Abstract
Many cellular cofactors have been documented to be critical for various stages of viral replication. Using high throughput proteomic assays, we have previously identified Bruton’s tyrosine kinase (BTK) as a host protein that was uniquely up-regulated in the plasma membrane of HIV-1 infected T-cells. Here, we have further characterized the BTK expression in HIV-1 infection and show that this cellular factor is specifically expressed in infected myeloid cells. Significant up-regulation of the phosphorylated form of BTK was observed in infected cells. Using size exclusion chromatography, we found BTK to be virtually absent in the uninfected U937 cells, however new BTK protein complexes were identified and distributed in both high molecular weight (~600 kDa) and a small molecular weight complex (~60–120 kDa) in the infected U1 cells. BTK levels were highest in cells either chronically expressing virus or induced/infected myeloid cells and that BTK translocated to the membrane following induction of the infected cells. BTK knockdown in HIV-1 infected cells using siRNA resulted in selective death of infected, but not uninfected, cells. Using BTK specific antibody and small molecule inhibitors including LFM-A13 and a FDA approved compound, Ibrutinib (PCI – 32765), we have found that HIV-1 infected cells are sensitive to apoptotic cell death and result in a decrease in virus production. Overall, our data suggests that HIV-1 infected cells are sensitive to treatments targeting BTK expressed in infected cells.
Keywords: HIV-1 replication, BTK, host cell protein, inhibitor, cell death
Introduction
The human immunodeficiency virus (HIV-1) is the causative agent of acquired immunodeficiency syndrome (AIDS) that infected more than 60 million people worldwide (UNAIDS Report 2012). Despite the success of combined antiretroviral therapy (cART) in treating HIV-1 infection and preventing progression to AIDS (Bagasra, 2006; Chun et al, 1999; Cohen, 2005; Jordan et al, 2003), limitations include the generation of resistant viral strains. Moreover, even though the cART is efficient in eradicating the circulating virus in plasma by inhibiting productive infection, the cART is not able to completely inhibit virus production from the infected cells (Richman et al, 2009). HIV-1 is capable of maintaining latent infection in reservoirs such as resting CD4+ memory and naive T cells, myeloid cells and CD34+ multipotent hematopoietic stem cells (Carter et al, 2010; Cobos-Jimenez et al, 2011; Evans et al, 2013; Gougeon et al, 2012; Wightman et al, 2010). Thus, the identification of new markers of non-productive HIV-1 infection as well as determination of the new potential targets for therapeutic interventions is the critical tasks of the current HIV/AIDS research.
