Abstract
“Cu–CF3” species have been used historically for a broad spectrum of nucleophilic trifluoromethylation reactions. Although recent advancements have employed ligands to stabilize and harness the reactivity of this key organometallic intermediate, the ability of a ligand to differentiate a regiochemical outcome of a Cu–CF3-mediated or -catalyzed reaction has not been previously reported. Herein, we report the first example of a Cu-catalyzed trifluoromethylation reaction in which a ligand controls the regiochemical outcome. More specifically, we demonstrate the ability of bipyridyl-derived ligands to control the regioselectivity of the Cu-catalyzed nucleophilic trifluoromethylation reactions of propargyl electrophiles to generate trifluoromethylallenes. This method provides a variety of di-, tri- and tetra-substituted trifluoromethylallenes, which can be further modified to generate complex fluorinated substructures.
Graphical Abstract
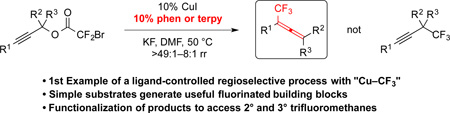
Copper-mediated and -catalyzed nucleophilic trifluoromethylation is a popular strategy for accessing CF3-based products.1 While the fundamental reactivity of Cu–CF3 with sp2- and activated sp3-electrophiles has long been established,2 recent advances have greatly improved the practical utility and economic viability of these methods.3–5 One important advancement involves the use of ligands to stabilize the reactive Cu–CF3 species, and to accelerate reactions with electrophiles.3,5,6 These two features allow reactions to proceed under milder conditions that tolerate a broad array of functional groups and heterocycles.3,5,6 While many of these new Cu-mediated and -catalyzed trifluoromethylation reactions display excellent chemoselectivity, ligands