Abstract
Gap junction channels can modify their activity in response to cell signaling pathways. Here, we demonstrate that Cx50 coupling, but not Cx46, increased when co-expressed with a constitutively active p110α subunit of PI3K in Xenopus oocytes. In addition, inhibition of PI3K signaling by blocking p110α, or Akt, significantly decreased gap junctional conductance in Cx50 transfected HeLa cells, with no effect on Cx46. Alterations in coupling levels were not a result of Cx50 unitary conductance, suggesting that changes in the number of active channels were responsible. These data indicate that Cx50 is specifically regulated by the PI3K signaling pathway.
Introduction
There are two main mechanisms of intercellular communication between adjacent cells: small molecules can be directly shared through gap junction channels linking the cytoplasm of neighboring cells, or, extracellular ligands binding to receptors can activate intracellular signaling pathways. Gap junction channels and cell signaling have been separately shown to be important elements for the maintenance of homeostasis and proper organ development in many organisms [1, 2], however, less is known about the interplay between them [3]. One organ that has been well studied because of its dependence on intercellular communication is the ocular lens. Due to its absence of a vascular system, the lens relies heavily on gap junction channels for cell-to-cell communication during growth and differentiation [4-6].
Transport of ions, metabolites, and small signaling molecules to all cells of the lens requires direct communication between neighboring cells by gap junction channels [7, 8]. Gap junctions are comprised of hexameric oligomers of connexin subunits that are inserted into the plasma membrane [9]. When two of these complexes from neighboring cells dock, they form a channel that connects the two cytoplasms [10, 11]. Out of the ∼20 connexin family members, three are present in the lens with distinct expression patterns: Connexin43 (Cx43) is expressed in the lens epithelium [12], Connexin46 (Cx46) is present in the differentiating and mature fiber cell types [13], and Connexin50 (Cx50) is expressed in all three cell types [14-16].
Genetic knockouts of lens connexins have identified the roles each have in development and homeostasis. Cx46 knockout mice developed severe nuclear cataracts [17]. Targeted deletion of Cx50 resulted in mild nuclear cataracts and an ocular growth defect [18]. Functional replacement of Cx50 with Cx46 restored ocular transparency but did not rescue the growth defect [19]. These results suggested that Cx46 and Cx50 lack redundancy and demonstrated the possibility that Cx50, specifically, was involved in proliferation and growth regulation of the lens [20, 21].
It was previously shown that manipulation of the MAPK signaling pathway differentially regulated Cx50 and Cx46 [22], increasing Cx50 mediated coupling with no effect on Cx46. This was consistent with the hypothesis that Cx50, but not Cx46, was interacting with growth signaling pathways. Here, we investigated the effects on gap junctional conductance produced by Cx50 or Cx46 when the PI3K signaling pathway was either activated or inhibited. In cells incubated with PIK-75 and Akt Inhibitor-VIII (Akti) to specifically block p110α and Akt, respectively, there was a significant decrease in Cx50- mediated gap junctional conductance that was not seen in Cx46 transfected cells. We demonstrated that Cx50 junctional coupling, but not Cx46, increased when co-expressed with a constitutively active PI3K subunit. Analysis of single channel conductance showed that alteration in Cx50 coupling mediated by PI3K did not result from changes in unitary conductance of the channel. These results suggest a mechanism of differential regulation of these two lens connexins by the PI3K signaling pathway.
Materials and Methods
In Vitro Transcription, Oocyte Microinjection, and Pairing
Cx50, Cx46, and constitutively active p110α-H1047R (caPI3K) [23] coding sequences were subcloned into pCS2+, linearized with NotI, and transcribed using the SP6 mMessage mMachine (Ambion, Austin, TX). Xenopus laevis oocytes were removed from adult females (Nasco, Fort Atkinson, WI), defolliculated by collagenase B and hyaluronidase digestion, and stage V-VI cells were selected and cultured in modified Barth's (MB) medium. Endogenous connexins were suppressed by injection of an antisense oligonucleotide to Xenopus Cx38 (10 ng/cell) [24] using a Nanoject II injector (Drummond, Broomall, PA). Oligo injected cells were subsequently injected with either Cx50, Cx46 cRNA (5 ng/cell), or H2O as a negative control 24 hours before pairing. Vitelline envelopes were removed, and oocytes were manually paired with vegetal poles apposed in MB medium. Paired cells were injected with caPI3K cRNA 4 hours before electrophysiology recordings. Gap junctional conductance measurements were taken 16-24 hours after pairing.