Using comparative proteomics analysis, we have previously identified the Bruton’s tyrosine kinase (BTK) as one of the 17 candidate proteins unique to latent HIV-1 infected T-cells, where BTK was localized to the cytoplasm and translocated to the plasma membrane upon viral activation (Berro et al, 2007). BTK is a member of the Tec family of non-receptor protein tyrosine kinases, and is encompassed within the Src superfamily, an important regulator of lymphocyte signaling (Berg et al, 2005; Mano, 1999). BTK is structurally characterized to contain the Src homology-1 (SH1) tyrosine kinase domain and additional protein-protein interacting domains SH2 and SH3 (Lindvall et al, 2005). Moreover, it harbors a pleckstrin homology (PH) domain located at the N-terminus which permits PH domain-mediated BTK translocation to the plasma membrane (Mohamed et al, 1999; Salim et al, 1996; Varnai et al, 1999). BTK characterization studies have been mostly performed in B cells, where it has been shown to influence multiple signaling pathways including calcium influx, cytoskeletal remodeling, apoptosis, survival, proliferation, differentiation, and gene expression (Contreras et al, 2007; Fluckiger et al, 1998; Liu et al, 2011; Matsuda et al, 2009; Satterthwaite et al, 1998; Uckun, 1998). Of interest, BTK has been shown to play a dual role in cell survival and apoptosis in a cell-type specific manner, conferring an anti-apoptotic effect in B cells while promoting apoptosis in HeLa cells (Islam and Smith, 2000; Uckun, 1998). BTK is primarily a cytoplasmic enzyme but can be recruited to the plasma membrane as a result of phosphoinositide 3-kinase (PI3K) activation in a PI3K-dependent manner (Mohamed et al, 1999; Mohamed et al, 2000; Salim et al, 1996; Scharenberg and Kinet, 1998; Varnai et al, 1999). PH-mediated membrane recruitment occurs through binding to phosphatidylinositol-3,4,5-trisphosphate (PIP3) after PI3K activation. BTK is required for proper tyrosine phosphorylation and activation of phospholipase C-γ (PLC-γ) which catalyzes the formation of inositol-3-4-5 triphosphate (IP3) and diacylglycerol, which in turn mediate calcium mobilization and activation of protein kinase C (PKC), respectively (Scharenberg and Kinet, 1998). The IP3 and PKC pathways lead to activation of transcription factors such as NF-κB, BAP-135 and TFII-I, that regulate gene expression required for B cell survival and cell cycle progression (Bajpai et al, 2000; Novina et al, 1999; Petro et al, 2000; Yang and Desiderio, 1997). In addition to a cytoplasmic and membrane location, BTK was also found in the nucleus (Mohamed et al, 2000; Saito et al, 2003; Webb et al, 2000b). Regulation of the nuclear translocation of this protein remains to be explored. However, a recent study showed that BTK nucleocytoplasmic shuttling requires its interaction with Lyn-interacting ankyrin repeat protein (Liar), utilizing a Crm1-mediated mechanism of nuclear export (Gustafsson et al, 2012).
In the present study we have characterized the expression profiles of BTK in different chronically HIV-1 infected cells including monocytes/macrophages. We demonstrate that BTK is upregulated during HIV-1 replication. Relevant to the BTK membrane translocation and nuclear shuttling activities observed earlier in B cells, we show that treatment with the PI3K inhibitors LY294002 and wortmannin in the presence of activated HIV-1 lead to decreased BTK translocation to the plasma membrane (Berro et al, 2007). We also show that selective depletion or inhibition of BTK kinase activity results in apoptosis of infected, but not uninfected cells. Taken together, our results suggest that viral regulation of receptor signaling can be mediated by redistribution of cytoplasmic and probably nuclear BTK to the cell membrane. Since BTK inactivation leads to selective apoptosis of HIV-1 infected cells, targeting the host BTK pathway could potentially serve as an attractive host-based therapeutic strategy for the elimination of latent HIV-1 infection.
