Abstract
Neurodegenerative disorders, such as spinocerebellar ataxias (SCAs) and Alzheimer’s disease (AD) represent a huge scientific and medical question, but the molecular mechanisms of these diseases are still not clear. There is increasing evidence that neuronal calcium signaling is abnormal in many neurodegenerative disorders. Abnormal neuronal calcium release from the endoplasmic reticulum may result in disturbances of cell homeostasis, synaptic dysfunction, and eventual cell death. Neuronal loss is observed in most cases of neurodegenerative diseases. Recent experimental evidence supporting the role of neuronal calcium signaling in the pathogenesis of SCAs and AD is discussed in this review.
1. Spinocerebellar ataxias
Spinocerebellar ataxias (SCAs) represent a group of progressive hereditary neurodegenerative diseases that differ from each other in clinical presentation and genetic basis. At present, about 30 different genes have been identified which can be the cause of these diseases [1]. In the case of some SCAs, molecular cloning methods revealed the expansion of CAG codons that leads to lengthening of polyglutamine (polyQ) tract in appropriate proteins, such as ataxins for SCA1, SCA2, SCA3 and SCA7 or α1A subunit of P/Q voltage-dependent calcium channel (VDCC) Cav2.1 for SCA6 [2]. These diseases relate to wide group of polyglutamine disorders. In addition to this, there are some types of SCAs caused by other DNA mutations with other trinucleotide repeat expansion, nucleotide repeats in non-coding regions of appropriate genes, or non-repeat mutations and deletions.
1.1 Spinocerebellar ataxia type 2 pathogenesis
In this section we will discuss SCA pathogenesis by the example of SCA2. This disorder is accompanied by a wide spectrum of severe clinical symptoms, such as ataxia of gait and stance, ataxia of limb movements, dysarthria, ophthalmoplegia, pyramidal and extrapyramidal disorders, muscular rigidity and other severe neurological symptoms [2–4]. Clinical investigations have shown that in SCA2 patients olivopontocerebellar atrophy (OPCA) is observed. OPCA is attended with the degeneration of Purkinje cells (PCs) – large neurons located in cerebellar cortex, also with the decay of inferior olive, pontine nuclei and pontocerebellar fibers – fibers that link pons with cerebellum. In clinical trials on humans different diagnostic tests were used: starting with general biochemical analysis, including additional screening-test for paraneoplastic antibodies to PCs and also neuro-ophthalmological examination, electroretinogram and electronystagmogram analysis and in some cases – autopsy [5].
MRI-morphometric examination of infratentorial region of the brain of SCA2 patients revealed significant atrophy of the cerebellar vermis, of the cerebellar hemispheres, of pons base, of middle cerebellar peduncle, of medulla oblongata, of cervical part of spinal cord and also hypertrophy of the fourth ventricle of the brain have been observed in all cases [6].
Some proteins with expanded polyQ tracts are neurotoxic, they disturb nuclear functions by means of misfolding or in other ways. Misfolding is linked with intranuclear inclusion formation. Immunolabeling of intranuclear inclusions revealed the presence of proteosomes, ubiquitin and chaperones and this fact indicates that these inclusions contain misfolded proteins which are exposed to ineffective proteolysis [7]. Ubiquitin-positive neuronal intranuclear inclusions are detected in brains of polyQ diseases patients in the case of Huntington’s disease [8], dentatorubral-pallidoluysian atrophy [9], SCA1 [10], SCA3 [11] and SCA7 [12]. However, ubiquitin-positive nuclear inclusions have not been detected in the brain of SCA2 patients [7]. Therefore, misfolding and disturbances in protein metabolism are not essential and there is some other mechanism of neurodegeneration that plays a key role in SCA2 pathogenesis.
1.2 Calcium signaling in cerebellar PCs
The assertion that calcium signaling plays an important role in PCs functioning can be confirmed by the fact that these neurons express a lot of different calcium-dependent proteins and enzymes. Thus, cerebellar PCs contain extremely high amounts of dendritic calbindin D-28k (CB) and somatic parvalbumin (PV). These proteins belong to the large family of EF-hand calcium-binding proteins (CaBPs) [13]. It was demonstrated that the loss of PV and CB leads to the alterations in Cav2.1 channels (P/Q-type VDCCs), encoded by CACNA1A gene [14].
Recently it was reported that regulation of calcium influx to PCs through VDCCs is very important for the right connection from a climbing fibre (CF) to a PC during postnatal development. These data were obtained via simultaneous whole-cell recordings and two-photon calcium imaging from PCs in vivo in wild type and PC-selective P/Q-type VDCC knockout mice [15]. At the same time, in earlier studies with a use of flavoprotein autofluorescence optical imaging and extracellular field potential recordings methods it was shown that derangements in the CF-PC circuitry contribute to neuronal abnormality in SCA1 mice different transgenic lines [16]. PCs also highly express calmodulin-binding transcription activator 1 (CAMTA1) and deletion of CAMTA1 gene in mice causes severe ataxia with PCs degeneration and cerebellar atrophy [17]. It’s commonly thought that long-term depression (LTD) at parallel fibre (PF) on a PC is the main basis for motor learning. PCs express calcium/calmodulin-dependent protein kinase II (CaMKII) and it has been observed that CaMKII activation leads to prolonged increase of cGMP, supporting the signaling mechanism of LTD induction by CaMKII [18].
Summing up, we can conclude that PCs express various calcium sensors to maintain intraneuronal calcium homeostasis. There are two general ways that calcium can get into the cytoplasm of PC. Both include the presence of glutamate, an excitatory neurotransmitter. The first way is calcium influx through VGCCs from the interstitial fluid. These channels are activated by the membrane depolarization, caused by the activation of AMPA receptors. The second way is the activation of metabotropic glutamate receptors (mGluR) which leads to calcium release from the endoplasmic reticulum (ER) via activating inositol 1,4,5-triphosphate receptors (InsP3R) and this calcium influx is called InsP3-induced calcium release (IICR).
1.2.1 InsP3-induced calcium release
InsP3R is an intracellular calcium channel that mediates ion release mainly from the ER. More often InsP3R is activated by InsP3 molecules and this leads to IICR. InsP3R is involved in the regulation of a large number of significant physiological processes including learning and memory, behavior, cell division and proliferation, differentiation, fertilization, development and cell death. There are three InsP3R subtypes, in neurons a predominant isoform is InsP3R type 1. There is evidence that dysfunction of InsP3R1 may play a key role in the pathogenesis of certain neurodegenerative diseases. The hyperactivation of InsP3R1 leads to enhanced calcium release from the ER. There is evidence to suggest that deranged neuronal calcium signaling might play an important role in pathogenesis of some neurodegenerative diseases such as Huntington’s disease (HD), SCAs and AD. To support this idea, experimental studies on transgenic mice were carried out. This demonstrated a connection between abnormal calcium signaling and neuronal cell death in experiments with HD, SCA2 and SCA3 transgenic mouse models [19]. Additional data in the literature indicate that abnormal neuronal calcium signaling may also play an important role in pathogenesis of SCA1, SCA5, SCA6, SCA14 and SCA15/16. These data suggested that IICR might be one of the causes of pathophysiological processes in neurons, leading to the neurodegeneration.