Dual Whole-Cell Voltage Clamp
Gap junctional conductance was measured by dual voltage clamp [25]. Current and voltage electrodes (1.2 mm diameter, omega dot; Glass Company of America, Millville, NJ) were pulled to a resistance of 1–2 MΩ with a vertical puller (Narishige, Tokyo, Japan) and filled with 3 M KCl, 10 mM EGTA, and 10 mM HEPES, pH 7.4. Voltage clamp recordings were performed using two GeneClamp 500 amplifiers controlled by pClamp software using a Digidata 1320A interface. (Axon Instruments, Foster City, CA). Both cells of a pair were clamped at -40 mV to eliminate any transjunctional potential. One cell was then subjected to alternating pulses of ±20 mV, while the current produced by the voltage change was measured in the second cell. The current delivered to the second cell was equal in magnitude to the junctional current (Ij), and gap junctional conductance (Gj) was calculated by dividing the measured current by the voltage difference between first cell (V1) and the second cell (V2), Gj =Ij/(V1-V2).
Preparation of Oocyte Samples and Western Blot
Oocytes were collected in 1 ml of ice-cold lysis buffer containing 5 mM Tris, pH 8.0, 5 mM EDTA, and protease inhibitors and homogenized using a series of mechanical passages through syringe needles of diminishing diameter (20, 22, and 26 Ga). Extracts were first centrifuged at 2000 × g at 4°C for 5 min, then the supernatants were centrifuged at 45,000 × g at 4°C for 30 min. Membrane pellets were resuspended in SDS sample buffer (2 μl/oocyte), separated on 10% SDS-PAGE gels, and transferred to nitrocellulose membranes. Protein blots were probed with antibodies specific for the carboxy tail of Cx46 (rabbit) [13, 14], or the cytoplasmic loop of Cx50 (goat; Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:1000 dilution followed by incubation with horseradish peroxidase-conjugated rabbit anti-goat, or goat anti-rabbit secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). In addition, blots were probed with mouse monoclonal phospho (p)-Akt (Ser 473) or rabbit total (t)-Akt antibodies (Cell Signaling Technology, Danvers, MA.) at 1:1000 dilution, followed by incubation with ECL-mouse secondary antibody or horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Densitometry quantifications were performed using ImageJ (NIH). Band densities of three independent experiments were analyzed and the normalized mean values plotted.
Transient transfection and Dual Whole-Cell Patch Clamp
HeLa cells were plated on glass coverslips, grown to 50% confluence and transiently transfected with 4–5 μg of Connexin 46 DNA subcloned into a pIRES2-eGFP vector (Clontech) using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Gap junctional conductance of Cx46 expressing cells was measured after overnight incubation. Cx50 stably transfected HeLa cells [26], were also used. Junctional conductance of cell pairs was measured using dual whole-cell patch clamp with Axopatch 1D patch-clamp amplifiers (Axon Instruments) at room temperature. Cells were bathed in a solution containing 137.7 mM NaCl, 5.4 mM KCl, 2.3 mM NaOH, 1 mM MgCl2, 5 mM HEPES, 10 mM glucose, pH 7.4. Patch electrodes with resistances of 3–5 MΩ were filled with internal solution containing 120 mM aspartic acid, 120 M KOH, 10 mM EGTA, 3 mM NaATP, and 5 mM HEPES, pH 7.2. Macroscopic and single-channel recordings were acquired using pClamp, sampled at 1–2 kHz and filtered at 0.2– 0.5 kHz. Analysis of recordings was performed with pClamp and Origin software (MicroCal Software, Northampton, MA). Each cell of a pair was initially held at a common holding potential of 0 mV. To evaluate junctional coupling, 200-ms hyperpolarizing pulses from the holding potential of 0 to ±20 mV were applied to one cell to establish a transjunctional voltage gradient (Vj), and junctional current was measured in the second cell (held at 0 mV). To selectively block PI3K signaling, cells were incubated in either 50 nM PIK-75 or 10 uM Akt inhibitor VIII (Akti, which blocks phosphorylation of Akt by maintaining it in an inactive conformer) for 12-48 hours prior to recordings. For unitary conductance measurements, alternating bipolar Vj pulses ranging from ±10 mV to ±110 mV were applied to low conductance cell pairs that had only one or two active channels. Single channel currents for the three treatment conditions were plotted against voltage and fit by linear regression to determine the slope which was equal to the unitary conductance. The correlation coefficients of all three linear fits were r2 ≥ 0.99.