Results
BTK expression is induced in HIV-1 infected cells
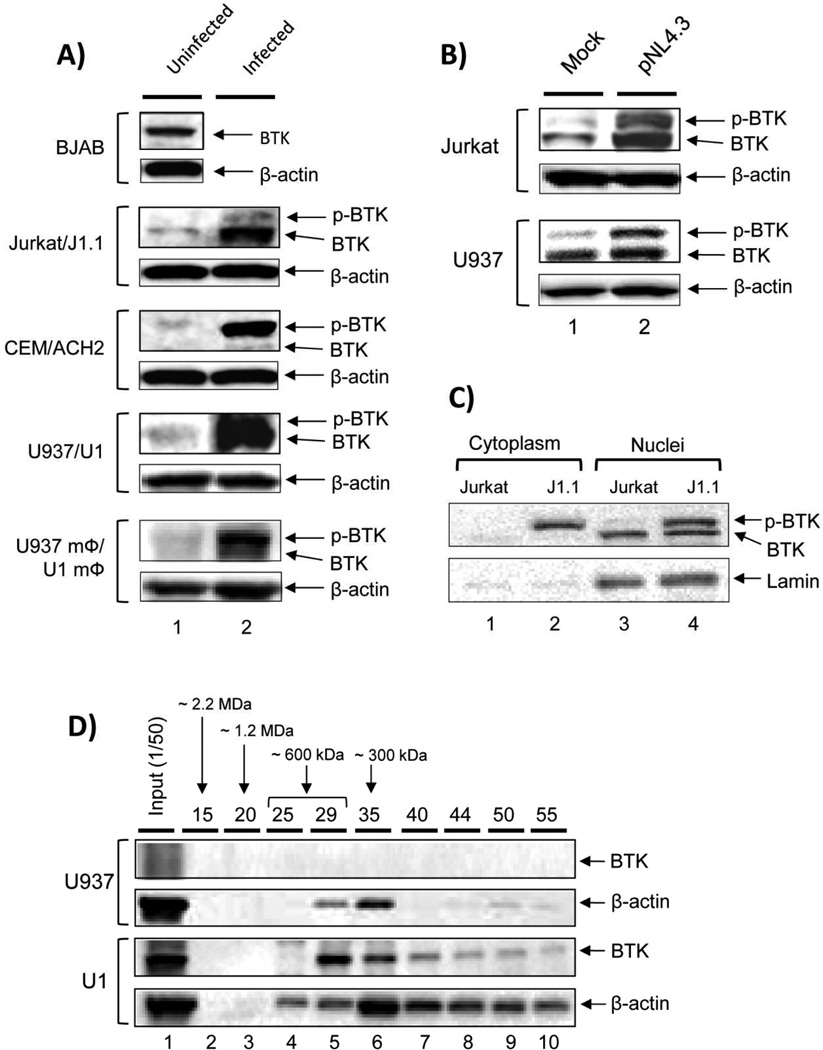
Using membrane proteomics analysis of HIV-1 infected T-cells, we have previously identified that amongst other proteins, BTK is upregulated in infected cells (Berro et al, 2007). In order to further explore the BTK expression profile during infection, western blot was performed in various cell types including uninfected (CEM and Jurkat) and HIV-1 infected T cells (positive control; ACH2 and J1.1), matched uninfected and infected monocytic cells (U937 and U1), and PMA-activated U937 and U1 cells that are considered as a model of monocyte derived macrophages (MDM model: U937 mΦ/U1 mΦ). The B cell line (BJAB) was used as a positive control for BTK expression. Results shown in Figure 1A indicate that BTK levels are much higher in the HIV-1 infected cells when compared to the uninfected counterparts for T cells, monocytes and MDM model cells. These results further show selective upregulation of BTK across a panel of cell lines that are chronically infected with HIV-1. To further examine BTK upregulation during de novo infection, we transfected the infectious HIV-1 clone pNL4.3 into Jurkat T cells (control) and monocytic U937 cells, and assessed the BTK distribution status by western blot. Results in Figure 1B indicate that there is significant upregulation of BTK expression at 48 hours post-infection (lane 2) when compared to mock-treated cells (lane 1). Even though BTK expression was detectable in the both uninfected T and monocytic cells, we observed significant upregulation of the phosphorylated form of BTK in all infected cells including both chronically (Fig. 1A) and newly infected (Fig. 1B) cell lines. Previously, we have shown in a latency model that increased BTK phosphorylation may be linked to its HIV-1-specific translocation and enrichment at the plasma membrane, where it becomes further phosphorylated (Berro et al, 2007). Finally, we were interested in exploring whether BTK localization was differently affected in HIV-1 infected cells. The western blot of cytoplasmic vs nuclear extracts, shown in Figure 1C revealed that BTK levels were increased in infection both at the cytoplasmic and nuclear fractions (lanes 2 and 4) when compared to uninfected fractions (lanes 1 and 3). Interestingly, BTK found in the cytoplasm of infected cells was mainly phosphorylated, whereas both phosphorylated and unphosphorylated forms of BTK were present in the nucleus. In contrast, BTK is found mainly in the nucleus of uninfected cells in an unphosphorylated form, with little to no BTK detectable in the cytoplasm. Thus, increased phosphorylation in de novo infection may be indicative of increased BTK activation and possible translocation to the plasma membrane where it partakes in the BTK signalosome, modulating signal transduction in a pro- or anti-apoptotic pathway (Islam and Smith, 2000).
Fig. 1. BTK expression is induced in HIV-1 infected cells.
