer VB, Benezra M, Licht JD, Boudreau NJ, Tsai KK, Welm AL, Feldman MD, Weber BL, Weaver VM. HOXA9 regulates BRCA1 expression to modulate human breast tumor phenotype. The Journal of clinical investigation. 2010;120:1535–1550. doi: 10.1172/JCI39534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ko SY, Barengo N, Ladanyi A, Lee JS, Marini F, Lengyel E, Naora H. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. The Journal of clinical investigation. 2012;122:3603–3617. doi: 10.1172/JCI62229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa BM, Smith JS, Chen Y, Chen J, Phillips HS, Aldape KD, Zardo G, Nigro J, James CD, Fridlyand J, Reis RM, Costello JF. Reversing HOXA9 oncogene activation by PI3K inhibition: epigenetic mechanism and prognostic significance in human glioblastoma. Cancer research. 2010;70:453–462. doi: 10.1158/0008-5472.CAN-09-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ko SY, Ladanyi A, Lengyel E, Naora H. Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype. The American journal of pathology. 2014;184:271–281. doi: 10.1016/j.ajpath.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bronsert P, Kohler I, Timme S, Kiefer S, Werner M, Schilling O, Vashist Y, Makowiec F, Brabletz T, Hopt UT, Bausch D, Kulemann B, Keck T, Wellner UF. Prognostic significance of Zinc finger E-box binding homeobox 1 (ZEB1) expression in cancer cells and cancer-associated fibroblasts in pancreatic head cancer. Surgery. 2014;156:97–108. doi: 10.1016/j.surg.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Zhang P, Sun Y, Ma L. ZEB1: at the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell cycle. 2015:0. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren D, Wang M, Guo W, Huang S, Wang Z, Zhao X, Du H, Song L, Peng X. Double-negative feedback loop between ZEB2 and miR-145 regulates epithelial-mesenchymal transition and stem cell properties in prostate cancer cells. Cell and tissue research. 2014;358:763–778. doi: 10.1007/s00441-014-2001-y. [DOI] [PubMed] [Google Scholar]
- 48.Sha L, Dong L, Lv L, Bai L, Ji X. HOXB9 promotes epithelial-to-mesenchymal transition via transforming growth factor-beta1 pathway in hepatocellular carcinoma cells. Clinical and experimental medicine. 2015;15:55–64. doi: 10.1007/s10238-014-0276-7. [DOI] [PubMed] [Google Scholar]
- 49.Yu M, Smolen GA, Zhang J, Wittner B, Schott BJ, Brachtel E, Ramaswamy S, Maheswaran S, Haber DA. A developmentally regulated inducer of EMT, LBX1, contributes to breast cancer progression. Genes & development. 2009;23:1737–1742. doi: 10.1101/gad.1809309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo J, Fu Z, Wei J, Lu W, Feng J, Zhang S. PRRX1 promotes epithelial-mesenchymal transition through the Wnt/beta-catenin pathway in gastric cancer. Medical oncology. 2015;32:393. doi: 10.1007/s12032-014-0393-x. [DOI] [PubMed] [Google Scholar]
- 51.Micalizzi DS, Christensen KL, Jedlicka P, Coletta RD, Baron AE, Harrell JC, Horwitz KB, Billheimer D, Heichman KA, Welm AL, Schiemann WP, Ford HL. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-beta signaling. The Journal of clinical investigation. 2009;119:2678–2690. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holland PW, Booth HA, Bruford EA. Classification and nomenclature of all human homeobox genes. BMC biology. 2007;5:47. doi: 10.1186/1741-7007-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mace KA, Hansen SL, Myers C, Young DM, Boudreau N. HOXA3 induces cell migration in endothelial and epithelial cells promoting angiogenesis and wound repair. Journal of cell science. 2005;118:2567–2577. doi: 10.1242/jcs.02399. [DOI] [PubMed] [Google Scholar]
- 54.Brock A, Krause S, Li H, Kowalski M, Goldberg MS, Collins JJ, Ingber DE. Silencing HoxA1 by intraductal injection of siRNA lipidoid nanoparticles prevents mammary tumor progression in mice. Science translational medicine. 2014;6:217ra212. doi: 10.1126/scitranslmed.3007048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgan R, Boxall A, Harrington KJ, Simpson GR, Michael A, Pandha HS. Targeting HOX transcription factors in prostate cancer. BMC urology. 2014;14:17. doi: 10.1186/1471-2490-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morgan R, Boxall A, Harrington KJ, Simpson GR, Gillett C, Michael A, Pandha HS. Targeting the HOX/PBX dimer in breast cancer. Breast cancer research and treatment. 2012;136:389–398. doi: 10.1007/s10549-012-2259-2. [DOI] [PubMed] [Google Scholar]
- 57.Patrick AN, Cabrera JH, Smith AL, Chen XS, Ford HL, Zhao R. Structure-function analyses of the human SIX1-EYA2 complex reveal insights into metastasis and BOR syndrome. Nature structural & molecular biology. 2013;20:447–453. doi: 10.1038/nsmb.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rhoads K, Arderiu G, Charboneau A, Hansen SL, Hoffman W, Boudreau N. A role for Hox A5 in regulating angiogenesis and vascular patterning. Lymphatic research and biology. 2005;3:240–252. doi: 10.1089/lrb.2005.3.240. [DOI] [PubMed] [Google Scholar]
- 59.Carrio M, Arderiu G, Myers C, Boudreau NJ. Homeobox D10 induces phenotypic reversion of breast tumor cells in a three-dimensional culture model. Cancer research. 2005;65:7177–7185. doi: 10.1158/0008-5472.CAN-04-1717. [DOI] [PubMed] [Google Scholar]