Results
Treatment with PIK-75 or Akti differentially regulated Cx50 and Cx46-mediated conductance
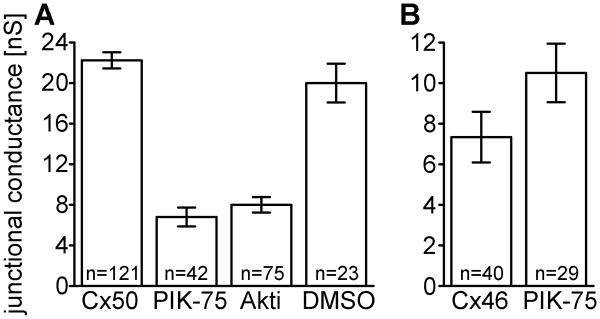
Previous studies have shown that manipulation of signaling pathways can affect gap junctional conductance [22, 27, 28]. To investigate whether inhibiting PI3K signaling affected gap junctional conductance, we treated HeLa cells stably expressing Cx50 [26], with PIK-75 to inhibit the p110α catalytic subunit of PI3K. As shown in Figure 1A, pairs expressing Cx50 without inhibitor had high coupling with a mean Gj of 22.2 nS. Treatment with PIK-75 for 24 hours significantly decreased conductance by 3-fold to a mean Gj of 6.8 nS (p< 0.05, Student's t test). To test if the decrease in conductance was caused by inhibiting PI3K directly, or inhibition of the signaling cascade, we treated Cx50 HeLa cells with Akt Inhibitor-VIII (Akti) to specifically inhibit Akt, a downstream effector of PI3K signaling. Inhibition of Akt also showed a 3-fold decrease in Cx50-mediated conductance with a mean Gj of 8 nS. DMSO was used as a vehicle for both inhibitors, and incubation of Cx50 expressing cells with DMSO alone had no effect on coupling. To evaluate the effects of inhibiting PI3K signaling on the other lens fiber connexin, we treated Cx46 expressing HeLa cells with PIK-75 for 24 hours. Cx46 conductance was not significantly reduced after incubation with PIK-75, with a mean Gj of 10.5 nS, compared to 7.3 nS with vehicle alone (Figure 1B, p> 0.05). Therefore, the inhibition of the catalytic subunit of PI3K, p110α, or its downstream effector, Akt, decreased Cx50-mediated coupling, but had not effect Cx46 gap junctional conductance. Similar results were obtained after 12 or 48 hours of inhibitor treatment (not shown).
Figure 1. Inhibition of PI3K signaling decreased junctional conductance mediated by Cx50 but not Cx46.
(A) Gap junctional conductance between HeLa cell pairs expressing Cx50 treated with an inhibitor of the p110α catalytic subunit of PI3K (PIK-75), Akt inhibitor VIII (Akti), or the vehicle (DMSO). Cell pairs expressing Cx50 showed a three-fold decrease in gap junctional coupling after treatment with either PIK-75, or Akti. Treatment with vehicle (DMSO) had no effect. (B) HeLa cells transiently transfected with Cx46 and treated with PIK-75 showed no significant difference in gap junctional coupling compared to controls. Data are the mean ± SE.
PIK-75 and Akti decreased Akt phosphorylation in Cx50 expressing HeLa cells
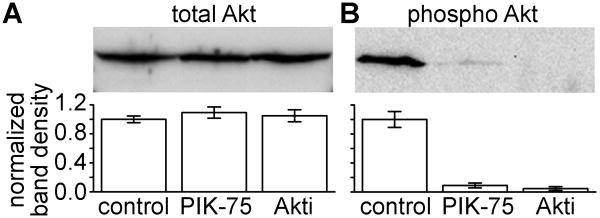
We evaluated the effectiveness of the inhibitors PIK-75 and Akti on down regulating PI3K signaling in HeLa cells stably expressing Cx50. After 24 hour treatment with either inhibitor or DMSO (used as control), Proteins were separated on SDS-PAGE gels, transferred to nitrocellulose membranes and probed for total Akt (t-Akt) and phosphorylated Akt (p-Akt). Cx50 expressing HeLa cells treated with either inhibitor showed no significant difference in t-Akt levels as compared to DMSO alone (Figure 2A). As expected, Cx50 HeLa cells treated with PIK-75 or Akti demonstrated significantly decreased p-Akt levels, 91% and 95% reduced, respectively, when compared to the vehicle treated sample (Figure 2B). Thus the inhibitors decreased activation of Akt in stably transfected Cx50 HeLa cells.
Figure 2. Treatment of Cx50 expressing HeLa cells with either a PI3K or an Akt inhibitor decreased levels of activated Akt protein.