1.2.2 Mutations in INSP3R1 gene
InsP3R functions could be clearly identified by the observation of InsP3R mutant mice. It was demonstrated that most InsP3R1 knock-out mice die in the period of prenatal development and mutant mice that managed to survive have severe ataxia and tonic or tonic-clonic seizures and die by the weaning period. An electroencephalogram study with these animals revealed that they suffer from epilepsy, indicating that InsP3R1 is required for important brain function [20].
In addition to InsP3R1 knock-out mice there is the opisthotonos (opt) mice colony. The opt mutation has appeared spontaneously in a one laboratory mice colony and is the only known, naturally occurring allele of opt. This mutant mouse reveals some features of ataxic and convulsive phenotype. Experimental data that were obtained in the results of genetic and molecular assays demonstrated that InsP3R1 is altered in the opt mice. The altered protein is believed to have lost several sites of modulation and is presented at significantly decreased levels in opt homozygotes. In the experimental study with opt mice it was shown that a strong calcium release from intracellular stores can be elicited in cerebellar PCs by the effect of mGluR agonist quisqualate (QA). The calcium response in opt homozygotes is less than in control littermates with the same QA application. Obtained results suggest that the ataxia and convulsions phenotype observed in opt mice may be caused by the physiological dysfunction of InsP3R1 [21].
Apart from said mutations in InsP3R1 gene there are Δ18 mice which have an in-frame deletion of 18 base pairs within exon 36 of InsP3R1 that results in the deletion in the regulatory domain. In order to corroborate the pathogenicity of this mutation, heterozygous Δ18 mice with heterozygous opt mice were crossed. Consequently, two litters of mice were obtained. From a total of 15 pups, four pups were affected means InsP3R1opt/Δ18 genotype. Remarkably, their phenotype was indistinguishable from that of the homozygous Δ18 and opt mice. Moreover, this phenotype was almost the same that in the InsP3R1 knock-out mice case. As with homozygous opt mice, translational reading frame was unaffected and the in-frame deletion in the internal coupling domain results in significantly reduction of the level of InsP3R1 expression in cerebellar Purkinje cells. So that these InsP3R1 mutations or deficiency results in the same autosomal recessive movement disorders [22]. By the way, it’s commonly thought that homozygous and heterozygous Δ18 mutant mice represent mouse models for SCA15/16 [23].
1.3 Spinocerebellar ataxia type 15/16
There is evidence that SCA 15/16 is caused by mutations in the InsP3R1 gene. SCA15 was described in 2001 as rarely occurring autosomal dominant ataxia with slow manifestation. The main sign of this disorder is head tremor. Via neuroradiological methods it was shown that the atrophy of the cerebellum, especially anterior and dorsal vermis, is observed in the case of SCA15. Actually, the significance of point deletions in InsP3R1 gene to cause SCA15 is not yet confirmed. It was demonstrated that “SCA16” is caused by the InsP3R1 gene mutation, and this autosomal dominant cerebellar ataxia (ADCA) subtype has now been subsumed into SCA15 [24]. SCA15 was originally detected in an Australian family. Subsequently, two Japanese families were mapped with this ataxia type. After that, partial deletions involving both the InsP3R1 gene and sulfatase modifying factor 1 (SUMF1) gene have been identified in Australian and British families with SCA15. Via gene dosage analysis, array-based comparative genomic hybridization analysis, gene expression and mutational analyses, a Japanese research group identified a 414-kb deletion including the entire InsP3R1 gene and exon 1 of SUMF1 in patients from one SCA15 family. The direct consequence of this mutation was around a 2-fold reduction in expression levels of InsP3R1 and SUMF1 mRNAs. This data confirmed that InsP3R1 is the causative gene for SCA15 [25]. The study conducted in UK on unrelated ADCA III families (n = 38) by the van de Leemput research group has revealed no InsP3R1 gene mutations. Based on these results it was concluded that point mutations or deletions in InsP3R1 gene are infrequent causes of ADCA III (namely, SCA15/16) [26]. In support of this, a deletion of InsP3R1 gene was found in 6 cases out of 333 (1.8%) families, corresponding to 13 SCA15 patients. Thus based on this research it was concluded that InsP3R1 gene deletions are rare and provoke around 1% of all ADCA [27].
1.4 Spinocerebellar ataxias type 2 and 3
SCA2 is an autosomal-dominant neurodegenerative disease caused by an expansion and translation of unstable CAG repeats in the gene encoding ataxin-2 from the normal 22 to more than 31 extra glutamine repeats [28]. Up until now the pathogenesis of SCA2 is not clear. Similar to wild type ataxin-2, polyglutamine-expanded mutant ataxin-2 protein is expressed in every cell type without severe aggregation and formation of inclusion bodies [29], but PCs in SCA2 patients are mostly affected with a loss of over 75% [2].
The experiments conducted by our research group have revealed that mutant ataxin-2 protein, but not wild type ataxin-2, interacts with the carboxyl terminus of the InsP3R1 (Fig. 1). These experiments were performed in a lipid bilayer model system, examining the effect of mutant ataxin-2 expression on InsP3R1 activation in single channel recordings of InsP3R1 co-expressed with mutant ataxin-2. It was discovered in these experiments that the presence of mutant ataxin-2 substantially sensitized InsP3R1 to activation by InsP3 [30]. Consistent with these findings, another group demonstrated that both wild type and mutant ataxin-2 proteins associate with ER membranes [31].
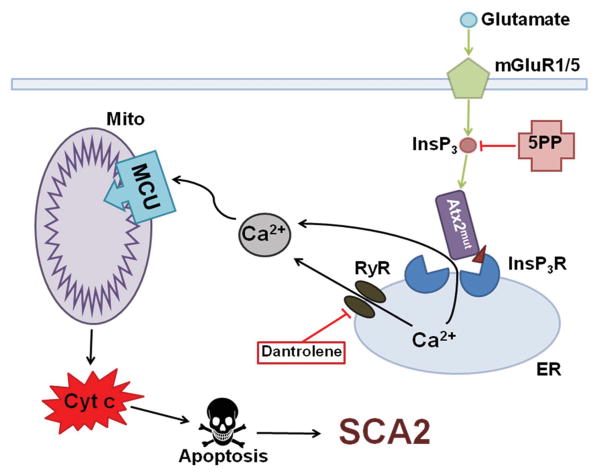
Figure 1.