(A) To assess BTK expression in a series of HIV-1 matched cell targets, 50 µg of whole cell extracts from B cells (BJAB), uninfected T cells (CEM and Jurkat), uninfected monocytic cells (U937 and U937-derived macrophage model cells, mΦ), HIV-1 infected T cells (ACH2 and J1.1), and infected monocytic cells (U1 and U1 mΦ) were run on a 4–20% SDS-PAGE and immunoblotted against BTK and β-actin. (B) Jurkat and U937 cells were transfected with the HIV-1 clone pNL4-3 using Effectene transfection reagent, harvested at 48 hr and lysed in Laemmli sample buffer. Twenty microgram of whole cell extract was assayed by western blot using α-BTK and α-β-actin antibodies. Phosphorylated BTK (p-BTK) is suggestive of BTK activation. (C) Cytosolic and nuclear extracts were prepared from Jurkat and J1.1 cells as described in Materials and Methods. The extracts were analyzed by western blot using anti-BTK antibody. Lamin A was used as a nuclear control. (D) Total cell lysates of both uninfected U937 and infected U1 cells were loaded onto a size-exclusion chromatography column and proteins separated in the presence of high salt buffer (500 mM NaCl) to minimize non-specific binding. No detergent was used during fractionation. A sampling (250 µl) of every 5th fraction from 10 – 55 was acetone precipitated and resuspended in 30 µl of Laemmli buffer, and then 15 µl were run on a gel for western blotting using BTK and β-actin antibody. More soluble actin was present in the U1 fractions as compared to uninfected U937 cells.
We finally asked whether BTK associated complexes could be modified between infected and uninfected cells. Our rationale came from our previous work where complexes such as Cdk9/T1 complex, GSK, and IKK were present in both large and small complexes after infection (Guendel et al, 2013; Kehn-Hall et al, 2011; Narayanan et al, 2012). Importantly, these novel small complexes were highly kinase active and were formed only after infection. Here, we used total cell lysates of both uninfected U937 and infected U1 cells and loaded onto a sizing column in the presence of high salt to minimize non-specific binding (500 mM NaCl). FPLC fractions were then precipitated and used for western blot analysis. Results of such an experiment are shown in Figure 1D, where BTK was virtually absent in the uninfected U937 cells, however the BTK protein complexes were distributed in both high molecular weight (~600 kDa) and a small molecular weight complex (~60–120 kDa, possibly the phosphorylated form) in the infected U1 cells. We also observed more actin distribution in the U1 infected cells compared to uninfected cells. The initial loading of actin onto the column was similar between infected and uninfected cells (lane 1). These data on distribution of BTK between large and small complexes further indicate that infection may cause a distribution between both active and inactive forms of BTK in cell.
Presence of BTK on cell surface membrane
We next examined the presence of BTK protein on both chronically infected and latent cells. We used J1-1 cells (Jurkat T-cell background) where wild type virus is secreted into the media from these cells and compared to U1 cells, which contain 2 copies of silent viruses (one wild type and one mutant). In both cases cells were grown to mid-log phase of growth and stained with BTK antibody, without any fixation. We also used U937 as control cells for infected U1 cells either with or without PMA treatment. Results in Figure 2A show that J1-1 cells (which actively secret virus) have a ~5 fold induction and presence of BTK on the membrane. Interestingly, U1 myeloid cells, that don’t secret virus, had minimal BTK on the surface, but upon activation BTK levels increased by about ~5 fold (Panel C) as compared to just a 70% increase for uninfected U937 cells. It is interesting to note that even though U1 cells contained higher levels of BTK protein, as determined by sizing column and western blots (Figure 1A and D), most of the protein becomes membrane associated after viral induction. Collectively, these results indicate that the BTK levels were highest in cells either chronically expressing virus or induced/infected myeloid cells and that BTK translocates to the membrane following induction of the infected cells.
Fig. 2. Extracellular staining of both infected and uninfected cells with BTK antibody.
(A) To assess BTK expression, chronically infected J1.1 cells (positive control) were used in these assays. Cells were not treated with either detergent or a fixative prior to staining, thereby preserving the natural state of the BTK localization on the membrane. These cells are chronically expressing virus and show a ~5-fold increase in surface BTK staining as compared to the uninfected parental Jurkat T-cell line. (B) Uninfected U937 cells (negative control) of myeloid lineage show baseline expression of surface BTK levels prior to differentiation with PMA treatment and an increase of 70% following PMA treatment. (C) Latently infected U1 cells (experimental) were used for staining with BTK antibodies. These cells do not normally express virus, however after the addition of PMA, they are differentiated to macrophages and express virus. These cells show low basal BTK surface staining and ~ 5-fold increase of BTK upon PMA stimulation.
