Abstract
Background
Nelarabine has shown impressive single agent clinical activity in T-cell acute lymphoblastic leukemia (T-ALL), but has been associated with significant neurotoxicities in heavily pre-treated patients. We showed previously that it was safe to add nelarabine to a BFM-86 chemotherapy backbone (AALL00P2). Children’s Oncology Group (COG) AALL0434 is a Phase III study designed to test the safety and efficacy of nelarabine when incorporated into a COG augmented BFM-based regimen, which increases exposure to agents with potential neurotoxicity compared to the historical AALL00P2 regimen.
Procedure
AALL0434 included a safety phase to assess nelarabine toxicity. Patients with high-risk (HR) T-ALL were randomized to receive Capizzi-style escalating methotrexate (MTX) plus pegaspargase or high dose (HD) MTX with/without six five-day courses of nelarabine. We report results from 94 patients who participated in the initial safety phase of the study.
Results
There were no differences in the incidence of peripheral motor neuropathies, sensory neuropathies or central neurotoxicities among those randomized to the nelarabine (n = 47) and non-nelarabine arms (n = 47).
Conclusions
The addition of nelarabine to COG-augmented BFM chemotherapy regimen is safe and feasible. The ongoing AALL0434 Efficacy Phase will determine whether the addition of nelarabine treatment improves outcome for patients with T-ALL.
Keywords: Nelarabine, T-ALL, BFM chemotherapy regimen
Introduction
Dose-intensified, multi-agent therapy, leads to long-term remissions for 80 to 85% of patients with T-lineage acute lymphoblastic leukemia (T-ALL).[1–6] However, patients with high risk (HR) T-ALL, characterized by elevated minimal residual disease (MRD) levels at the end of induction or beyond have inferior outcomes.[2,7] Relapsed T-ALL is rarely cured, suggesting that new and targeted therapies should be evaluated in T-ALL in first remission, particularly in patients with a poor response to initial therapy.[8–12] Nelarabine (Compound 506U78; IND# 52611) has shown impressive single agent clinical activity in relapsed T-ALL, but has been associated with significant central, sensory and motor neuropathies, especially when given to heavily pre-treated patients, [13] or used in combination with other therapies associated with neurotoxicity. We recently reported results of Children’s Oncology Group (COG) pilot study AALL00P2, which showed that was safe to add nelarabine to a BFM-86 based regimen in newly diagnosed T-ALL patients, with encouraging outcome data in a small number of HR T-ALL patients.[14]
Based on these encouraging data, we developed a randomized trial (COG AALL0434) of chemotherapy with/without nelarabine in children with newly diagnosed T-ALL. The COG augmented BFM (ABFM) regimen with single interim maintenance (IM) and delayed intensification (DI) phases was selected as the treatment backbone based on excellent results from CCG 1961 for patients with NCI HR B- and T-ALL.[15] Two different methods of methotrexate intensification have been shown to be effective in pediatric ALL, high dose (5 gm/m2 over 24 hours) methotrexate (HD MTX) with leucovorin rescue [16] and 6-Mercaptopurine (6-MP), [16–18], and Capizzi-style escalating intravenous methotrexate (CMTX) without leucovorin rescue plus pegaspargase. [15,19] AALL0434 employs a 2 × 2 factorial design, one question randomizing patients to either receive CMTX or HD MTX during IM, and the second randomizing patients to receive or not receive six 5-day courses of nelarabine. Because the COG ABFM backbone includes more intensive intrathecal chemotherapy and more frequent vincristine administration than was given on COG AALL00P2, AALL0434 included a safety phase, the results of which are reported here, to first assess the tolerability of adding nelarabine to the COG ABFM backbone containing either CMTX or HD MTX.
Methods
Patients
Patients aged 1 to 30.99 years with newly-diagnosed T-ALL (>25% marrow blasts or white blood cell count (WBC) ≥25,000/μL with ≥50% blasts) were eligible for the study. Patients may have received up to 600 cGy of emergent chest radiation to manage airway compromise; however, none in the Safety Phase required such treatment. Subjects with a prior seizure disorder requiring anti-convulsant therapy were assigned to non-nelarabine containing treatment arms. All patients and/or their legal guardians provided written informed consent as required by all federal, state and local institutional requirements. A total of 600 patients had enrolled on AALL0434 from January 2007 to February 2010, when the Safety Phase interim analysis was performed. Based on the study-defined risk criteria, 45 patients had low risk, 356 had intermediate risk, 94 had HR disease, 17 had failed induction, ten patients were not evaluable for randomization post induction, and 78 had not finished induction therapy. We report results from 94 patients who participated in the initial safety phase of the study. This study is registered at http://clinicaltrials.gov as NCT00408005.