Calcium hypothesis of SCA2 pathogenesis. Molecules of glutamate activate metabotropic glutamate receptor (mGluR), then inositol 1,4,5-triphosphate (InsP3) molecules release into the cytoplasm and activate inositol 1,4,5-triphosphate receptor (InsP3R) on the endoplasmic reticulum (ER) membrane. Next calcium influx from ER to the cytoplasm is observed and this is InsP3-induced calcium release (IICR). It was shown that mutant ataxin-2 protein (Atx2mut), but not wild type Atx2 binds with InsP3R and increases its sensitivity to InsP3 molecules. Hyperactivation of InsP3R causes deranged calcium signaling in PCs. Ca2+ ions, greatly increased in its number, are pumped to the mitochondria (Mito) through the mitochondrial calcium uniporter (MCU) that leads to mitochondrial swelling, followed later by the rupture of outer mitochondrial membrane and next pro-apoptotic factors like cytochrome c (Cyt c) come into the cytoplasm and initiate apoptosis. Hence, dramatically increased IICR can be suppressed by adenoassociated virus-mediated expression of the InsP3-phosphatase enzyme (5PP) which converts InsP3 into non-active form InsP2. Also calcium release from ER can be reduced via the use of dantrolene, a blocker of ryanodine receptors (RyRs).
In addition to experiments conducted on lipid bilayers, calcium imaging experiments in primary PCs cultured from SCA2-58Q transgenic and wild-type mice were performed. It was shown that there was a significant increase in calcium release from ER stores via InsP3R1 in the case of mutant mice PCs, but this effect was not observed in wild-type mice PCs. To block ryanodine receptors (RyRs) and ER calcium release in PC cultures ryanodine or dantrolene were added (Fig. 1). The effect of mutant ataxin-2 expression was immediately reversed as ER calcium release returned to wild type levels. Thus it was suggested that dantrolene and ryanodine provided this protection by inhibiting Ca2+ signals that is induced from IICR that is amplified by the RyR [30].
The test the hypothesis that Ca2+ release from the PC ER plays a key role in the development of SCA2, chronic suppression of InsP3R-mediated Ca2+ signaling was achieved by adeno-associated virus-mediated expression of the InsP3-phosphatase enzyme (5PP) in PCs of a SCA2-58Q transgenic mouse model (Fig. 1) [32]. It was determined that recombinant 5PP overexpression alleviated age-dependent dysfunction in the firing pattern of SCA2 PCs. Further it was discovered that chronic 5PP overexpression also rescued age-dependent motor incoordination and PC death in SCA2 mice. These findings support the important role of supranormal Ca2+ signaling in SCA2 pathogenesis and suggest that partial inhibition of IICR could provide therapeutic benefit for the patients afflicted with SCA2 and possibly other SCAs [32].
SCA3 is also an autosomal-dominant neurodegenerative disorder caused by a polyglutamine expansion in the carboxy-terminal of ataxin-3 protein [33]. Morphologically, SCA3 is characterized by degeneration of spinocerebellar tracts, dentate nucleus, pontine and other brainstem nuclei, substantia nigra and pallidum [34]. In SCA3 patients, magnetic resonance imaging (MRI) techniques revealed cerebellar atrophy, which is less pronounced than in SCA2 in combination with brainstem atrophy. It’s interesting, that, unlike SCA2 pathogenesis, nuclear inclusions have been found containing mutant ataxin-3 in neurons of affected brain regions. The clinical picture of SCA3 is characterized by a wide range of clinical manifestations, similar to SCA2 symptoms [35]. It was shown that ataxin-3 protein contains 3 ubiquitin-interactions motifs and an amino-terminal Josephin domain, that testifies to ataxin-3 functions as deubiquitinating enzyme (DUB). Moreover, it was demonstrated that the polyQ-expansion has no effect on DUB activity of ataxin-3 [36], so it is likely that neuropathology in SCA3 develops due to gain of toxic function. Normal function of ataxin-3 has been shown to be the repression of transcription via chromatin binding, interaction with histone deacetylase 3, and histone deacetylation [37]. Further in transfected mouse neuroblastoma N2a and human embryonic kidney 293T cells it was discovered that the aggregation of mutant ataxin-3 could be suppressed by the inhibition of the Ca2+-dependent protease, calpain [38]. Similar to mutant ataxin-2, in a lipid bilayer model system it was shown that mutant ataxin-3 but not wild type ataxin-3 binds to and activates InsP3R1. Thus, calcium release from ER through InsP3R1 is rising. Moreover, long-term feeding of SCA3-YAC-84Q transgenic mice with Ca2+ stabilizer dantrolene alleviated age-dependent motor coordination deficits in this mice and prevented neuronal loss in affected neurons [39]. Also, just as in the SCA2 case, these improvements can be explained by inhibiting Ca2+ signals that is induced from abnormal IICR that is extra amplified by the RyR in neurons.
These results support the hypothesis that abnormal neuronal Ca2+ signaling through the InsP3R1 may play a key role in the pathogenesis of many polyglutamine expansion neurodegenerative disorders [19, 40]. The same hypothesis also supported by an independent genetic analysis of human ataxias [41].
2. Alzheimer disease
Alzheimer disease (AD) is a neurodegenerative disorder that affects the human brain. AD destroys brain areas that are involved in memory formation, consolidation and storage. There are two forms of AD: sporadic AD, the main risk factor being advanced age; and familial AD (FAD) that is genetically inherited and caused by mutations in genes encoding amyloid precursor protein (APP), presenilin 1 (PS1) and presenilin 2 (PS2) proteins. Presenilins together with nicastrin, APH-1 and PEN-2 form the γ-secretase complex. This protease complex is transported to the cell surface and endosomes where it cleaves several substrates including APP. Cleavage of APP by β- and γ-secretase constitutes the amyloidogenic pathway that leads to production of toxic amyloid beta (Aβ) peptides. Aβ peptides self-aggregate and form oligomeric species (nowadays considered to be the most toxic species) as well as fibrils. The amyloid hypothesis is the dominant one; however, all attempts that tried to clear the brain of Aβ aggregates have failed. Therefore, other hypotheses including the calcium hypothesis of AD are gaining popularity.