Stably transfected Cx50 HeLa cells were treated with PIK-75, Akti, or vehicle for 24 hours then lysed in SDS buffer and ran on SDS-PAGE gels. Proteins were transferred to nitrocellulose membrane and probed for p-Akt and t-Akt. Band density measurements were averages of three independent experiments. (A) PIK-75 and Akti had no effect on total Akt. (B) Both PIK-75 and Akti decreased Akt phosphorylation. DMSO was used as a vehicle for the inhibitor, and had no effect on either p-Akt or t-Akt.
Constitutive activation of PI3K signaling specifically increased Cx50-mediated conductance in Xenopus oocytes
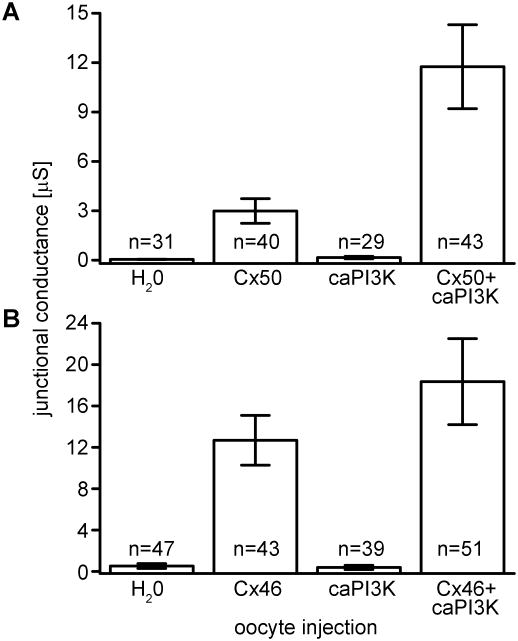
To test the effects that activation of the signaling pathway has on coupling of paired cells expressing Cx50 or Cx46, we used a constitutively active PI3K mutant construct, caPI3K, [23], and the Xenopus oocyte expression system. Control oocyte pairs injected with anti-sense oligonucleotide and water or caPI3K had nominal junctional conductance (Figure 3A). Cell pairs injected with Cx50 cRNA had a mean conductance of 3.0 μS. When cells expressing Cx50 were subsequently injected with caPI3K, gap junctional conductance significantly increased 4-fold to a mean Gj of 11.8 μS (p< 0.05). To determine if this increase was Cx50 specific, we co-injected Cx46 and caPI3K and measured conductance in paired oocytes. Cell pairs injected with anti-sense oligo and caPI3K had negligible conductance (Figure 3B). Unlike Cx50, there was no significant difference in conductance in Cx46 expressing cells following PI3K activation, with mean Gj's of 12.7 μS and 18.4 μS respectively (p> 0.05). These experiments demonstrated that constitutive activation of the PI3K signaling increased junctional conductance mediated by Cx50, but not Cx46.
Figure 3. Activation of PI3K signaling specifically increased junctional conductance mediated by Cx50.
PI3K signaling was stimulated by co-expression of the constitutively active catalytic subunit p110αH1047R (caPI3K) in paired Xenopus oocytes expressing either Cx50 or Cx46. (A) Gap junctional conductance of pairs co-expressing Cx50 and caPI3K showed increased gap junctional conductance compared to pairs expressing Cx50 alone. Cells injected with water or caPI3K alone had negligible junctional conductance. (B) Xenopus oocyte pairs co-expressing Cx46 and caPI3K showed no significant difference in coupling compared to pairs with Cx46 alone. Data are the mean ± SE.