Safety Phase Treatment Plan
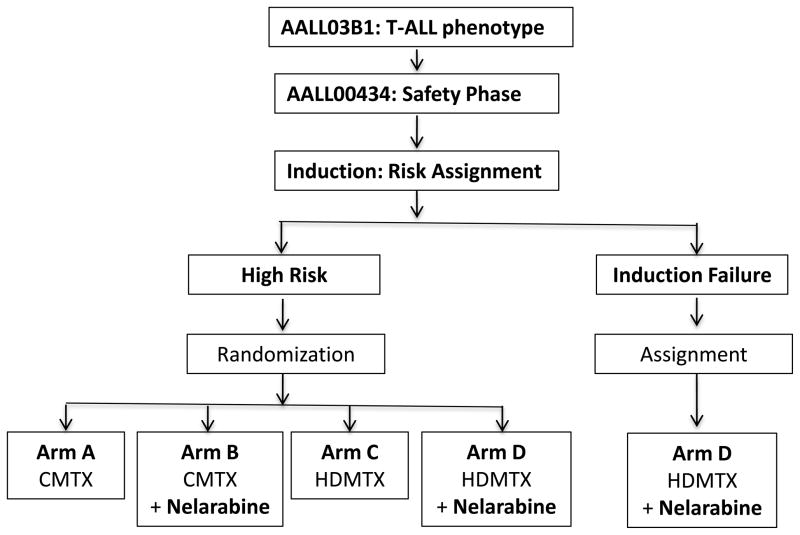
All patients received an identical 4-week, 4-drug induction phase which consisted of prednisone 30 mg/m2/dose twice daily for 28 days; vincristine 1.5 mg/m2/dose (max 2 mg) days 1, 8, 15, and 22; daunorubicin 25 mg/m2/dose days 1, 8, 15, and 22 and pegaspargase 2500 IU/m2/dose x1, days 4 or 5 or 6. Age-adjusted intrathecal (IT) cytarabine was given on day 1, and age-adjusted IT MTX on days 8 and 29 for CNS1 and 2 disease, and additionally on days 15 and 22 for those with CNS3 disease. Following induction, patients were given treatment per the treatment arms described in Table I. At the end of induction, patients were assigned to low, intermediate or high-risk groups based on early response. Patients with low-risk disease were defined as having NCI standard risk (age 1–9.99 years and initial WBC <50,000/microliter) clinical features without CNS or testicular disease, an M1 marrow (<5% blasts) by day 15, and day 29 minimal residual disease (MRD) <0.1%, as measured in a COG Reference Laboratory using flow cytometric techniques. Patients with intermediate risk T-ALL were those with any NCI high risk features (age ≥10 years and/or initial WBC ≥50,000/microliter), any CNS or testicular disease, any marrow response by D15 and day 29 MRD < 1%. Patients with HR-T-ALL were defined as those having either an M2 marrow (5–25% blasts) or MRD ≥1% on Day 29 of induction therapy. Following completion of induction therapy, patients with HR disease received the ABFM backbone (Figure 1) with randomization to Arms A, B, C or D (Table I) using a 2 × 2 factorial design. All received prophylactic cranial radiation (1,200 cGy for CNS1 and CNS2; 1,800 cGy for CNS3) either during Consolidation for those randomized to Capizzi MTX (Arms A or B), or during DI if randomized to receive HD MTX (Arms C or Arm D). Patients with HR disease with central nervous system leukemia (CNS3; ≥5 white blood cells/mm3 cerebrospinal fluid with blasts present on cytospin) or testicular involvement were non-randomly assigned to receive HD MTX and randomized to receive/not receive nelarabine (Arms C or D). Only patients with HR T-ALL without CNS3 disease were included in the Safety Phase analysis. Those with induction failure (>25% marrow blasts at day 29) were non-randomly assigned to HD MTX and nelarabine (Arm D). Because such patients were considered to be dissimilar from patients who achieved a first remission, they were not included in the Safety Phase analysis (Figure 1).
Table I.
Arms A–D in the AALL0434 Safety Phase Factorial Design
| Arm | Consolidation | Interim Maintenance | Delayed Intensification | Maintenance† |
|---|---|---|---|---|
|
Arm D N = 23 |
Nelarabine: 650 mg/m2 days 1–5 & 43–47. | HD MTX: 5,000 mg/m2 with leucovorin rescue; days 1, 15, 29 & 43.+ | Nelarabine: 650 mg/m2 IV days 29–33. | Nelarabine: 650 mg/m2 IV days 29–33; first three cycles. |
|
Arm C N = 23 |
HD MTX: 5,000 mg/m2 with leucovorin rescue; days 1, 15, 29 & 43.+ | |||
|
Arm B N = 24 |
Nelarabine: 650 mg/m2 days 1–5 & 43–47. | Capizzi MTX: 100 mg/m2 Days 1, 11, 21, 31 & 41 (escalating by 50 mg/m2 dose) ‡ | Nelarabine: 650 mg/m2 IV days 29–33. | Nelarabine: 650 mg/m2 IV days 29–33; first three cycles. |
| Arm A N = 24 | Capizzi MTX: 100 mg/m2 Days 1, 11, 21, 31 & 41 (escalating by 50 mg/m2 dose)‡ | |||
| All Arms | IT MTX - days 15, 22, 57, & 64. | IT MTX - days 1 & ~29. | IT MTX - days 1, 36 & 43. | IT MTX - day 1 |
| VCR: 1.5 mg/m2 IV days 22, 64 & 71. | VCR: 1.5 mg/m2 IV days 1, 15, 29 & 43. | VCR 1.5 mg/m2 IV days 1, 8, 15, & 50. | VCR: IV 1.5 mg/m2 days 1 & 57. | |
| PEG Asp 2500 IU/m2 IM days 22 & 64. | ‡ PEG Asp: 2500 units/m2 IM days 2 & 22. | PEG Asp 2500 IU/m2 IM day 4 & 43. | ||
| Cyclophosphamide: 1000 mg/m2 IV days 8 & 50. | Cyclophosphamide: 1000 mg/ m2 IV day 36. | |||
| ARA-C: 75 mg/m2 days 8–11, 15–18, 50–53 & 57–60. | ARA-C: 75 mg/m2 IV or SubQ days 36–39 & 43– 46. | |||
| 6MP: 60 mg/m2 days 8– 21 & 50–63. | + 6MP: 25 mg/m2 days 1– 56. | 6TG: 60 mg/ m2 days 36–49 (omit for CRT). | 6MP: 75 mg/ m2 days 1– 84 (not with nelarabine). | |
| DEX: 5 mg/m2 BID days 1–7 & 15–21. | PRED: 20 mg/m2 BID days 1–5, 29–33 & 57–61 | |||
| . | DOXO: 25 mg/ m2 days 1, 8 &15. | |||
| MTX: 20 mg/m2 PO days 8, 15, 22, 29, 36, 43, 50, 57, 64, 71 & 78 (except during nelarabine administration) | ||||
| Cranial Radiation: 1,200 cGy for CNS1-2c patients on Arms A and B; 1800 cGy for CNS3 Weeks 3–4 (A) or 4–5 (B) | Cranial Radiation: 1,200 cGy for CNS1-2c patients on Arms C and D; 1800 cGy for CNS3 Week 7, Day 50 (C, D) |
Boys received three (3) years and girls received two (2) years of Maintenance Phase therapy, ending of the anniversary of Interim Maintenance start date.