2.1 Deranged calcium signaling in AD
Disruption of calcium (Ca2+) signaling has been proposed to be involved in the pathogenesis of AD [42]. Ca2+ is a major second messenger that is involved in many cellular processes starting form dividing of cells during embryogenesis and ending in cell death. Due to the fact that Ca2+ is so important for living cells and especially for neurons different questions impugning Ca2+ hypothesis arise. For example, is it possible to specifically block Ca2+ channels in the brain and do not affect function of similar channels in other excitable tissues such as muscles? To answer this question it is important to understand the full signaling pathway that is disrupted in neurodegenerative disorder, in our case in AD. Moreover, the knowledge of function of every single molecule involved in the signaling pathway is necessary, as the example of the γ-secretase inhibitor semagacestat demonstrates [43]. Semagacestat-treated AD patients displayed significantly worsened functional ability, were predisposed to cancer, infections and inflammation when compared to placebo-treated patients [43]. The reasons of this are a) APP is not the major substrate for γ-secretase, γ-secretase also cleaves Notch protein, b) γ-secretase is not a single protease it has four variants [44] probably with different substrate preferences [45]. Eventual blocking of Notch signaling caused the side effects (gastrointestinal, infection, and skin cancer related) observed in treated patients [46].
2.2 Γ-secretase inhibitors as modifying therapy of AD
Another direction for development of AD modifying therapies is the search of specific inhibitors of β-secretase BACE1 (beta site amyloid precursor protein cleaving enzyme 1). A number of β-secretase inhibitors have been proposed [47–50]. However, after the dramatic story with γ-secretase inhibitors more attention is being put into the investigation of potential side effects of BACE 1 inhibitors. Similarly to γ-secretase, BACE1 has multiple substrates [51–55] and a big catalytic site [56] that make development of pharmaceutical blockers difficult. There are data showing that loss of BACE1 leads to developmental impairments such as peripheral hypomyelination [52, 53], defects of axon guidance [54, 57, 58], disrupted synaptic functions [59, 60], retardation of growth, and increased early lethality [61]. Recently it has been shown in mice that blocking of BACE1 with high doses (100 mg/kg) of the inhibitors SCH1682496 (from Merck) or LY2811376 (from Lilly) caused a decrease in spine formation of layer V pyramidal neurons, a reduced rate of spontaneous and miniature excitatory postsynaptic currents in pyramidal neurons and reduced hippocampal long-term potentiation [62]. However, administration of lower doses (30 mg/kg) of the same BACE1 inhibitors did not cause such side effects [62]. Taken together, it seems to be a long way until specific BACE1 inhibitors that could be applied to AD patients will be developed.
2.3 New therapeutic targets in AD treatment
What other directions of the calcium hypothesis may bring new therapeutic targets? We recently have shown that presenilins function as a passive low conductance ER Ca2+ leak channels [63, 64]. The “leak channel hypothesis” of presenilin function was initially received with skepticism [65], but was recently validated in independent studies [66, 67]. Some but not all FAD associated mutations in PS disrupt this function leading to overfilling of ER with Ca2+ [63, 64, 68, 69]. To compensate for ER store overloading the neuron can upregulate the function of other ER Ca2+ releasing channels such as InsP3R1 and RyRs. Certainly the involvement of these channels in AD pathogenesis has been shown. It has been observed that mutant presenilins activate the InsP3R [70]. Increased expression of RyR has been reported in multiple studies including FAD models as well as in AD patients [69, 71–74]. Consequently it has been proposed that upregulation of RyRs expression at early stages of AD may be a compensatory mechanism for early Ca2+ dysregulation and synaptic failure [75], however over the time overactivation of RyRs become toxic. There is a number of studies that administered an antagonist of RyR dantrolene to mouse models of AD and observed a therapeutic effect [76–78]. However, there is another study from our lab showing that long-term oral feeding of dantrolene induced plaques formation and resulted in loss of hippocampal synaptic markers [69]. Since dantrolene does not effectively block neuronal subtypes of RyR 2 and 3, being specific only for skeletal muscle RyR1 [79], the development of highly specific antagonists of neuronal RyRs are needed. In the absence of such inhibitors our laboratory recently took an advantage of RyR3 knockout mice. These genetic experiments with AD mouse models revealed that RyR3 plays protective role at early stages of AD pathology but becomes detrimental at late stages of pathology [80]. These results suggested that RyR inhibitors are likely to be therapeutically useful at late stages but not at early stages of the disease.
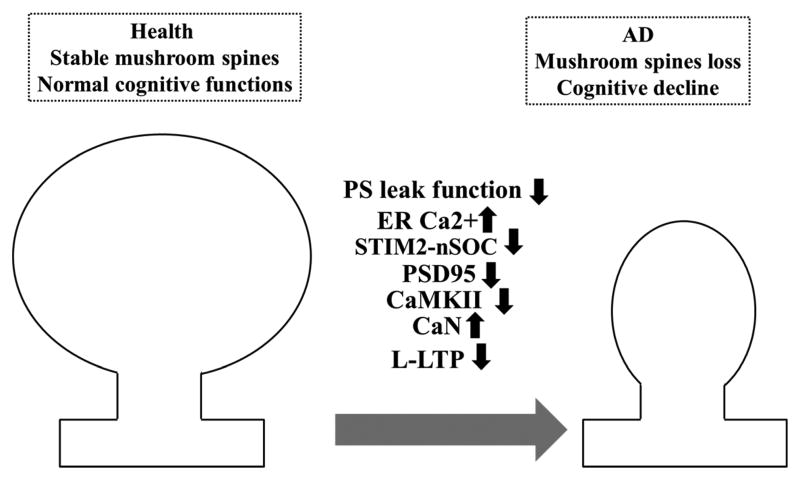
Another source of Ca2+ for the cell is store-operated calcium entry (SOCE) which has been first observed in non-excitable cells (reviewed in [81]) and lately in neurons [82, 83]. SOCE is mediated by SOC channels. Well described SOC channels are STIM1-regulated Orai1/TRPC [84]. SOC channels are unique in the nature of their activation. They are activated in response to lowering of Ca2+ content in the ER. Recent evidence suggests that SOCE may play a role in the pathogenesis of neurodegenerative disorders, particularly in AD [85–88]. In our recent publication we have observed that in response to ER Ca2+ overload neuronal SOC (nSOC) is downregulated in the M146V-KI (KI) mouse model of AD [89]. Moreover, we observed that the homologue of STIM1, STIM2 plays a dominant role in mediating nSOC in hippocampal spines. Why do neurons need nSOC when they already have so many sources of Ca2+ such as NMDAR, AMRAR, VGCC? The answer is that NMDAR, AMRAR, VGCC are sources of fast and massive Ca2+ influx and active only when the neuron is depolarized, they are silent at rest. As we observed, neurons need constant Ca2+ influx in order to support the stability of mushroom spines [89], neuronal structures involved in memory storage [90]. Such a source of constant Ca2+ influx in hippocampal spines is nSOC [89, 91]. Our results suggested that nSOC activity is necessary for proper function of Ca2+/calmodulin-dependent protein kinase II (CaMKII) [89]. We discovered that activity of synaptic CaMKII is reduced in response to nSOC decrease [89]. CaMKII is a so called memory molecule. Its activity is associated with formation of long-term potentiation (LTP) and it is highly expressed in synaptic spines. Most likely the decrease in CaMKII expression is mirrored by increased expression of calcineurin (CaN). Indeed, CaN activity is enhanced in aging neurons and plays an important role in increased long-term depression (LTD) [92, 93]. The same pathway is disrupted in aging neurons, and in sporadic AD brains [89, 94]. Importantly, genetic rescue of this pathway in KI mice restores mushroom spines as well as expression of synaptic proteins PSD95 and rescues CaMKII activity [89]. Thus, we propose that in KI neurons M146V mutation in presenilin 1 causes disruption of ER Ca2+ leak function of presenilin 1, which causes ER Ca2+ overload, compensatory downregulation of STIM2 protein and inhibition of nSOC. This leads to reduced activity of synaptic CaMKII, loss of mushroom spines and cognitive decline (Fig. 2). Reduction of CaMKII activity could explain impaired late form of LTP observed in KI mice [95]. We propose that the pharmacological activators of STIM2-nSOC constitute attractive therapeutic targets for AD.