have not previously influenced regiochemical outcomes of reactions. Herein, we report the first example of a regioselective trifluoromethylation reaction in which a ligand overrides the intrinsic reactivity of unligated “Cu–CF3” with electrophiles. Further, we show that the products can serve as useful synthetic building blocks by providing access to 2° trifluoromethanes that are otherwise difficult to synthesize.
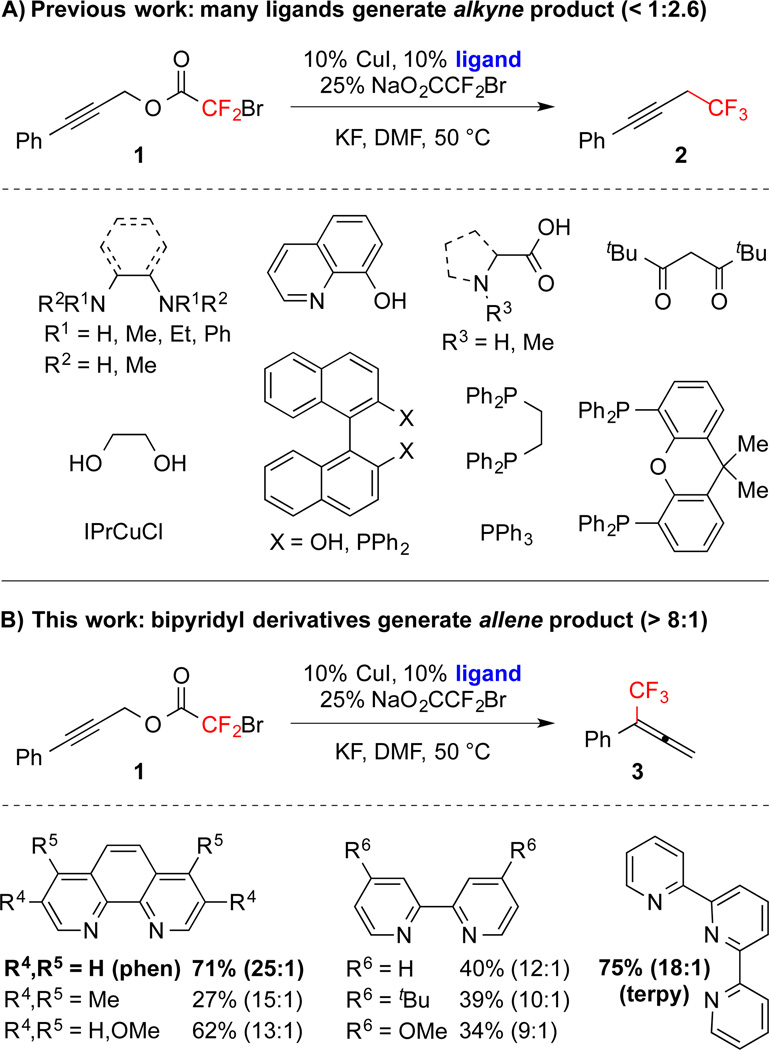
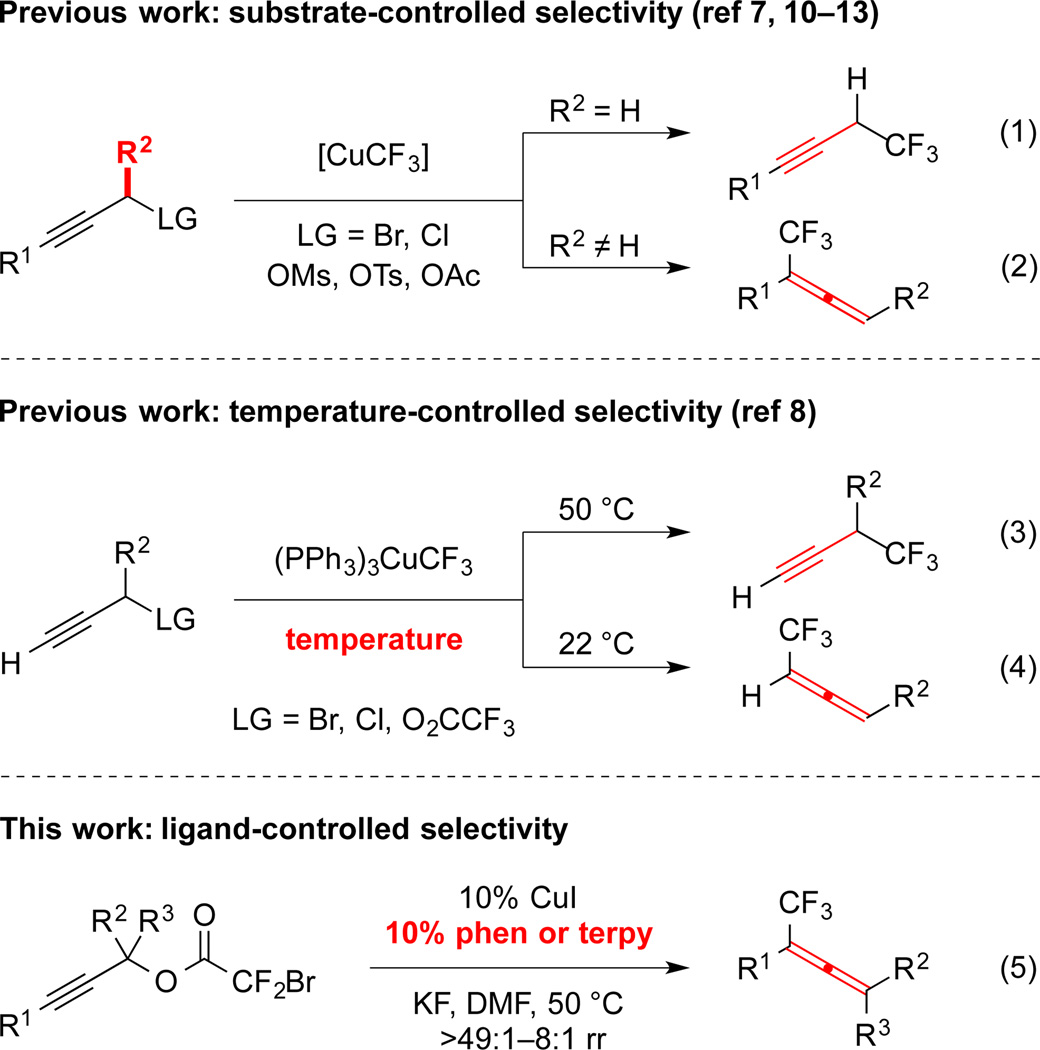
Propargyl electrophiles, including –Br,7–9 –Cl,8–11 –OMs,12 –OTs,10 –OAc,13 and –O2CCF2X (X = F, Cl, Br), 8,14,15c react using either catalytic11,15c or stoichiometric7–10,12–14 “Cu–CF3” to generate propargyl and/or allenyl products with minimal control of regiochemistry. Unsubstituted propargyl electrophiles provide trifluoromethylallene;9,10,14 however, reactions of substituted substrates display distinct selectivities. In most cases, the product distribution is dictated by the substitution pattern of the substrate with 1° electrophiles providing propargyl products, and with 2° and 3° electrophiles providing allenyl products (eq 1–2).7,10–14 In contrast, using a Cu/PPh3-based system, modulation of the reaction temperature can control the regioisomeric ratio of branched and linear products (eq 3–4).8 However, for many cases, the intrinsic reactivity of the substrate overrides the control by temperature, and thus, many allenyl products are not accessible.8
In our own work aimed at developing decarboxylative strategies for fluoroalkylation,15 we reported a CuI/N,N’-dimethylethylenediamine catalyzed trifluoromethylation reaction of propargyl bromodifluoroacetates to generate propargyl trifluoromethanes preferentially.15c