Constitutive activation of PI3K signaling did not alter Cx46 or Cx50 protein expression
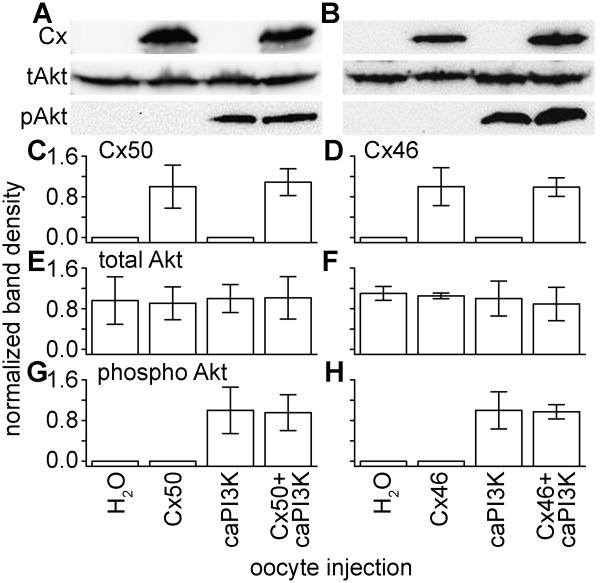
The increased conductance mediated by Cx50 when co-expressed with caPI3K could have been a result of increased protein expression. To test this, western blots of oocyte samples injected with caPI3K and Cx50, or Cx46, were probed with antibodies for connexin protein, total Akt, and phospho-Akt. Immunoblots confirmed expression of Cx50 and Cx46 in oocytes injected with connexin cRNA, and showed that co-injection of caPI3K had no effect on the protein levels of either connexin (Figures 4A and 4B). This was validated by band densitometry analysis, confirming that there were no significant differences in connexin protein levels when co-expressed with caPI3K (Figure 4C and 4D). Average band densities of total Akt levels were equal in all samples (Figure 4E and 4F). The amount of p-Akt in cells injected with connexin and caPI3K were not significantly different than the level of p-Akt in oocytes injected with caPI3K alone (Figure 4G and 4H). These results verified activation of Akt by the caPI3K. More importantly, these data suggested that the increase in Cx50 conductance was not due to a change of connexin protein expression.
Figure 4. Injection of oocytes with caPI3K increased activation of Akt without effecting connexin or t-Akt protein levels.
(A) Immunoblotting confirmed the expression of Cx50 and increased levels of p-Akt, with no change in t-Akt levels between all of the samples. (B) Expression of Cx46 did not change when co-expressed with caPI3K. (C) Densitometry measurements (averages of three independent experiments) showed there was no significant difference in Cx50 protein when co-expressed with caPI3K. (D) Quantification of Cx46 protein levels confirmed they were not affected by Akt activation. (E), (F) Total Akt expression is used as a control. (G) (H) Quantification of p-Akt bands verified equal activation of Akt in oocytes co-expressing caPI3K and connexin proteins.
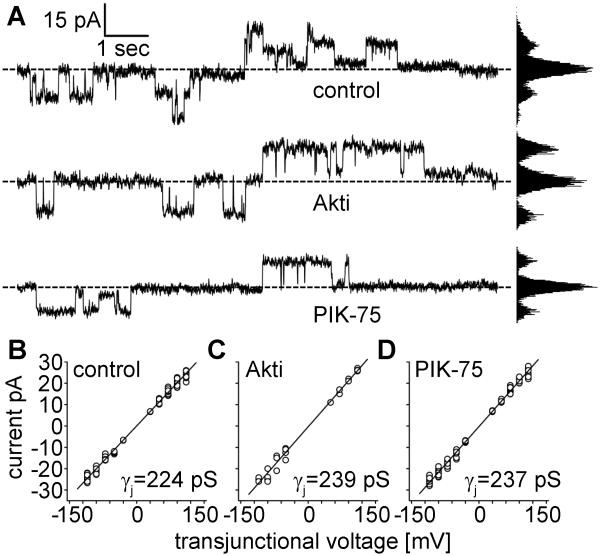
Treatment with PI3K signaling inhibitors did not reduce Cx50 unitary conductance
Our experiments demonstrated that inhibitors of PI3K signaling reduced Cx50 gap junctional conductance. There are two possible mechanisms that could have produced the decrease in junctional conductance following treatment with PIK-75 and Akti; 1) a reduction in single channel conductance, or 2) a decrease in the number of active channels. To differentiate between these possibilities, Cx50 single channel currents were recorded in poorly coupled cell pairs. Figure 5A shows representative traces of single channel currents in response to ±70 mV pulses in each of the treatment conditions. Similar channel activity was detected in all conditions with no obvious differences in the times channels were in the open state. All points histograms revealed that the channels spent the majority of time in the closed configuration at this voltage as previously reported for Cx50 [29]. Single channel currents of control (n=71), Akti (n=25), and PIK-75 (n=109) treated cells were plotted against transjunctional voltage and fit by linear regression to determine unitary conductance Figures 5B-D. The unitary conductance of Cx50 had values of 224 pS for control cells in the absence of inhibitors, 239 pS after treatment with Akti, and 237 pS for PIK-75 treated cells. These data suggest that the macroscopic effects in Cx50 gap junctional conductance during PI3K signaling inhibition were not due to a reduction of single channel conductance, but instead caused by a decrease in the number of active channels.
Figure 5. PI3K signaling inhibition did not reduce Cx50 single channel conductance.