BOLD text identifies drug dosing and timing that varied within the arms. “~” relative date of therapy administration, adjusted with relation to other phase-defined therapies for each arm. Nelarabine was not given within two weeks of other chemotherapies known to cause central or peripheral toxicities. CNS1: no morphological evidence for blastsCNS2a-c: < 5TNC AND blasts by morphology, modified per Steinherz-Bleyer formula. [32] CNS3: > 5 TNC chamber count AND morphological presence of leukemic blasts.
Figure 1.
Safety Phase AALL0434 Study Schema
Only the High Risk patients were randomized to receive or not receive nelarabine at a dose of 650 mg/m2/day for 5 days during the Consolidation, Delayed Intensification and Maintenance phases of therapy. All patients received only one Delayed Intensification course and all High Risk Induction Failure patients receive prophylactic cranial radiation (1,200 cGy) either during Consolidation (if randomized to treatment Arm A (CMTX) or Arm B (CMTX + Nel)) or Delayed Intensification (if randomized to treatment Arm C (HDMTX) or Arm D (HDMTX + Nel). All patients for whom induction failed were assigned to receive treatment on Arm D, but were not included in the Safety Phase analysis. All patients with HR disease classified as CNS3 were assigned to receive HD MTX on either Arm C (HDMTX) or Arm D (HDMTX + Nel) and received cranial radiation therapy (CRT) (1,800 cGy) during Delayed Intensification. Patients with testicular leukemia were assigned to receive HDMTX on either Arm C (HDMTX) or Arm D (HDMTX + Nel), and received testicular radiation therapy (TRT) (2,400 cGy) during Consolidation therapy, if testicular disease had not resolved by the end of Induction therapy.
Dose limiting toxicities were monitored in all patients, with targeted monitoring of specific additional toxicities among patients with HR disease. Specific reporting procedures were implemented to alert the study team to grade 3 (or higher) toxicities that were attributable to nelarbine. The study design included a planned interim analysis after at least 40 Patients with HR disease (10 each on each arm) had received treatment through Week 44. This time-point was chosen because it allowed for resolution of radiation-induced somnolence syndrome. Safety and toxicity rules were defined in advance with a primary monitoring rule to halt the nelarabine randomization for any death that could be clearly attributed to nelarabine. Secondary monitoring rules called for cessation of the nelarabine randomization with either 10% grade 4 or 20% grade 3 peripheral neuropathies that did not resolve within one week after receiving nelarabine. If and when the Data and Safety Monitoring Committee (DSMC) determined that nelarabine could be safely incorporated into the AALL0434 study regimen, the study was designed to open an Efficacy Phase during which all high and intermediate risk patients would participate in the nelarabine randomization.
MRD Determination of HR Status
Bone marrow morphology was assessed by light microscopy at each participating institution. MRD was determined at a single COG Reference Laboratory (University of Washington) on samples shipped overnight by express carrier using methods similar to those described previously for B-ALL.[20] For patients with HR disease, mid- and end-Consolidation MRD was determined. However, these data were collected to assess the overall arm-related treatment efficacy, and were not reported to the treating physician, and have not yet been unblinded. Patients who did not have Day 29 MRD and marrow morphological assessments were deemed inevaluable for post-induction therapy. To assure accurate MRD detection, all cases were assessed for end-induction drift in MRD antigen expression.[21]
Toxicity Reporting Metrics and Procedures
At the completion of each phase of treatment, adverse event data were submitted to COG through the COG remote data entry system. Each adverse event was coded using the appropriate CTCAE metrics for versions 3 and 4. All grade 3 and higher adverse events occurring during each phase of treatment were captured with protocol specific exceptions. Exceptions included required reporting of any grade CNS toxicities and peripheral neuropathies regardless of treatment arm and no reporting required for any hematological toxicity unless it was grade 3 or higher and also resulted in a delay of treatment for greater than one week or hospitalization. The modified Balis Scale was used to grade neurotoxic events that could be attributed to any bioactive agent used in this study. The protocol included a comprehensive Adverse Event and Potential Risks listing for nelarabine, with specific instructions on the reporting of expected and unexpected events. Adverse events meeting specific, pre-determined criteria were required to be reported to the Cancer Therapeutics Evaluation Program and the Food and Drug Agency using the AdEERS reporting system. Investigators were required to contact the Study Chairs with grade 3 (or higher) non-hematological adverse events that were possibly, probably or definitely attributable to nelarabine.
Statistical Analysis for the Safety Phase Cohort
For the study’s interim safety analysis, all adverse events (AEs) observed for the first 57 patients with HR disease who had completed therapy through Maintenance Cycle 3 were reported to the COG DSMC. To expand the Safety Phase experience with nelarabine, we have also analyzed AEs for 37 additional HR T-ALL patients who continued to be randomized to each of the four study arms during the study’s planned interim analysis. These additional patients were also followed through Maintenance Cycle 3. In total 94 patients were evaluated for differences in toxicity as part of the Safety Phase interim analysis, AEs were compared between HR T-ALL patients randomized to receive CMTX (Arm A) or HD MTX (Arm C) without nelarabine, and those randomized to receive nelarabine with CMTX (Arm B) or HDMTX (Arm D) regimens. For the Safety Phase statistical analyses, events for targeted toxicities were compared between the study arms using two-sided Fisher’s chi-squared analyses. Additionally, individual drug toxicity attributions are described to distinguish events related to specific agents.