Figure 2.
The schema representing recently discovered signaling pathway that is disrupted in M146V-KI mouse model of AD. M146V mutation in presenilin 1 (PS) causes loss of ER Ca2+ leak function of PS, that leads to downregulation of neuronal store-operated Ca2+ entry mediated by STIM2-dependent nSOC channels. nSOC is necessary for the stability of mushroom spines that store memory. Reduced nSOC causes decrease in expression of CaMKII (well-studied “memory” molecule) [89]. Lowering of CaMKII activity could underlie impaired late form of LTP observed in KI mice [95]. Eventually this leads to mushroom spine loss and cognitive decline.
Conclusion
Calcium signaling is involved in the regulation of important physiological processes, including learning and memory, behavior, cell division and proliferation, differentiation, development and cell death. Derangement in calcium signaling plays a significant role in numerous neurodegenerative diseases such as different types of SCAs and AD. In SCAs perturbation of InsP3R functions results in abnormal Ca2+ signaling, more often in the increasing of IICR from ER. So InsP3R represents a potentially effective drug target for treatment of some polyglutamine neurodegenerative diseases via normalization of the enhanced Ca2+ signaling in affected neurons.
To date, attempts have been made to repress IICR in the context of neurodegenerative diseases. Thus, a chronic suppression of IICR was achieved by viral expression of the InsP3-phosphatase enzyme in PCs of the SCA2-58Q mouse model. This resulted in improvement of the neuropathological and behavioral phenotypes of the disease [32]. Also IICR might be pharmacologically inhibited with caffeine [96] and by therapeutic levels of lithium in the presence of neuronal calcium sensor-1 [97]. Of course, there are several other hypothetical approaches that could affect the IICR. So, future studies will provide us more information about physiological functions of InsP3R in physiology and pathology and will give us new ways to search for treatments for these incurable neurodegenerative diseases.
There is much evidence to suggest that deranged Ca2+ signaling plays a significant role in AD pathology. Based on experimental results it can be concluded that the various calcium-dependent channels and pumps in the ER take part in the alterations of intracellular Ca2+ signaling in AD. Some changes in the Ca2+ signaling observed in AD can lead to neuronal degeneration, but some alterations in calcium level may be compensatory. Further study of the role of deregulated calcium signaling in neurodegeneration in AD will be very important for the future design of effective disease-modifying therapeutics.
Abnormal calcium signaling plays an important role in pathogenesis of neurodegenerative disorders
In spinocerebellar ataxias activity of inositol 1,4,5-trisphosphate receptor is enhanced, resulting in excessive calcium release from endoplasmic reticulum
In Alzheimer’s disease calcium levels in endoplasmic reticulum are elevated as a result of familial mutations in presenilns or aging
Compensatory downregulation of store-operated calcium entry results in destabilization of synaptic spines in Alzhiemer’s neurons
Calcium signaling modulators have a therapeutic potential for treatment of ataxias, Alzheimer’s disease and other neurodegenerative disorders
Acknowledgments
We are grateful to members of Ilya Bezprozvanny laboratory for advice and suggestions and to Leah Taylor and Polina Plotnikova for administrative assistance. IB is a holder of the Carl J. and Hortense M. Thomsen Chair in Alzheimer’s Disease Research. PE is a holder of Presidential Stipend 2354.2013.4. This work was supported by the Dynasty Foundation grant DP–B-11/13 (EP), NIH grants R01NS07437, R01NS056224, R01NS080152 (IB), by the contract with the Russian Ministry of Science 11.G34.31.0056 (IB), and by the Russian Scientific Fund grant 14-25-00024 (IB).
List of abbreviations
- AD
Alzheimer’s disease
- ADCA
autosomal dominant cerebellar ataxia
- AMPA receptor
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- APP
amyloid precursor protein
- Atx2mut
mutant ataxin-2
- Aβ peptides
amyloid beta peptides
- BACE1
beta site amyloid precursor protein cleaving enzyme 1
- CaBPs
calcium-binding proteins
- CaMKII
calcium/calmodulin-dependent protein kinase II
- CAMTA1
calmodulin-binding transcription activator 1
- CaN
calcineurin
- Ca2+
calcium
- CB
calbindin D-28k
- CF
climbing fibre
- cGMP
cyclic guanosine monophosphate
- Cyt c
cytochrome c
- DUB
deubiquitinating enzyme
- ER
endoplasmic reticulum
- FAD
familial Alzheimer disease
- HD
Huntington’s disease
- IICR
inositol 1,4,5-triphosphate-induced calcium release
- InsP3
inositol 1,4,5-triphosphate
- InsP3R
inositol 1,4,5-triphosphate receptor
- KI
knock-in
- LTD
long-term depression
- LTP
long-term potentiation
- MCU
mitochondrial calcium uniporter
- mGluR
metabotropic glutamate receptor
- Mito
mitochondria
- MRI
Magnetic resonance imaging
- NMDAR
N-methyl-D-aspartate receptor
- nSOC
neuronal store-operated calcium
- OPCA
olivopontocerebellar atrophy
- Opt
opisthotonos
- PC
Purkinje cell
- PF
parallel fibre
- polyQ
polyglutamine
- PS
presenilin
- PV
parvalbumin
- Q
glutamine
- QA
quisqualate
- RyRs
ryanodine receptors
- SCA
spinocerebellar ataxia
- SOCE
store-operated calcium entry
- SOC channels
store-operated calcium channels
- STIM1
stromal interaction molecule 1
- SUMF1
sulfatase modifying factor 1
- VDCC
P/Q voltage-dependent calcium channel
- 5PP
inositol 1,4,5-triphosphate-phosphatase enzyme
Footnotes
Disclosures: IB is a paid consultant to Ataxion and TEVA in the field of neurodegeneration.