For this reaction, a wide variety of N-, O- and P-based ligands provided propargyl products with modest regioselectivity (Figure 1A). However, the use of 1,10-phenanthroline-based and 2,2’-bipyridine-based ligands reversed the regioselectivity of the transformation, and afforded trifluoromethylallene 3 with high regioselectivity (Figure 1B). For these bipyridines and phenanthrolines, the use of ligands bearing electron-donating aliphatic and methoxy groups did not significantly modulate the selectivity of reactions. Thus, the geometric influence of the bipyridyl substructure presumably controlled the regioselectivity of the transformation. However, these electron-donating groups decreased the activity of the catalysts. Thus, 1,10-phenanthroline (phen) and terpyridine (terpy) were identified as the optimal ligands for the current transformation.
Figure 1.
Ligand-controlled Regioselective Trifluoromethylation
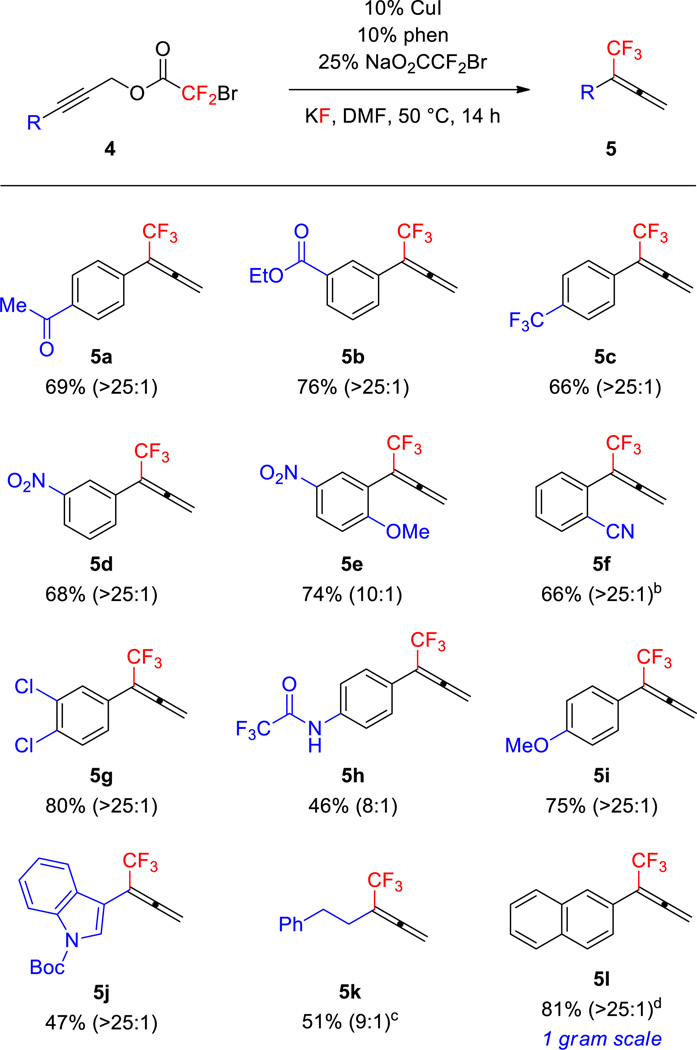
Employing phen as a ligand, various 1° propargyl bromodifluoroacetates were converted to 1,1-disubstituted trifluoromethylallenes with good to excellent selectivity (Figure 2). Initial efforts focused on the synthesis of 1-aryl-1-trifluoromethylallenes, which cannot be selectively accessed via other Cu-mediated or -catalyzed processes,7–14 and otherwise requires multi-step sequences that afford low yields of product.16 Propargyl electrophiles conjugated with electron-rich, -neutral, and -deficient aromatic moieties all formed allene products in excellent selectivity (5a–d, 5g–j).17 When the reaction was conducted on a gram-scale, good yield and excellent selectivity were maintained (5l). In contrast to substrates bearing m- and p-substituted aryl moieties, substrates