Results
Ninety-four patients randomized during the Safety Phase to receive/not receive nelarabine were fully evaluable and are the subject of this report. Interestingly, 24.7% of all enrollees were identified as having ≥1% end-induction MRD, a finding similar to European TALL studies in which MRD was determined through PCR quantification.[7] Forty-seven were randomized not to receive nelarabine, 24 with Capizzi (Arm A) and 23 with HD MTX (Arm C), and 47 were randomized to receive nelarabine, 24 received Capizzi (Arm B) and 23 HD MTX (Arm D) (Table I). Toxicities were compared between patients who were randomized to regimens with nelarabine (Arms B and D) and without nelarabine (Arms A and C). Since randomization occurred at the end of Induction therapy and nelarabine was not administered during this phase, AEs from Induction were excluded from analysis. Otherwise, all adverse event data submitted during the Safety Phase were included in the analyses; sensory, motor and central toxicities are shown in Table II.
Table II.
Sensory, Motor and Central Neuropathies
| Sensory Neuropathies | Grade 1 | Grade 2 | Grade 3 | Grade 4 | P value* | |
|---|---|---|---|---|---|---|
| C | Nelarabine | 3 (DEF, POS, UNR) | 2 (POS, UNR) | 0 | 0 | 1.00 |
| No Nelarabine | 3 | 1 | 0 | 0 | ||
| IM | Nelarabine | 2 (UNL, UNR) | 1 (UNR) | 1 (POS) | 0 | 1.00 |
| No Nelarabine | 2 | 2 | 0 | 0 | ||
| DI | Nelarabine | 3 (UNR x 2, UNL) | 0 | 3 (UNLx3) | 0 | 0.74 |
| No Nelarabine | 1 | 3 | 0 | 0 | ||
| M1 | Nelarabine | 2 (POS, UNL) | 3 (POS, UNLx2) | 1 (UNL) | 0 | 0.74 |
| No Nelarabine | 1 | 2 | 1 | 0 | ||
| M2 | Nelarabine | 2 (UNLx2) | 0 | 0 | 0 | 1.00 |
| No Nelarabine | 1 | 0 | 0 | 0 | ||
| M3 | Nelarabine | 2 (POS, UNL) | 0 | 0 | 0 | 1.00 |
| No Nelarabine | 1 | 1 | 0 | 0 | ||
| Motor Neuropathies | ||||||
| C | Nelarabine | 2 (PROB, POS) | 3 (PROB, POS, UNL) | 1 (POS) | 0 | 0.74 |
| No Nelarabine | 2 | 2 | 0 | 0 | ||
| IM | Nelarabine | 1 (UNL) | 5 (UNLx2, UNRx3) | 1 (POS) | 0 | 0.16 |
| No Nelarabine | 1 | 1 | 0 | 0 | ||
| DI | Nelarabine | 1 (UNR) | 4 (UNLx3, UNR) | 3 (PR, UNLx2) | 0 | 0.36 |
| No Nelarabine | 1 | 3 | 0 | 0 | ||
| M1 | Nelarabine | 1 (UNL) | 1 (UNL) | 1 (UNL) | 0 | 1.00 |
| No Nelarabine | 1 | 1 | 1 | 0 | ||
| M2 | Nelarabine | 1 (UNL) | 0 | 0 | 0 | 1.00 |
| No Nelarabine | 0 | 1 | 0 | 1 | ||
| M3 | Nelarabine | 0 | 0 | 0 | 0 | - - |
| No Nelarabine | 0 | 0 | 0 | 0 | ||
| Central Neuropathies | ||||||
| C | Nelarabine | 0 | 4 (UNR, UNL, POS x2) | 0 | 0 | 0.12 |
| No Nelarabine | 0 | 0 | 0 | 0 | ||
| IM | Nelarabine | 1 (UNR) | 0 | 0 | 0 | 0.20 |
| No Nelarabine | 0 | 2 | 2 | 1 | ||
| DI | Nelarabine | 2 (UNRx2) | 1 (PROB) | 1 (POS) | 0 | 0.68 |
| No Nelarabine | 0 | 1 | 1 | 0 | ||
| M1 | Nelarabine | 1 (UNR) | 1 (POS) | 0 | 0 | 0.50 |
| No Nelarabine | 0 | 0 | 0 | 0 | ||
| M2 | Nelarabine | 0 | 0 | 0 | 0 | 1.00 |
| No Nelarabine | 0 | 0 | 1 | 0 | ||
| M3 | Nelarabine | 0 | 0 | 0 | 0 | - - |
| No Nelarabine | 0 | 0 | 0 | 0 | ||
UNR = unrelated, UNL = unlikely, POS = possible, PROB = probable, and DEF = definite;
P values: Fisher’s Exact test.
Safety analyses
Sensory Neuropathy
There were 22 sensory AEs in all phases of therapy on the non-nelarabine containing arms (8 and 14, respectively, on Arms A and C), and 27 sensory AEs in all phases of therapy among patients randomized to receive nelarabine (14 and 13, respectively, on Arms B and D). The AEs are shown by phase of therapy in Table II, sensory neuropathy. Of the six grade 3 sensory neuropathies, only one was possibly attributable to nelarabine, while a second AE in the same patient was attributed to MTX. No grade 4 sensory AEs were reported for any patient randomized to +/− nelarabine during the AALL0434 Safety Phase.