Other authors have no financial interests related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bezprozvanny I, Klockgether T. Therapeutic prospects for spinocerebellar ataxia type 2 and 3. Drugs of the Future. 2010;34(12):991–999. doi: 10.1358/dof.2009.034.12.1443434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schols L, et al. Autosomal dominant cerebellar ataxias: clinical features, genetics, and pathogenesis. Lancet Neurol. 2004;3(5):291–304. doi: 10.1016/S1474-4422(04)00737-9. [DOI] [PubMed] [Google Scholar]
- 3.Filla A, et al. Spinocerebellar ataxia type 2 in southern Italy: a clinical and molecular study of 30 families. J Neurol. 1999;246(6):467–71. doi: 10.1007/s004150050385. [DOI] [PubMed] [Google Scholar]
- 4.Lastres-Becker I, Rub U, Auburger G. Spinocerebellar ataxia 2 (SCA2) Cerebellum. 2008;7(2):115–24. doi: 10.1007/s12311-008-0019-y. [DOI] [PubMed] [Google Scholar]
- 5.Geschwind DH, et al. The prevalence and wide clinical spectrum of the spinocerebellar ataxia type 2 trinucleotide repeat in patients with autosomal dominant cerebellar ataxia. Am J Hum Genet. 1997;60(4):842–50. [PMC free article] [PubMed] [Google Scholar]
- 6.Burk K, et al. Autosomal dominant cerebellar ataxia type I clinical features and MRI in families with SCA1, SCA2 and SCA3. Brain. 1996;119(Pt 5):1497–505. doi: 10.1093/brain/119.5.1497. [DOI] [PubMed] [Google Scholar]
- 7.Rubinsztein DC, Wyttenbach A, Rankin J. Intracellular inclusions, pathological markers in diseases caused by expanded polyglutamine tracts? J Med Genet. 1999;36(4):265–70. [PMC free article] [PubMed] [Google Scholar]
- 8.Ho LW, et al. The molecular biology of Huntington’s disease. Psychol Med. 2001;31(1):3–14. doi: 10.1017/s0033291799002871. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji S. Dentatorubral-pallidoluysian atrophy. Handb Clin Neurol. 2012;103:587–94. doi: 10.1016/B978-0-444-51892-7.00041-3. [DOI] [PubMed] [Google Scholar]
- 10.Persengiev S, Kondova I, Bontrop RE. Functional Annotation of Small Noncoding RNAs Target Genes Provides Evidence for a Deregulated Ubiquitin-Proteasome Pathway in Spinocerebellar Ataxia Type 1. J Nucleic Acids. 2012;2012:672536. doi: 10.1155/2012/672536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Switonski PM, et al. A new humanized ataxin-3 knock-in mouse model combines the genetic features, pathogenesis of neurons and glia and late disease onset of SCA3/MJD. Neurobiol Dis. 2015;73:174–88. doi: 10.1016/j.nbd.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Mohan RD, Abmayr SM, Workman JL. Pulling complexes out of complex diseases: Spinocerebellar Ataxia 7. Rare Dis. 2014;2:e28859. doi: 10.4161/rdis.28859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwaller B, Meyer M, Schiffmann S. ‘New’ functions for ‘old’ proteins: the role of the calcium-binding proteins calbindin D-28k, calretinin and parvalbumin, in cerebellar physiology. Studies with knockout mice. Cerebellum. 2002;1(4):241–58. doi: 10.1080/147342202320883551. [DOI] [PubMed] [Google Scholar]
- 14.Kreiner L, et al. Compensatory regulation of Cav2.1 Ca2+ channels in cerebellar Purkinje neurons lacking parvalbumin and calbindin D-28k. J Neurophysiol. 2010;103(1):371–81. doi: 10.1152/jn.00635.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kano M, et al. Calcium-dependent regulation of climbing fibre synapse elimination during postnatal cerebellar development. J Physiol. 2013;591(Pt 13):3151–8. doi: 10.1113/jphysiol.2012.248252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes JA, et al. Abnormalities in the climbing fiber-Purkinje cell circuitry contribute to neuronal dysfunction in ATXN1[82Q] mice. J Neurosci. 2011;31(36):12778–89. doi: 10.1523/JNEUROSCI.2579-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long C, et al. Ataxia and Purkinje cell degeneration in mice lacking the CAMTA1 transcription factor. Proc Natl Acad Sci U S A. 2014;111(31):11521–6. doi: 10.1073/pnas.1411251111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawaguchi SY, Hirano T. Gating of long-term depression by Ca2+/calmodulin-dependent protein kinase II through enhanced cGMP signalling in cerebellar Purkinje cells. J Physiol. 2013;591(Pt 7):1707–30. doi: 10.1113/jphysiol.2012.245787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezprozvanny I. Role of inositol 1,4,5-trisphosphate receptors in pathogenesis of Huntington’s disease and spinocerebellar ataxias. Neurochem Res. 2011;36(7):1186–97. doi: 10.1007/s11064-010-0393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto M, et al. Ataxia and epileptic seizures in mice lacking type 1 inositol 1,4,5-trisphosphate receptor. Nature. 1996;379(6561):168–71. doi: 10.1038/379168a0. [DOI] [PubMed] [Google Scholar]
- 21.Street VA, et al. The type 1 inositol 1,4,5-trisphosphate receptor gene is altered in the opisthotonos mouse. J Neurosci. 1997;17(2):635–45. doi: 10.1523/JNEUROSCI.17-02-00635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van de Leemput J, et al. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 2007;3(6):e108. doi: 10.1371/journal.pgen.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown SA, Loew LM. Computational analysis of calcium signaling and membrane electrophysiology in cerebellar Purkinje neurons associated with ataxia. BMC Syst Biol. 2012;6:70. doi: 10.1186/1752-0509-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Storey E, Gardner RJ. Spinocerebellar ataxia type 15. Handb Clin Neurol. 2012;103:561–5. doi: 10.1016/B978-0-444-51892-7.00037-1. [DOI] [PubMed] [Google Scholar]
- 25.Hara K, et al. Total deletion and a missense mutation of ITPR1 in Japanese SCA15 families. Neurology. 2008;71(8):547–51. doi: 10.1212/01.wnl.0000311277.71046.a0. [DOI] [PubMed] [Google Scholar]
- 26.van de Leemput J, et al. Sequencing analysis of the ITPR1 gene in a pure autosomal dominant spinocerebellar ataxia series. Mov Disord. 2010;25(6):771–3. doi: 10.1002/mds.22970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marelli C, et al. SCA15 Due to Large ITPR1 Deletions in a Cohort of 333 White Families With Dominant Ataxia. Archives of Neurology. 2011;68(5):637–643. doi: 10.1001/archneurol.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulst SM, et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat Genet. 1996;14(3):269–76. doi: 10.1038/ng1196-269. [DOI] [PubMed] [Google Scholar]