HeLa cells stably expressing Cx50 were treated with either Akti or PIK-75 for 24 hours. Junctional currents were measured by bipolar Vj pulses of a range of voltages from ±10 mV to ±110 mV in cell pairs that had only one or two active channels. (A) Representative traces of single channel currents in response to ±70 mV pulses in control, Akti, or PIK-75 treated cells. (B-D) Single channel currents of the three treatment conditions were plotted against voltage and values were fitted by linear regression. Unitary conductance (the slope) did not decrease in Akti treated or PIK-75 treated cells, as compared to control; with values of 239 pS, 237 pS, and 224 pS, respectively. The correlation coefficients of all three linear fits were r2=0.99
Discussion
These data provide evidence that PI3K signaling interacted with Cx50, but not Cx46, and specifically regulated its activity. Using the Xenopus oocyte expression system, we demonstrated that Cx50 gap junctional conductance increased when co-expressed with a constitutively active PI3K subunit, caPI3K. In contrast, activation of PI3K signaling did not have any effect on Cx46 expressing oocyte pairs. Connexin expressing HeLa cells incubated with PIK-75 and Akti showed a three-fold decrease in Cx50-mediated gap junctional conductance when treated with either inhibitor, with no effect on the conductance of Cx46 channels. Lastly, the unitary conductance of Cx50 expressing cells was not reduced after treatment with either inhibitor. Therefore, the decrease in Cx50 gap junctional conductance was not due to reduction of unitary channel conductance, but suggested a decrease in the number of active channels. These results suggest a mechanism of preferential regulation of Cx50, but not Cx46, by the PI3K signaling pathway.
Both Cx50 and Cx46 are phosphoproteins with multiple consensus sequences for phosphorylation by protein kinases [30]. Our data showed that the modification of Cx50 gap junction activity occurs downstream of Akt activation. The activation of Akt could lead to phosphorylation of Cx50, possibly altering its transport, assembly, or stability in the membrane. A study by Dunn et al. showed that after Cx43 is phosphorylated by Akt, gap junctional conductance increased due to stabilization of the complex on the plasma membrane [31]. This group later identified the site of Akt phosphorylation on Cx43 and determined that this post translational modification disrupts the interaction between Cx43 and ZO-1. Since ZO-1 is a scaffolding protein shown to slow gap junction formation, the disruption by Akt phosphorylation allowed larger gap junction plaques to develop [32]. A similar mechanism could explain increased Cx50 gap junctional activity when PI3K signaling is constitutively activated in our experiments. However, a previous study showed decreased Cx50 channel formation when ZO-1 is knocked down by RNA interference [33]. It is possible that ZO-1 is required for connexon transport and insertion into the plasma membrane but has an inhibitory effect on gap junction plaque formation. Noteworthy, Cx46 channels were not affected by removal of its ZO-1 binding site [33]. Nonetheless, our results corroborate an increasing number of studies that have shown a modification of gap junction activity by the activation of signaling pathways [34, 35], specifically, we showed that PI3K-Akt activation upregulates Cx50 gap junctional conductance.
The regulation of development and homeostasis of lens cells by growth factor signaling pathways has been well studied [27, 36, 37]. One example that has been extensively researched in the lens is FGF signaling. The response to FGF receptor activation was the initiation of the Ras-MAPK and the PI3K-Akt signaling pathways [38]. While FGF induced relatively low levels of Akt activation in primary lens cells, treatment with vitreous humor strongly stimulated Akt [39]. Our lab has shown that both the MAPk and PI3K pathways preferentially regulate Cx50 gap junctional conductance with no effect on Cx46-mediated coupling. Furthermore, proper activation of these pathways has been shown to be necessary for lens epithelial cell proliferation and fiber cell differentiation [40, 41].
In the model discussed by Le and Musil (2001), the equatorial fiber cells of the lens have the highest response to FGF signaling, which increases gap junctional coupling in these regions [42]. The asymmetry produced by high conductance levels at the equator and low conductance at the poles is required for the lens micro-circulation system [43]. The differential regulation of Cx50 and Cx46 by PI3K signaling could be a mechanism the lens uses to produce or maintain this asymmetry, by specifically upregulating Cx50-mediated coupling in the differentiating equatorial fiber cells, without affecting the conductance of Cx46. Recently, it has been shown that over activation of PI3K signaling by deletion of its antagonist, phosphatase and tensin homolog (PTEN), resulted in lens swelling and cataractogenesis caused by abnormal ion transport and reduced Na+/K+-ATPase activity [44]. Taken together with the data presented here, these studies suggested that normal, functional PI3K signaling plays an important role in intercellular transport in the lens to maintain homeostasis, and preserve visual function.