bearing o-substituted aryl systems afforded products in lower selectivity (ca. 10:1; 5e–f). Using phen as a ligand, a 1° aliphatic-substituted substrate was not effectively converted to product; however, the use of terpy as a ligand provided trifluoromethylallene 5k in synthetically useful yield and selectivity. The reaction tolerated many important functional groups, including carbonyl groups (5a, 5b, 5h, 5j), nitro groups (5d, 5e), nitriles (5f), and ethers (5i). The carbonyl-containing groups are particularly interesting, because they are prone to react with free CF3− to provide β,β,β-trifluoroethyl alcohols.1d,4,18 Since products of 1,2-addition were not observed in these reactions, free −CF3 must not have existed in solution. Therefore, generation of the reactive (phen)Cu-CF3 species likely involved an inner-sphere process that does not generate free −CF3.
Figure 2.
Reactions of Primary Propargyl Bromodifluoroacetates Generate 1,1-Disubstituted Trifluoromethylallenesa
aConditions: 4a–l (1 equiv), CuI (10 mol %), phen (10 mol %), NaO2CCF2Br (25 mol %), KF (2 equiv), DMF (1.0 M), 50 °C, 14 h. The numbers in parentheses represent the ratios of allene:alkyne in purified product as determined by 1H NMR spectroscopy. b 12:1 Mixture of allene:alkyne prior to chromatographic purification as determined by 19F NMR spectroscopy. c Terpy (10 mol %) employed as a ligand. d Reaction conducted on a 7 mmol scale.
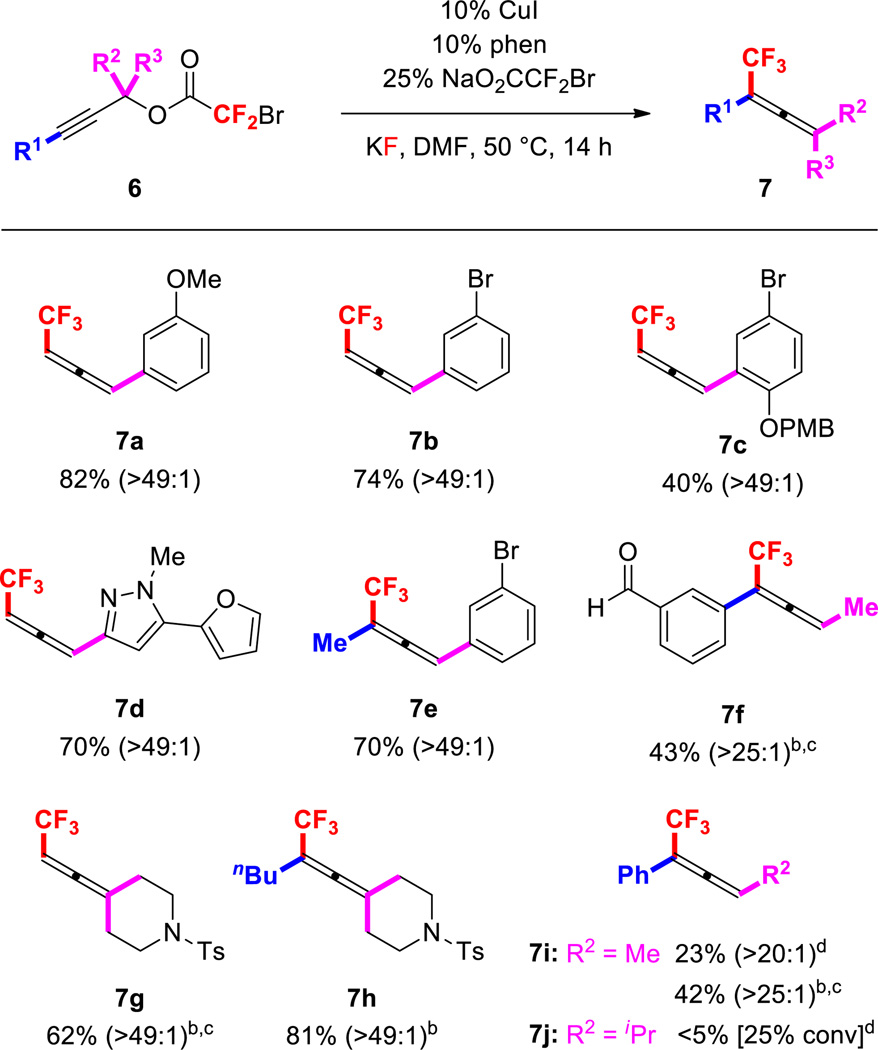
Utilizing similar reaction conditions to those used for 1° bromodifluoroacetates, 2° and 3° propargyl electrophiles were also regioselectively converted to di- and tri-substituted trifluoromethylallenes in high regioisomeric ratios (Figure 3). Generally, 2° 1-aryl propargyl substrates