Motor Neuropathy
There were 18 motor neuropathy AEs in all phases and of any grade reported on Arms A (n =12) and C (n = 6), which did not include nelarabine, and 28 reported on the nelarabine-containing Arms B (n = 18) and D (n = 10)(Table II, motor neuropathies). There were only seven grade 3 motor neuropathy toxicities reported. For the six patients who were treated on nelarabine-containing arms, three of the grade 3 AEs were attributed to nelarabine. For the three AEs that were attributed to nelarabine, one occurred in a patient on Arm B who developed a grade 3 motor neuropathy during Interim Maintenance therapy, and two occurred in patients who were treated on Arm D, one in Consolidation and the other during DI phase therapy. The only grade 4 motor neuropathy occurred in a patient on a non-nelarabine containing arm. Most neurotoxicities that were attributed to nelarabine either occurred at the time of exposure, or within two weeks following its administration. Statistical analyses show no differences in the incidence of motor neuropathies rates between Arms A and B or between Arms C and D among all 94 patients with HR disease.
Central Neuropathies
Encephalopathy, seizure, stroke, extrapyramidal tract symptoms, acute mental status changes and somnolence were considered central neurotoxicities, each of which has been attributed to chemotherapeutic agents, including nelarabine (Table II, central neuropathies). There was one grade 4 central neurotoxicity described in a patient who did not receive nelarabine, which presented as acute mental status changes during IM phase therapy with CMTX. The central neurotoxicities attributed to nelarabine included two grade 2 episodes of tremor that were possibly related to nelarabine given during consolidation; one grade 2 episode of somnolence, probably attributable to nelarabine, and one grade 3 episode of cranial nerve V dysfunction during DI therapy, possibly related. Central neurotoxicities on the non-nelarabine containing arms were most frequently attributed to MTX when administered as CMTX, HD MTX or as intrathecal MTX.
Two-sided Fisher’s Exact tests were performed for sensory grades 1–3 (there were no grade 4), motor grades 1–4 and central grades 1–4 toxicites between nelarabine and non-nelarabine containing arms through Maintenance Cycle 3. There were no statistical differences between occurrences of all neurological AEs, regardless of attribution for any of the comparisons. However, neurotoxicities were more commonly reported for DI phase therapy, most likely the result of the cumulative exposure to potentially neurotoxic agents used in this and previous phases of treatment (Table I). For patients treated on Arms A and C (no nelarabine) having any grade neurological adverse event, 14 of 22 (64%) experienced a complete resolution of their symptoms after one reporting period. For the remaining eight cases, only one reported a grade 1 sensory neuropathy that persisted beyond Maintenance Cycle 3. For the patients treated on Arms B and D (nelarabine), 13 of 22 (59%) had a complete resolution of their symptoms after one reporting period. For the remaining nine cases, only one patient had a grade 1 motor neuropathy that persisted beyond Maintenance Cycle 3. These results show that over 90% of reported neurotoxicities resolved during Maintenance phase therapy.
Non-neurological toxicities
All arms of the protocol are myelosuppressive and associated with infectious toxicities. The types and grades of hematological toxicities were the same between the nelarabine and non-nelarabine arms, with two exceptions. Grade 3 and 4 anemia and thrombocytopenia was more pronounced in the non-nelarabine containing arms during DI phase therapy (Supplemental Table I, P = 0.03 and 0.001, respectively). Additional toxicities including febrile neutropenia and AST/ALT elevations were similar in the study arms with/without nelarabine.
Death as a First Event
Among all 94 HR T-ALL study subjects, one patient died during induction and eight patients died after being randomized at end induction to the four treatment arms. On Arm A, one patient died from an opportunistic infection that developed during the Consolidation phase of therapy. Three patients died on Arm C; two with progressive disease, and one from transplant-related complications. Three patients died on Arm D, two from progressive disease. The third developed a superior sagittal sinus thrombosis associated with pegaspargase during Consolidation, was treated with low molecular weight heparin, and died following a left temporoparietal hemorrhage. No patients died from complications related to nelarabine. Based on the analyses of toxicity observed during the Safety Phase among HR T-ALL patients treated with ABFM chemotherapy including either CMTX or HD MTX with/without nelarabine, we concluded that nelarabine administration was safe. The efficacy phase of AALL0434 opened to accrual in October 2010. Accrual to AALL0434 was completed in July 2014.
Nelarabine dose-delivery
To assess the feasibility of completing all assigned nelarabine doses, all patients who were enrolled on Arms B and D were tracked through Maintenance Cycle 3 for neurological AEs attributable to nelarabine versus other agents. On Arm B, 15 of 24 patients (63%) received all 30 scheduled doses of nelarabine. Among the nine patients who did not receive all scheduled doses of nelarabine, two had their nelarabine stopped, one for grade 2 diploplia that occurred concomitantly with a nelarabine course administered in Delayed Intensification, and one for grade 3 sensory and motor neuropathies that were attributed to nelarabine when administered during Consolidation Cycle 2. One patient with a non-nelarabine associated neurotoxicity developed somnolence after receiving lorazepam on Day 2 of nelarabine during Maintenance Cycle 3. Two patients were ineligible for nelarabine therapy because of an underlying seizure disorder, one discovered before nelarabine was administered, and one discovered after Consolidation Cycle 1. Four patients did not receive all scheduled doses of nelarabine for reasons of family withdrawal of consent, physician preference and relapsed disease. Overall, 549 of 720 possible doses were delivered on Arm B, for a dose-intensity of 76% (Table III).
Table III.