- 29.Huynh DP, et al. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet. 2000;26(1):44–50. doi: 10.1038/79162. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 2. J Neurosci. 2009;29(29):9148–62. doi: 10.1523/JNEUROSCI.0660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Loo S, et al. Ataxin-2 associates with rough endoplasmic reticulum. Exp Neurol. 2009;215(1):110–8. doi: 10.1016/j.expneurol.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 32.Kasumu AW, et al. Chronic suppression of inositol 1,4,5-triphosphate receptor-mediated calcium signaling in cerebellar purkinje cells alleviates pathological phenotype in spinocerebellar ataxia 2 mice. J Neurosci. 2012;32(37):12786–96. doi: 10.1523/JNEUROSCI.1643-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paulson HL, et al. Machado-Joseph disease gene product is a cytoplasmic protein widely expressed in brain. Ann Neurol. 1997;41(4):453–62. doi: 10.1002/ana.410410408. [DOI] [PubMed] [Google Scholar]
- 34.Stevanin G, Durr A, Brice A. Clinical and molecular advances in autosomal dominant cerebellar ataxias: from genotype to phenotype and physiopathology. Eur J Hum Genet. 2000;8(1):4–18. doi: 10.1038/sj.ejhg.5200403. [DOI] [PubMed] [Google Scholar]
- 35.Coutinho P, Andrade C. Autosomal dominant system degeneration in Portuguese families of the Azores Islands. A new genetic disorder involving cerebellar, pyramidal, extrapyramidal and spinal cord motor functions. Neurology. 1978;28(7):703–9. doi: 10.1212/wnl.28.7.703. [DOI] [PubMed] [Google Scholar]
- 36.Burnett B, Li F, Pittman RN. The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum Mol Genet. 2003;12(23):3195–205. doi: 10.1093/hmg/ddg344. [DOI] [PubMed] [Google Scholar]
- 37.Evert BO, et al. Ataxin-3 represses transcription via chromatin binding, interaction with histone deacetylase 3, and histone deacetylation. J Neurosci. 2006;26(44):11474–86. doi: 10.1523/JNEUROSCI.2053-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haacke A, Hartl FU, Breuer P. Calpain inhibition is sufficient to suppress aggregation of polyglutamine-expanded ataxin-3. J Biol Chem. 2007;282(26):18851–6. doi: 10.1074/jbc.M611914200. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, et al. Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J Neurosci. 2008;28(48):12713–24. doi: 10.1523/JNEUROSCI.3909-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasumu A, Bezprozvanny I. Deranged Calcium Signaling in Purkinje Cells and Pathogenesis in Spinocerebellar Ataxia 2 (SCA2) and Other Ataxias. Cerebellum (London, England) 2012;11(3):630–9. doi: 10.1007/s12311-010-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schorge S, et al. Human ataxias: a genetic dissection of inositol triphosphate receptor (ITPR1)-dependent signaling. Trends Neurosci. 2010;33(5):211–9. doi: 10.1016/j.tins.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khachaturian ZS. Calcium, membranes, aging, and Alzheimer’s disease. Introduction and overview. Ann N Y Acad Sci. 1989;568:1–4. doi: 10.1111/j.1749-6632.1989.tb12485.x. [DOI] [PubMed] [Google Scholar]
- 43.Doody RS, et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med. 2013;369(4):341–50. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 44.De Strooper B. Aph-1, Pen-2, and Nicastrin with Presenilin generate an active gamma-Secretase complex. Neuron. 2003;38(1):9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 45.De Strooper B, Chavez Gutierrez L. Learning by Failing: Ideas and Concepts to Tackle gamma-Secretases in Alzheimer’s Disease and Beyond. Annu Rev Pharmacol Toxicol. 2015;55:419–37. doi: 10.1146/annurev-pharmtox-010814-124309. [DOI] [PubMed] [Google Scholar]
- 46.Henley DB, et al. Safety profile of semagacestat, a gamma-secretase inhibitor: IDENTITY trial findings. Curr Med Res Opin. 2014;30(10):2021–32. doi: 10.1185/03007995.2014.939167. [DOI] [PubMed] [Google Scholar]
- 47.May PC, et al. Robust central reduction of amyloid-beta in humans with an orally available, non-peptidic beta-secretase inhibitor. J Neurosci. 2011;31(46):16507–16. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang HB, Li RN, Shen Y. beta-Secretase: its biology as a therapeutic target in diseases. Trends in Pharmacological Sciences. 2013;34(4):215–225. doi: 10.1016/j.tips.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan J, et al. Structure-based design of beta-site APP cleaving enzyme 1 (BACE1) inhibitors for the treatment of Alzheimer’s disease. J Med Chem. 2013;56(11):4156–80. doi: 10.1021/jm301659n. [DOI] [PubMed] [Google Scholar]
- 50.Yan R, Vassar R. Targeting the beta secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 2014;13(3):319–29. doi: 10.1016/S1474-4422(13)70276-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Q, Sudhof TC. Cleavage of amyloid-beta precursor protein and amyloid-beta precursor-like protein by BACE 1. J Biol Chem. 2004;279(11):10542–50. doi: 10.1074/jbc.M310001200. [DOI] [PubMed] [Google Scholar]
- 52.Hu X, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nat Neurosci. 2006;9(12):1520–5. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 53.Willem M, et al. Control of peripheral nerve myelination by the beta-secretase BACE1. Science. 2006;314(5799):664–6. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 54.Hitt B, et al. beta-Site amyloid precursor protein (APP)-cleaving enzyme 1 (BACE1)-deficient mice exhibit a close homolog of L1 (CHL1) loss-of-function phenotype involving axon guidance defects. J Biol Chem. 2012;287(46):38408–25. doi: 10.1074/jbc.M112.415505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim DY, et al. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol. 2007;9(7):755–64. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vassar R, Kandalepas PC. The beta-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimers Res Ther. 2011;3(3):20. doi: 10.1186/alzrt82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao L, et al. The precision of axon targeting of mouse olfactory sensory neurons requires the BACE1 protease. Sci Rep. 2012;2:231. doi: 10.1038/srep00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rajapaksha TW, et al. The Alzheimer’s beta-secretase enzyme BACE1 is required for accurate axon guidance of olfactory sensory neurons and normal glomerulus formation in the olfactory bulb. Mol Neurodegener. 2011;6:88. doi: 10.1186/1750-1326-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laird FM, et al. BACE1, a major determinant of selective vulnerability of the brain to amyloid-beta amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J Neurosci. 2005;25(50):11693–709. doi: 10.1523/JNEUROSCI.2766-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savonenko AV, et al. Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc Natl Acad Sci U S A. 2008;105(14):5585–90. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dominguez D, et al. Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J Biol Chem. 2005;280(35):30797–806. doi: 10.1074/jbc.M505249200. [DOI] [PubMed] [Google Scholar]