provided 1,3-disubstituted trifluoromethylallenes in synthetically useful yields and excellent selectivities (7a–e). In addition, the standard conditions converted a 2° substrate to a trisubstituted allene product (7e); however, the standard conditions did not effectively transform several challenging substrates. For example, substrates bearing aliphatic groups at the α position reacted sluggishly, and provided low yields of allene products (7f–j). For these less reactive 2° and 3° alkyl-substituted bromodifluoroacetates, the use of terpyridine as a ligand and/or more forcing conditions (60 °C, 24 h) facilitated the formation of trisubstituted (7f–g, i) and tetrasubstituted (7h) allenes. Notably, the decarboxylative trifluoromethylation reaction tolerated aryl bromides (7b–c, 7e), which can undergo Cu-catalyzed nucleophilic trifluoromethylation under similar conditions.3a Although substrates bearing free amines decomposed under the reaction conditions, protection of these groups as amides, carbamates, and sulfonamides permitted catalyst turnover (5h, 5j, 7g–h). Finally, the catalyst system tolerated several important heterocycles, including indole (5j), pyrazole (7d), and furan (7d), which may be useful for the design of biological probes and agrochemicals.
Figure 3.
Reactions of Substituted Propargyl Bromodifluoroacetates Provide Di- and Tri-Substituted Trifluoromethylallenes
a Conditions: 6a–i (1 equiv), CuI (10 mol %), phen (10 mol %), NaO2CCF2Br (25 mol %), KF (2 equiv) DMF (1.0 M), 50 °C, 14 h. The numbers in parentheses represent the ratios of allene:alkyne in purified product as determined by 1H NMR spectroscopy. b 60 °C, 24 h. c Terpy (10 mol %) employed as a ligand. d Estimated by 19F NMR spectroscopy.