Nelarabine dose intensity
| Arm B, n = 24 (actual doses received) | Total doses | |
|---|---|---|
| Full nelarabine exposure, n = 15 (30 each) | 450 | |
| Attenuated nelarabine exposure, n = 9 | ||
| # Neurotoxic AEs, attributable to nelarabine, n = 2 (10, 12) | 22 | |
| # Neurotoxic AEs, not attributable to nelarabine, n = 1 (27) | 27 | |
| # Underlying seizure disorder, n = 2 (0, 5) | 5 | |
| * Other, n = 4 (0, 10, 10, 25) | 45 | |
| 549 | ||
| Received/possible: 549/720 = 76% | ||
| Arm D, n = 23 (actual doses received) | ||
| Full nelarabine exposure, n = 15 (30 each) | 450 | |
| Attenuated nelarabine exposure, n = 8 | ||
| # Neurotoxic AEs, attributable to nelarabine, n = 2 (10, 20) | 30 | |
| # Neurotoxic AEs, not attributable to nelarabine, n = 1 (10) | 10 | |
| * Other, n = 5 (5, 15, 15, 20, 20) | 75 | |
| 565 | ||
| Received/possible: 565/690 = 82% | ||
Per study guidelines, grade 3 (or higher) neurotoxic adverse events that did not resolve to grade 2 or less, attributable to any neurotoxin, precluded the continued use of nelarabine (n = 6), as did a seizure disorder (n = 2).
Other = Relapse (n = 6) or death from septic complications (n = 1), and as ascribed by the physician, non-compliance with the therapy (n = 1) and long delays in therapy (n = 1).
On Arm D, 15 of 23 patients (65%) were able to receive all scheduled doses of nelarabine. Of the two patients in whom nelarabine was discontinued due to drug-specific neurotoxicities, one had grade 3 motor neuropathies, and the other had grade 3 muscle pain. One patient was taken off therapy in Interim Maintenance for grade IV toxicities that were attributed to HD-MTX and complicated by metoclopramide-induced dystonia. Of the five patients who did not receive all scheduled nelarabine doses, the reasons cited for discontinuing nelarabine administration included infectious complications, physician preference, and for relapsed disease. Overall, 565 of 690 doses were delivered, yielding an overall dose intensity of 82% (Table III). In total, 79% of all intended doses of nelarabine were given for patients on Arms B and D.
Discussion
T-cell lymphoid malignancies are clinically and biologically distinct from B-lymphoid malignancies.[22,23] Historically, the diagnosis of T-ALL portended a worse prognosis than other forms of childhood ALL.[23–25] Over the past three decades, the introduction of intensive, high-dose, multi-agent pulse chemotherapy has significantly improved event-free survival for patients with T-ALL from 15–20% to 75% or higher, as shown by numerous cooperative group studies, including the CCG 1961[15], the POG 9404[1], the DFCI [18,26], St. Jude Children’s Research Hospital[27], the UKALL[4] and the BFM.[2,28] While earlier studies indicated that nelarabine may be associated with significant neurotoxicity in heavily pre-treated relapsed T-ALL patients, [13] we now extend the findings of AALL00P2[14] to show that it can be safely used in newly diagnosed patients with T-ALL receiving the COG ABFM backbone with either CMTX or HD MTX during a single IM phase.
As a targeted therapy for T-lymphoblastic disease, nelarabine (2-amino-9-B-D-arabinofuranosyl-6-methoxy-9H-purine) is a water-soluble prodrug of araG (9-B-arabinofuranosylguanine) and is cytotoxic to T-lymphoblasts at micromolar concentrations.[10,29,30] In the development of the AALL0434 study, the ABFM backbone was chosen as it provided data to support the omission of CRT in low-risk patients, and included a lower total anthracycline dose. [1,31] The ABFM backbone was also most similar to the pilot study, AALL00P2, which incorporated nelarabine into a multi-agent backbone, and to the COG AALL0232 study for patients with HR B-ALL. The use of the same ABFM backbone was reasoned to facilitate the continued comparison of response measures, including the significance of MRD among patients with T- and B-ALL.[7,32]
COG AALL00P2 previously showed that it was safe to add 5-day courses of nelarabine (400 mg/m2/day and 650 mg/m2/day) to a modified BFM-86 backbone in newly diagnosed T-ALL patients. [14] Here, we show that in newly diagnosed T-ALL patients, it is safe to add six 5-day courses of nelarabine 650 mg/m2/day to the COG ABFM backbone that includes more intensive use of agents with potential neurotoxicity than does the BFM-86 regimen used in AALL00P2. In general, most neurotoxicities progressively accumulated during the Consolidation, IM and DI phases, and were largely attributed to vincristine. Regardless of attribution, the neuropathies gradually resolved in subsequent phases of Maintenance therapy. As shown in Supplemental Table II, substantial increases in steroid exposure, vincristine doses and number of IT MTX administrations distinguish the AALL0434 ABFM backbone from the AALL00P2 BFM-86 regimen. Overall, approximately 80% of nelarabine doses were delivered. Cessation of therapy was for a variety of reasons, but the most common being due to relapsed disease. The AALL0434 efficacy phase has now opened, with both the intermediate and HR groups that comprise about 90% of T-ALL patients, randomly assigned to four treatment arms. The COG experience with nelarabine in AALL00P2 and AALL0434 demonstrates that while this agent has significant neurotoxicity in heavily pre-treated T-ALL patients, it is well-tolerated in newly diagnosed patients. AALL0434 will determine whether or not nelarabine treatment improves the outcome of children, adolescents and young adults with newly diagnosed T-ALL.
Supplementary Material
Hematological Toxicities
Total cumulative CNS toxin exposure on AALL00P2 and AALL0434
Acknowledgments
This work was supported by grants CA13539 and CA98543 from the National Institutes of Health to the COG. The authors wish to thank Dr. Malcolm Smith and the members of the Cancer Therapeutic Evaluation Program (CTEP) for their helpful oversight of the protocol during its development, and to members the Federal Drug Administration for help in supporting the evaluation of nelarabine in a Phase III clinical trial. We also wish to than Dr. Mark Russo and GlaxoSmithKline for kindly providing nelarabine to the study subjects through CTEP, and to the patients and families who participated in the study.