Highlights.
Inhibition of PI3K signaling reduces Cx50 gap junctional conductance.
Constitutive activation of PI3K signaling increases Cx50-mediated coupling.
Cx46 conductance levels are not regulated by PI3K signaling.
Acknowledgments
This study was supported by a grant from the National Institutes of Health EY013163 (T.W.W.)
Footnotes
Authors contributions: TWW conceived and supervised the study; PRB and TWW designed experiments; JMM and H-ZW performed experiments; RZL provided new tools and reagents; PRB and TWW analyzed data; JMM and TWW wrote the manuscript; TWW made manuscript revisions
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans WH, Martin PE. Gap junctions: structure and function (Review) Mol Membr Biol. 2002;19(2):121–36. doi: 10.1080/09687680210139839. [DOI] [PubMed] [Google Scholar]
- 2.Raju R, et al. A Network Map of FGF-1/FGFR Signaling System. J Signal Transduct. 2014;2014:962962. doi: 10.1155/2014/962962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei CJ, Xu X, Lo CW. Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol. 2004;20:811–38. doi: 10.1146/annurev.cellbio.19.111301.144309. [DOI] [PubMed] [Google Scholar]
- 4.Goodenough DA. The crystalline lens. A system networked by gap junctional intercellular communication. Semin Cell Biol. 1992;3(1):49–58. doi: 10.1016/s1043-4682(10)80007-8. [DOI] [PubMed] [Google Scholar]
- 5.Mathias RT, White TW, Gong X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol Rev. 2010;90(1):179–206. doi: 10.1152/physrev.00034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthoud VM, et al. Roles and regulation of lens epithelial cell connexins. FEBS Lett. 2014;588(8):1297–303. doi: 10.1016/j.febslet.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodenough DA, Dick JS, 2nd, Lyons JE. Lens metabolic cooperation: a study of mouse lens transport and permeability visualized with freeze-substitution autoradiography and electron microscopy. J Cell Biol. 1980;86(2):576–89. doi: 10.1083/jcb.86.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donaldson P, Kistler J, Mathias RT. Molecular solutions to mammalian lens transparency. News Physiol Sci. 2001;16:118–23. doi: 10.1152/physiologyonline.2001.16.3.118. [DOI] [PubMed] [Google Scholar]
- 9.Harris AL. Emerging issues of connexin channels: biophysics fills the gap. Q Rev Biophys. 2001;34(3):325–472. doi: 10.1017/s0033583501003705. [DOI] [PubMed] [Google Scholar]
- 10.Bruzzone R, White TW, Paul DL. Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem. 1996;238(1):1–27. doi: 10.1111/j.1432-1033.1996.0001q.x. [DOI] [PubMed] [Google Scholar]
- 11.Fleishman SJ, et al. A Calpha model for the transmembrane alpha helices of gap junction intercellular channels. Mol Cell. 2004;15(6):879–88. doi: 10.1016/j.molcel.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 12.Beyer EC, et al. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108(2):595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paul DL, et al. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991;115(4):1077–89. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White TW, et al. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell. 1992;3(7):711–20. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dahm R, et al. Gap junctions containing alpha8-connexin (MP70) in the adult mammalian lens epithelium suggests a re-evaluation of its role in the lens. Exp Eye Res. 1999;69(1):45–56. doi: 10.1006/exer.1999.0670. [DOI] [PubMed] [Google Scholar]
- 16.Rong P, et al. Disruption of Gja8 (alpha8 connexin) in mice leads to microphthalmia associated with retardation of lens growth and lens fiber maturation. Development. 2002;129(1):167–74. doi: 10.1242/dev.129.1.167. [DOI] [PubMed] [Google Scholar]
- 17.Gong X, et al. Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell. 1997;91(6):833–43. doi: 10.1016/s0092-8674(00)80471-7. [DOI] [PubMed] [Google Scholar]
- 18.White TW, Goodenough DA, Paul DL. Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J Cell Biol. 1998;143(3):815–25. doi: 10.1083/jcb.143.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White TW. Unique and redundant connexin contributions to lens development. Science. 2002;295(5553):319–20. doi: 10.1126/science.1067582. [DOI] [PubMed] [Google Scholar]
- 20.Sellitto C, Li L, White TW. Connexin50 is essential for normal postnatal lens cell proliferation. Invest Ophthalmol Vis Sci. 2004;45(9):3196–202. doi: 10.1167/iovs.04-0194. [DOI] [PubMed] [Google Scholar]