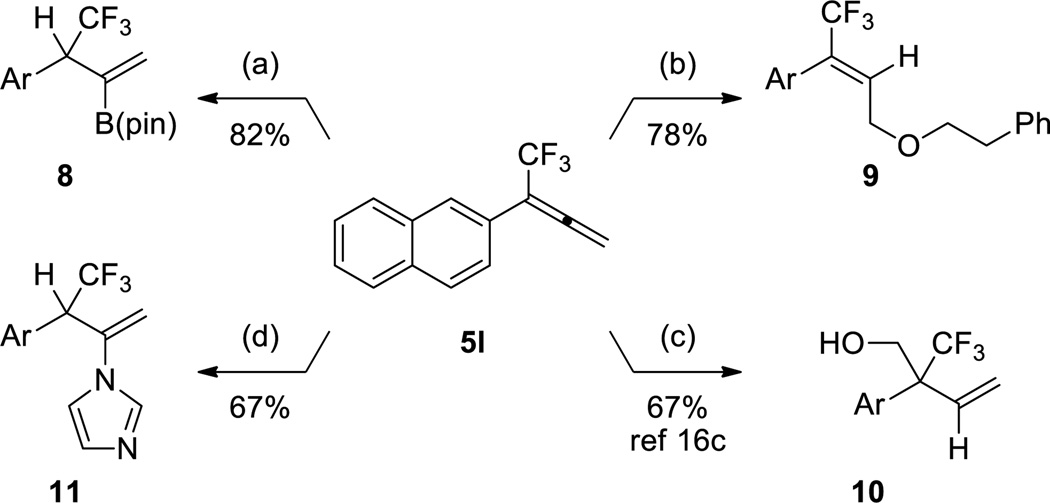
Allenes can serve as a useful building block for accessing complex substructures,19 and in recent years, considerable attention has focused on both the synthesis of allenes,20 and transformations of allene-based building blocks.21 Given the synthetic potential of allenes, trifluoromethylallenes should be useful synthetic precursors for various fluorinated motifs. However, few modern transformations of trifluoromethylallenes have been disclosed,16c, 22 which restricts the use of these fluorinated substructures as intermediates in synthetic sequences. To showcase the potential synthetic utility of trifluoromethylallenes, 5l was subjected to metal-catalyzed hydrofunctionalization reactions to generate C–B,23 C–O,24 C–N,25 and C–C16c bonds (Scheme 2). In all cases, the reactions of 5l provided products (8–11) in good yields and excellent regioselectivity,26 with minimal optimization of previously reported systems.27 In most cases, the regioselectivities of the transformations matched those of previous reports;23–24 however, the product of the hydroamination reaction did not match the predicted regiochemical outcome,25 indicating that some reactions of trifluoromethylallenes may generate unique products (Scheme 2, d). Nonetheless, all functionalization reactions provide trifluoromethyl-containing products that might otherwise be challenging to prepare.
Scheme 2.
Direct Conversion of Trifluoromethylallenes to Functionalized Trifluoromethylated Motifs
a B2(pin)2 (1.1 equiv), CuCl (5 mol %), IPr•HCl (5 mol %), NaOtBu (40 mol %), MeOH (6 equiv), THF, 23 °C. b 2-phenylethanol (1.1 equiv), AuIPrCl (10 mol %), AgOTf (10 mol %), PhMe, 23 °C. c (CH2O)n (2 equiv), RuHCl(CO)(PPh3)3 (5 mol %), dppm (5 mol %), iPrOH (4 equiv), PhMe, 105 °C. d Imidazole (1.2 equiv), [PdCl(C3H5)]2 (2.5 mol %), dppf (5 mol %), THF, 80 °C.
In conclusion, the use of bipyridyl-derived ligands overrode the intrinsic regioselectivity of Cu-catalyzed trifluoromethylation reactions of propargyl electrophiles, and provided di-, tri-, and tetra-substituted trifluoromethylallenes bearing synthetically important functional groups. More broadly, this transformation serves as the first example of a Cu-catalyzed trifluoromethylation reaction in which a ligand controls the regiochemical outcome. Ongoing work in our laboratory aims to understand the basis by which the ligands control the regiochemistry of the reaction.
Supplementary Material
Scheme 1.
Substrate and Temperature-controlled Regioselective Trifluoromethylation
ACKNOWLEDGMENT
We thank the donors of the Herman Frasch Foundation for Chemical Research (701-HF12) and the American Chemical Society Petroleum Research Fund (52073-DNI1) for support of this project. We gratefully acknowledge the NIGMS Training Grant on Dynamic Aspects of Chemical Biology (T32 GM08545) for a graduate traineeship (B.R.A.). Additional financial assistance was provided by the University of Kansas Office of the Provost, Department of Medicinal Chemistry, and General Research Fund (2301795). The NMR instruments were supported by NIH Shared Instrumentation Grant # S10RR024664 and NSF Major Research Instrumentation Grant # 0320648. We gratefully acknowledge Aspira Scientific for providing HO2CCF2Br. We thank Casey L. Henderson and Caleb D. Vogt of The University of Kansas Department of Medicinal Chemistry for assistance assisting with the preparation of substrates.