Footnotes
COI Statement: None of the authors has declared competing financial or intellectual interests in relation to his manuscript.
References
- 1.Asselin BL, Devidas M, Wang C, Pullen J, Borowitz MJ, Hutchison R, Lipshultz SE, Camitta BM. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children’s Oncology Group (POG 9404) Blood. 2011;118(4):874–883. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrappe M, Valsecchi MG, Bartram CR, Schrauder A, Panzer-Grumayer R, Moricke A, Parasole R, Zimmermann M, Dworzak M, Buldini B, Reiter A, Basso G, Klingebiel T, Messina C, Ratei R, Cazzaniga G, Koehler R, Locatelli F, Schafer BW, Arico M. Late MRD response determines relapse risk overall and in subsets of childhood T-cell ALL: results of the AIEOP-BFM-ALL 2000 study. Blood. 2011;118(8):2077–2084. doi: 10.1182/blood-2011-03-338707. [DOI] [PubMed] [Google Scholar]
- 3.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, Reaman GH, Carroll WL. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(14):1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vora A, Goulden N, Wade R, Mitchell C, Hancock J, Hough R, Rowntree C, Richards S. Treatment reduction for children and young adults with low-risk acute lymphoblastic leukaemia defined by minimal residual disease (UKALL 2003): a randomised controlled trial. Lancet Oncol. 2013;14(3):199–209. doi: 10.1016/S1470-2045(12)70600-9. [DOI] [PubMed] [Google Scholar]
- 5.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 6.Whitlock JA, Silverman LB. Childhood T-all: it’s time to move on. Blood. 2011;118(4):828–829. doi: 10.1182/blood-2011-05-348375. [DOI] [PubMed] [Google Scholar]
- 7.Willemse MJ, Seriu T, Hettinger K, d’Aniello E, Hop WC, Panzer-Grumayer ER, Biondi A, Schrappe M, Kamps WA, Masera G, Gadner H, Riehm H, Bartram CR, van Dongen JJ. Detection of minimal residual disease identifies differences in treatment response between T-ALL and precursor B-ALL. Blood. 2002;99(12):4386–4393. doi: 10.1182/blood.v99.12.4386. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MH, Johnson JR, Massie T, Sridhara R, McGuinn WD, Jr, Abraham S, Booth BP, Goheer MA, Morse D, Chen XH, Chidambaram N, Kenna L, Gobburu JV, Justice R, Pazdur R. Approval summary: nelarabine for the treatment of T-cell lymphoblastic leukemia/lymphoma. Clin Cancer Res. 2006;12(18):5329–5335. doi: 10.1158/1078-0432.CCR-06-0606. [DOI] [PubMed] [Google Scholar]
- 9.DeAngelo DJ, Yu D, Johnson JL, Coutre SE, Stone RM, Stopeck AT, Gockerman JP, Mitchell BS, Appelbaum FR, Larson RA. Nelarabine induces complete remissions in adults with relapsed or refractory T-lineage acute lymphoblastic leukemia or lymphoblastic lymphoma: Cancer and Leukemia Group B study 19801. Blood. 2007;109(12):5136–5142. doi: 10.1182/blood-2006-11-056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez CO, Jr, Stellrecht CM, Gandhi V. Mechanisms for T-cell selective cytotoxicity of arabinosylguanine. Blood. 2003;102(5):1842–1848. doi: 10.1182/blood-2003-01-0317. [DOI] [PubMed] [Google Scholar]
- 11.Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick NJ, Camitta BM, Gaynon PS, Carroll WL. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children’s Oncology Group Study[corrected] J Clin Oncol. 2008;26(24):3971–3978. doi: 10.1200/JCO.2008.16.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivera GK, Zhou Y, Hancock ML, Gajjar A, Rubnitz J, Ribeiro RC, Sandlund JT, Hudson M, Relling M, Evans WE, Pui CH. Bone marrow recurrence after initial intensive treatment for childhood acute lymphoblastic leukemia. Cancer. 2005;103(2):368–376. doi: 10.1002/cncr.20743. [DOI] [PubMed] [Google Scholar]
- 13.Berg SL, Blaney SM, Devidas M, Lampkin TA, Murgo A, Bernstein M, Billett A, Kurtzberg J, Reaman G, Gaynon P, Whitlock J, Krailo M, Harris MB. Phase II study of nelarabine (compound 506U78) in children and young adults with refractory T-cell malignancies: a report from the Children’s Oncology Group. J Clin Oncol. 2005;23(15):3376–3382. doi: 10.1200/JCO.2005.03.426. [DOI] [PubMed] [Google Scholar]
- 14.Dunsmore KP, Devidas M, Linda SB, Borowitz MJ, Winick N, Hunger SP, Carroll WL, Camitta BM. Pilot Study of Nelarabine in Combination With Intensive Chemotherapy in High-Risk T-Cell Acute Lymphoblastic Leukemia: A Report From the Children’s Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(22):2753–2759. doi: 10.1200/JCO.2011.40.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seibel NL, Steinherz PG, Sather HN, Nachman JB, Delaat C, Ettinger LJ, Freyer DR, Mattano LA, Jr, Hastings CA, Rubin CM, Bertolone K, Franklin JL, Heerema NA, Mitchell TL, Pyesmany AF, La MK, Edens C, Gaynon PS. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111(5):2548–2555. doi: 10.1182/blood-2007-02-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamps WA, Bokkerink JP, Hahlen K, Hermans J, Riehm H, Gadner H, Schrappe M, Slater R, van den Berg-de Ruiter E, Smets LA, de Vaan GA, Weening RS, van Weerden JF, van Wering ER, den der Does-van den Berg A. Intensive treatment of children with acute lymphoblastic leukemia according to ALL-BFM-86 without cranial radiotherapy: results of Dutch Childhood Leukemia Study Group Protocol ALL-7 (1988–1991) Blood. 1999;94(4):1226–1236. [PubMed] [Google Scholar]