- 62.Filser S, et al. Pharmacological Inhibition of BACE1 Impairs Synaptic Plasticity and Cognitive Functions. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Tu H, et al. Presenilins form ER calcium leak channels, a function disrupted by mutations linked to familial Alzheimer’s disease. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nelson O, et al. Mutagenesis mapping of the presenilin 1 calcium leak conductance pore. J Biol Chem. 2011;286(25):22339–47. doi: 10.1074/jbc.M111.243063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shilling D, et al. Lack of evidence for presenilins as endoplasmic reticulum Ca2+ leak channels. J Biol Chem. 2012;287:10933–10944. doi: 10.1074/jbc.M111.300491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bandara S, Malmersjo S, Meyer T. Regulators of Calcium Homeostasis Identified by Inference of Kinetic Model Parameters from Live Single Cells Perturbed by siRNA. Science signaling. 2013;6(283):ra56. doi: 10.1126/scisignal.2003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bezprozvanny I. Presenilins and calcium signaling-systems biology to the rescue. Sci Signal. 2013;6(283):pe24. doi: 10.1126/scisignal.2004296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nelson O, et al. Familial Alzheimer’s disease mutations in presenilins: effects on endoplasmic reticulum calcium homeostasis and correlation with clinical phenotypes. J Alzheimers Dis. 2010;21(3):781–93. doi: 10.3233/JAD-2010-100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, et al. Role of presenilins in neuronal calcium homeostasis. J Neurosci. 2010;30(25):8566–80. doi: 10.1523/JNEUROSCI.1554-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheung KH, et al. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP(3) receptor channel gating. Neuron. 2008;58(6):871–83. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelliher M, et al. Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer’s disease neurofibrillary and beta-amyloid pathologies. Neuroscience. 1999;92(2):499–513. doi: 10.1016/s0306-4522(99)00042-1. [DOI] [PubMed] [Google Scholar]
- 72.Bruno AM, et al. Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2012;33(5):1001 e1–6. doi: 10.1016/j.neurobiolaging.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stutzmann GE, et al. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J Neurosci. 2006;26(19):5180–9. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakroborty S, et al. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J Neurosci. 2009;29(30):9458–70. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chakroborty S, et al. Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer’s disease mice. J Neurosci. 2012;32(24):8341–53. doi: 10.1523/JNEUROSCI.0936-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peng J, et al. Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett. 2012;516(2):274–9. doi: 10.1016/j.neulet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakroborty S, et al. Stabilizing ER Ca2+ Channel Function as an Early Preventative Strategy for Alzheimer’s Disease. PLoS One. 2012;7(12) doi: 10.1371/journal.pone.0052056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oules B, et al. Ryanodine Receptor Blockade Reduces Amyloid-beta Load and Memory Impairments in Tg2576 Mouse Model of Alzheimer Disease. J Neurosci. 2012;32(34):11820–11834. doi: 10.1523/JNEUROSCI.0875-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krause T, et al. Dantrolene--a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59(4):364–73. doi: 10.1111/j.1365-2044.2004.03658.x. [DOI] [PubMed] [Google Scholar]
- 80.Liu J, et al. The role of ryanodine receptor type 3 in a mouse model of Alzheimer disease. Channels (Austin) 2014;8(3):230–42. doi: 10.4161/chan.27471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85(2):757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 82.Kachoei BA, et al. A store-operated Ca(2+) influx pathway in the bag cell neurons of Aplysia. J Neurophysiol. 2006;96(5):2688–98. doi: 10.1152/jn.00118.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29(1):197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 84.Liao Y, et al. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104(11):4682–7. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leissring MA, et al. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J Cell Biol. 2000;149(4):793–8. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoo AS, et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27(3):561–72. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 87.Herms J, et al. Capacitive calcium entry is directly attenuated by mutant presenilin-1, independent of the expression of the amyloid precursor protein. J Biol Chem. 2003;278(4):2484–9. doi: 10.1074/jbc.M206769200. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto S, et al. Transient receptor potential channels in Alzheimer’s disease. Biochim Biophys Acta. 2007;1772(8):958–67. doi: 10.1016/j.bbadis.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 89.Sun S, et al. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron. 2014;82(1):79–93. doi: 10.1016/j.neuron.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17(3):381–6. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 91.Bezprozvanny I, Hiesinger PR. The synaptic maintenance problem: membrane recycling, Ca2+ homeostasis and late onset degeneration. Mol Neurodegener. 2013;8:23. doi: 10.1186/1750-1326-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Foster TC, et al. Calcineurin links Ca2+ dysregulation with brain aging. J Neurosci. 2001;21(11):4066–73. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jouvenceau A, Dutar P. A role for the protein phosphatase 2B in altered hippocampal synaptic plasticity in the aged rat. J Physiol Paris. 2006;99(2–3):154–61. doi: 10.1016/j.jphysparis.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 94.Reese LC, et al. Dysregulated phosphorylation of Ca(2+)/calmodulin-dependent protein kinase II-alpha in the hippocampus of subjects with mild cognitive impairment and Alzheimer’s disease. J Neurochem. 2011;119(4):791–804. doi: 10.1111/j.1471-4159.2011.07447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Auffret A, et al. Progressive age-related impairment of the late long-term potentiation in Alzheimer’s disease presenilin-1 mutant knock-in mice. J Alzheimers Dis. 2010;19(3):1021–33. doi: 10.3233/JAD-2010-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bezprozvanny I, Bezprozvannaya SG, Ehrlich BE. Caffeine-induced inhibition of inositol (1,4,5)-trisphosphate-gated calcium channels from cerebellum. Molec Biol Cell. 1994;5:97–103. doi: 10.1091/mbc.5.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schlecker C, et al. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J Clin Invest. 2006;116(6):1668–74. doi: 10.1172/JCI22466. [DOI] [PMC free article] [PubMed] [Google Scholar]