Footnotes
ASSOCIATED CONTENT
Experimental protocols and characterization data for all compounds are available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
REFERENCES
- 1.Recent reviews: Tomashenko OA, Grushin VV. Chem. Rev. 2011;111:4475. doi: 10.1021/cr1004293. Liu T, Shen Q. Eur. J. Org. Chem. 2012:6679. Liang T, Neumann CN, Ritter T. Angew. Chem. Int. Ed. 2012;52:8214. doi: 10.1002/anie.201206566. Liu X, Xu C, Wang M, Liu Q. Chem. Rev. 2015;115:683. doi: 10.1021/cr400473a. Alonso C, Martínez de Marigorta E, Rubiales G, Palacios F. Chem. Rev. 2015;115:1847. doi: 10.1021/cr500368h..
- 2.(a) Kobayashi Y, Kumadaki I. Tetrahedron Lett. 1969;10:4095. [Google Scholar]; (b) Kobayashi Y, Yamamoto K, Kumadaki I. Tetrahedron Lett. 1979;20:4071. [Google Scholar]; (c) Burton DJ, Wiemers DM. J. Am. Chem. Soc. 1985;107:5014. [Google Scholar]; (d) Wiemers DM, Burton DJ. J. Am. Chem. Soc. 1986;108:832. [Google Scholar]; (e) Chen Q-Y, Wu S-W. J. Chem. Soc. Chem. Commun. 1989:705. [Google Scholar]
- 3.Shelf-stable stoichiometric reagents: Morimoto H, Tsubogo T, Litvinas ND, Hartwig JF. Angew. Chem. Int. Ed. 2011;50:3793. doi: 10.1002/anie.201100633. Tomashenko OA, Escudero-Adán EC, Belmonte MM, Grushin VV. Angew. Chem. Int. Ed. 2011;50:7655. doi: 10.1002/anie.201101577.
- 4.[Cu–CF3] generated from CHF3: Zanardi A, Novikov MA, Martin E, Benet-Buchholz J, Grushin VV. J. Am. Chem. Soc. 2011;133:20901. doi: 10.1021/ja2081026. Lishchynskyi A, Novikov MA, Martin E, Escudero-Adán EC, Novák P, Grushin VV. J. Org. Chem. 2013;78:11126. doi: 10.1021/jo401423h..
- 5.Examples of Cu-catalyzed nucleophilic trifluoromethylation reactions: Oishi M, Kondo H, Amii H. Chem. Commun. 2009:1909. doi: 10.1039/b823249k. Kondo H, Oishi M, Fujikawa K, Amii H. Adv. Synth. Catal. 2011;353:1247. Knauber T, Arikan F, Röschenthaler G-V, Gooßen LJ. Chem. Eur. J. 2011;17:2689. doi: 10.1002/chem.201002749. Gonda Z, Kovács S, Wéber C, Gáti T, Mészáros A, Kotschy A, Novák Z. Org. Lett. 2014;16:4268. doi: 10.1021/ol501967c..
- 6.Dubinina GG, Furutachi H, Vicic DA. J. Am. Chem. Soc. 2008;130:8600. doi: 10.1021/ja802946s. [DOI] [PubMed] [Google Scholar]
- 7.Kawai H, Furukawa T, Nomura Y, Tokunaga E, Shibata N. Org. Lett. 2011;13:3596. doi: 10.1021/ol201205t. [DOI] [PubMed] [Google Scholar]
- 8.Zhao TSN, Szabó K. J. Org. Lett. 2012;14:3966. doi: 10.1021/ol3017287. [DOI] [PubMed] [Google Scholar]
- 9.Bouillon J-P, Maliverney C, Merényi R, Viehe HG. J. Chem. Soc. Perkin Trans. 1. 1991:2147. [Google Scholar]
- 10.Burton DJ, Hartgraves GA, Hsu J. Tetrahedron Lett. 1990;31:3699. [Google Scholar]
- 11.Miyake Y, Ota S-i, Shibata M, Nakajima K, Nishibayashi Y. Chem. Commun. 2013;49:7809. doi: 10.1039/c3cc44434a. [DOI] [PubMed] [Google Scholar]
- 12.Jiang X, Qing F-L. Beilstein J. Org. Chem. 2013;9:2862. doi: 10.3762/bjoc.9.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji Y-L, Kong J-J, Lin J-H, Xiao J-C, Gu Y-C. Org. Biomol. Chem. 2014;12:2903. doi: 10.1039/c3ob42575d. [DOI] [PubMed] [Google Scholar]
- 14.Duan J-X, Chen Q-Y. J. Chem. Soc. Perkin Trans. 1. 1994:725. [Google Scholar]
- 15.(a) Ambler BR, Altman RA. Org. Lett. 2013;15:5578. doi: 10.1021/ol402780k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang M-H, Orsi DL, Altman RA. Angew. Chem. Int. Ed. 2015;54:2361. doi: 10.1002/anie.201410039. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ambler BR, Peddi S, Altman RA. Synthesis. 2014;46:1938. doi: 10.1055/s-0033-1339128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Werner H, Wiedemann R, Laubender M, Windmüller B, Steinert P, Gevert O, Wolf J. J. Am. Chem. Soc. 2002;124:6966. doi: 10.1021/ja012479g. [DOI] [PubMed] [Google Scholar]; (b) Han HY, Kim MS, Son JB, Jeong IH. Tetrahedron Lett. 2006;47:209. [Google Scholar]; (c) Sam B, Montgomery TP, Krische MJ. Org. Lett. 2013;15:3790. doi: 10.1021/ol401771a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Generally >25:1 selectivity as determined by 19F NMR spectroscopy of the crude reaction mixtures.