- 17.Salzer WL, Devidas M, Carroll WL, Winick N, Pullen J, Hunger SP, Camitta BA. Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: a report from the children’s oncology group. Leukemia. 2010;24(2):355–370. doi: 10.1038/leu.2009.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldberg JM, Silverman LB, Levy DE, Dalton VK, Gelber RD, Lehmann L, Cohen HJ, Sallan SE, Asselin BL. Childhood T-cell acute lymphoblastic leukemia: the Dana-Farber Cancer Institute acute lymphoblastic leukemia consortium experience. J Clin Oncol. 2003;21(19):3616–3622. doi: 10.1200/JCO.2003.10.116. [DOI] [PubMed] [Google Scholar]
- 19.Nachman JB, Sather HN, Sensel MG, Trigg ME, Cherlow JM, Lukens JN, Wolff L, Uckun FM, Gaynon PS. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338(23):1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 20.Borowitz MJ, Devidas M, Hunger SP, Bowman WP, Carroll AJ, Carroll WL, Linda S, Martin PL, Pullen DJ, Viswanatha D, Willman CL, Winick N, Camitta BM. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111(12):5477–5485. doi: 10.1182/blood-2008-01-132837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roshal M, Fromm JR, Winter S, Dunsmore K, Wood BL. Immaturity associated antigens are lost during induction for T cell lymphoblastic leukemia: implications for minimal residual disease detection. Cytometry Part B, Clinical cytometry. 2010;78(3):139–146. doi: 10.1002/cyto.b.20511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uckun FM, Sensel MG, Sun L, Steinherz PG, Trigg ME, Hareema NA, Sather HN, Reaman GH, Gaynon PS. Biology and treatment of childhood T-Lineage acute lymphoblastic leukemia. Blood. 1998;91:735–746. [PubMed] [Google Scholar]
- 23.Borowitz MJ, Dowell BL, Boyett JM, Pullen DJ, Crist WM, Humphrey GB, Metzgar RS. Clinicopathologic aspects of E-rosette negative T-cell acute lymphocytic leukemia. A Pediatric Oncology Group Study. Jounal of Clinical Oncology. 1986;4:170–177. doi: 10.1200/JCO.1986.4.2.170. [DOI] [PubMed] [Google Scholar]
- 24.Reinherz EL, Hussey RE, Schlossman SF. A monoclonal antibody blocking human T cell function. Eur J Immunol. 1980;10(10):758–762. doi: 10.1002/eji.1830101006. [DOI] [PubMed] [Google Scholar]
- 25.Roper M, Crist WM, Metzgar R, Ragab AH, Smith S, Starling K, Pullen J, Leventhal B, Bartolucci AA, Cooper MD. Monoclonal antibody characterization of surface antigens in childhood T- cell lymphoid malignancies. Blood. 1983;61(5):830–837. [PubMed] [Google Scholar]
- 26.Schorin MA, Blattner S, Gelber RD, Tarbell NJ, Donnelly M, Dalton V, Cohen HJ, Sallan SE. Treatment of childhood acute lymphoblastic leukemia: results of Dana- Farber Cancer Institute/Children’s Hospital Acute Lymphoblastic Leukemia Consortium Protocol 85–01. J Clin Oncol. 1994;12(4):740–747. doi: 10.1200/JCO.1994.12.4.740. [DOI] [PubMed] [Google Scholar]
- 27.Pui CH, Campana D, Pei D, Bowman WP, Sandlund JT, Kaste SC, Ribeiro RC, Rubnitz JE, Raimondi SC, Onciu M, Coustan-Smith E, Kun LE, Jeha S, Cheng C, Howard SC, Simmons V, Bayles A, Metzger ML, Boyett JM, Leung W. Treating childhood acute lymphoblastic leukemia without cranial irradiation. The New England journal of medicine. 2009;360(26):2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiter A, Schrappe M, Ludwig WD, Hiddemann W, Sauter S, Henze G, Zimmermann M, Lampert F, Havers W, Niethammer D, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84(9):3122–3133. [PubMed] [Google Scholar]
- 29.Buie LW, Epstein SS, Lindley CM. Nelarabine: a novel purine antimetabolite antineoplastic agent. Clin Ther. 2007;29(9):1887–1899. doi: 10.1016/j.clinthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 30.Kisor DF, Plunkett W, Kurtzberg J, Mitchell B, Hodge JP, Ernst T, Keating MJ, Gandhi V. Pharmacokinetics of nelarabine and 9-beta-D-arabinofuranosyl guanine in pediatric and adult patients during a phase I study of nelarabine for the treatment of refractory hematologic malignancies. J Clin Oncol. 2000;18(5):995–1003. doi: 10.1200/JCO.2000.18.5.995. [DOI] [PubMed] [Google Scholar]
- 31.CDC Definitions of Nosocomial Infections: Association for Practioners in Infection Control and Epidemiology, Inc. Infection Control and Applied Epidemiology: Principles and Practice. St. Louis: Mosby; 1996. pp. A1–A20. [Google Scholar]
- 32.van der Velden VH, Jacobs DC, Wijkhuijs AJ, Comans-Bitter WM, Willemse MJ, Hahlen K, Kamps WA, van Wering ER, van Dongen JJ. Minimal residual disease levels in bone marrow and peripheral blood are comparable in children with T cell acute lymphoblastic leukemia (ALL), but not in precursor-B-ALL. Leukemia. 2002;16(8):1432–1436. doi: 10.1038/sj.leu.2402636. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hematological Toxicities
Total cumulative CNS toxin exposure on AALL00P2 and AALL0434