- 21.White TW, et al. Optimal lens epithelial cell proliferation is dependent on the connexin isoform providing gap junctional coupling. Invest Ophthalmol Vis Sci. 2007;48(12):5630–7. doi: 10.1167/iovs.06-1540. [DOI] [PubMed] [Google Scholar]
- 22.Shakespeare TI, et al. Interaction between Connexin50 and mitogen-activated protein kinase signaling in lens homeostasis. Mol Biol Cell. 2009;20(10):2582–92. doi: 10.1091/mbc.E08-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Z, et al. Increased persistent sodium current due to decreased PI3K signaling contributes to QT prolongation in the diabetic heart. Diabetes. 2013;62(12):4257–65. doi: 10.2337/db13-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebihara L. Expression of gap junctional proteins in Xenopus oocyte pairs. Methods Enzymol. 1992;207:376–80. doi: 10.1016/0076-6879(92)07026-k. [DOI] [PubMed] [Google Scholar]
- 25.Spray DC, Harris AL, Bennett MV. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981;77(1):77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berthoud VM, et al. Loss of function and impaired degradation of a cataract-associated mutant connexin50. Eur J Cell Biol. 2003;82(5):209–21. doi: 10.1078/0171-9335-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280(1):1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Boswell BA, Le AC, Musil LS. Upregulation and maintenance of gap junctional communication in lens cells. Exp Eye Res. 2009;88(5):919–27. doi: 10.1016/j.exer.2008.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srinivas M, et al. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J Physiol. 1999;517(Pt 3):673–89. doi: 10.1111/j.1469-7793.1999.0673s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shearer D, et al. Posttranslational modifications in lens fiber connexins identified by off-line-HPLC MALDI-quadrupole time-of-flight mass spectrometry. Invest Ophthalmol Vis Sci. 2008;49(4):1553–62. doi: 10.1167/iovs.07-1193. [DOI] [PubMed] [Google Scholar]
- 31.Dunn CA, et al. Activation of Akt, not connexin 43 protein ubiquitination, regulates gap junction stability. J Biol Chem. 2012;287(4):2600–7. doi: 10.1074/jbc.M111.276261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunn CA, Lampe PD. Injury-triggered Akt phosphorylation of Cx43: a ZO-1-driven molecular switch that regulates gap junction size. J Cell Sci. 2014;127(Pt 2):455–64. doi: 10.1242/jcs.142497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chai Z, Goodenough DA, Paul DL. Cx50 requires an intact PDZ-binding motif and ZO-1 for the formation of functional intercellular channels. Mol Biol Cell. 2011;22(23):4503–12. doi: 10.1091/mbc.E11-05-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419(2):261–72. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36(7):1171–86. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robinson ML. An essential role for FGF receptor signaling in lens development. Semin Cell Dev Biol. 2006;17(6):726–40. doi: 10.1016/j.semcdb.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez G, et al. Conditional mutations of beta-catenin and APC reveal roles for canonical Wnt signaling in lens differentiation. Invest Ophthalmol Vis Sci. 2009;50(10):4794–806. doi: 10.1167/iovs.09-3567. [DOI] [PubMed] [Google Scholar]
- 38.Goetz R, Mohammadi M. Exploring mechanisms of FGF signalling through the lens of structural biology. Nat Rev Mol Cell Biol. 2013;14(3):166–80. doi: 10.1038/nrm3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q, et al. MAPK/ERK1/2 and PI3-kinase signalling pathways are required for vitreous-induced lens fibre cell differentiation. Exp Eye Res. 2009;88(2):293–306. doi: 10.1016/j.exer.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weber GF, Menko AS. Phosphatidylinositol 3-kinase is necessary for lens fiber cell differentiation and survival. Invest Ophthalmol Vis Sci. 2006;47(10):4490–9. doi: 10.1167/iovs.06-0401. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q, McAvoy JW, Lovicu FJ. Growth factor signaling in vitreous humor-induced lens fiber differentiation. Invest Ophthalmol Vis Sci. 2010;51(7):3599–610. doi: 10.1167/iovs.09-4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le ACN. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. The Journal of Cell Biology. 2001;154(1):197–216. doi: 10.1083/jcb.200101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol Rev. 1997;77(1):21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- 44.Sellitto C, et al. AKT activation promotes PTEN hamartoma tumor syndrome-associated cataract development. J Clin Invest. 2013;123(12):5401–9. doi: 10.1172/JCI70437. [DOI] [PMC free article] [PubMed] [Google Scholar]