- 18.Prakash GKS, Yudin AK. Chem. Rev. 1997;97:757. doi: 10.1021/cr9408991. [DOI] [PubMed] [Google Scholar]
- 19.Neff RK, Frantz DE. ACS Catal. 2014;4:519. [Google Scholar]
- 20.Recent reviews: Brummond KM, DeForrest JE. Synthesis. 2007:795. Ogasawara M. Tetrahedron: Asymmetry. 2009;20:259. Yu S, Ma S. Chem. Commun. 2011;47:5384. doi: 10.1039/c0cc05640e. Allen AD, Tidwell TT. Chem. Rev. 2013;113:7287. doi: 10.1021/cr3005263..
- 21.Recent reviews: Hashmi ASK. Angew. Chem. Int. Ed. 2000;39:3590. Zimmer R, Dinesh CU, Nandanan E, Khan FA. Chem. Rev. 2000;100:3067. doi: 10.1021/cr9902796. Ma S. Chem. Rev. 2005;105:2829. doi: 10.1021/cr020024j. Ma S. Acc. Chem. Res. 2009;42:1679. doi: 10.1021/ar900153r. Aubert C, Fensterbank L, Garcia P, Malacria M, Simonneau A. Chem. Rev. 2011;111:1954. doi: 10.1021/cr100376w. Krause N, Winter C. Chem. Rev. 2011;111:1994. doi: 10.1021/cr1004088. Lu T, Lu Z, Ma Z-X, Zhang Y, Hsung RP. Chem. Rev. 2013;113:4862. doi: 10.1021/cr400015d. Yang W, Hashmi ASK. Chem. Soc. Rev. 2014;43:2941. doi: 10.1039/c3cs60441a. Kitagaki S, Inagaki F, Mukai C. Chem. Soc. Rev. 2014;43:2956. doi: 10.1039/c3cs60382b. Le Bras J, Muzart J. Chem. Soc. Rev. 2014;43:3003. doi: 10.1039/c3cs60379b. Adams CS, Weatherly CD, Burke EG, Schomaker JM. Chem. Soc. Rev. 2014;43:3136. doi: 10.1039/c3cs60416k..
- 22.For recent examples of transformations of trifluoromethylallenes see: Watanabe Y, Yamazaki T. Synlett. 2009;20:3352. Li P, Liu Z-J, Liu J-T. Tetrahedron. 2010;66:9729. Zeng R, Ma Z, Fu C, Ma S. Adv. Synth. Catal. 2014;356:1343. Li G, Gagare PD, Ramachandran PV. Tetrahedron Lett. 2014;55:5736..
- 23.Meng F, Jung B, Haeffner F, Hoveyda AH. Org. Lett. 2013;15:1414. doi: 10.1021/ol4004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Widenhoefer RA. Org. Lett. 2008;10:2079. doi: 10.1021/ol800646h. [DOI] [PubMed] [Google Scholar]
- 25.Xu K, Thieme N, Breit B. Angew. Chem. Int. Ed. 2014;53:2162. doi: 10.1002/anie.201309126. [DOI] [PubMed] [Google Scholar]
- 26.The major isomers were formed in >19:1 selectivity as determined by 1H NMR spectroscopy.
- 27.Only the reaction to form 9 required a higher catalyst loading and extended reaction time. 1
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.