Selective BTK depletion induces apoptosis
Due to dual role of BTK regarding apoptosis, we were interested to determine if inhibiting BTK expression through siRNA knockdown would have an effect on cell survival. The HLM-1 cells (Sadaie et al, 1990) were transfected with two siRNAs against BTK (BTK #1 and BTK #2) and cultured for 24, 48, and 72 hrs post-transfection. In the absence of induction, HLM-1 cell line is negative for virus particle production but displays basal transcription (i.e. latent phenotype). Small interfering RNA against luciferase was used as a negative control. Results in Figure 3A indicate that BTK #1 siRNA transiently but dramatically decreased the presence of phosphorylated BTK at 24 and 48 hrs post-transfection, respectively (lanes 4 and 5), while BTK #2 siRNA decreased level of this BTK form only at 24 hours (lane 7). The non-phosphorylated BTK was detected as a minor form of this enzyme in these cells. To evaluate if loss of BTK resulted in apoptosis in these cells, we performed western blot analysis against caspase 3 and PARP, canonical markers of the apoptotic cascade activation. The activation of caspase 3 leads to downstream cleavage events of cytoplasmic and nuclear substrates including PARP, attributing morphological features of apoptotic cell death (Huppertz et al, 1999). Results in Figure 3B indicate that upon BTK selective depletion, cleavage of both procaspase-3 and PARP was observed (lanes 2–3 and 5–6) in contrast to their respective luciferase control samples (lanes 1 and 4) or when the RNAimediated knockdown had resolved at 72 hours post-transfection (lanes 8–9). As a further confirmation, we then treated HLM-1 cells with either siRNA and performed a cell viability assay. Results in Figure 3C indicate that either siRNAs were capable of inhibiting or apoptosing cell growth after 72 hrs of treatment. Taken together, these data indicate that selective down-regulation of BTK, and especially the phosphorylated form of this enzyme, triggers programmed cell death pathways suggesting that BTK may play an anti-apoptotic role in HIV-1 infected cells.
Fig. 3. siRNA mediated BTK knockdown induces apoptosis in HIV-1 infected cells.
(A) Latently infected HLM-1 cells were used for selective depletion with siLuc (control) or two different siRNAs targeting BTK (siBTK #1 and siBTK #2). Log phase growing cells (5×105/ml) were transfected with either siLuc or siBTK (10 nm) using Effectene. Cells were harvested at 24, 48 and 72 h post-transfection, and 20 µg of whole cell lysates were resolved by SDS-PAGE and immunoblotted against BTK to assay for effective siRNA-mediated BTK downregulation. (B) Similar to panel A, where cell lysates were further probed with α-caspase 3 and α-PARP antibodies as markers of program cell death. Western blots against β-actin were performed as a loading control. (C) Similar to panel A, where HLM-1 cells were transfected with siLuc, siBTK #1 and siBTK #2 (50 nM). Viability was assessed using CellTiter-Glo assay 72 hours post-treatment. Results are shown as mean of three independent experiments ± SD.
Treatment with BTK antibody results in selective death of HIV-1 infected cells
Monoclonal antibodies against plasma membrane proteins that are overexpressed in cancer cells, such as VEGF and EGFR, have been successfully used as targeted therapeutics (Ludwig et al, 2003). In the case of HIV-1 infection, there are currently two humanized monoclonal antibodies for CCR5, Pro-140 and HGS Ab004, which inhibit viral entry (Castagna et al, 2005; Lederman et al, 2006) that are in clinical trial. Since BTK localizes specifically to the plasma membrane of HIV-1 infected cells, we tested whether antibody treatment against BTK could induce cell death in HIV-1 infected cell lines. A variety of uninfected and infected cell lines were incubated with a titration of monoclonal BTK antibody or IgG as a non-specific target control. Twenty-four hours post-treatment, trypan blue staining and CellTiter-Glo assays were used to assess cellular viability or growth. Results in Figure 4A depict a panel of cells treated with α-BTK antibodies and control IgG for 24 hours. Infected T cells (control; ACH2 and J1.1) and monocytes (U1) displayed >35% loss of cell viability upon treatment with BTK antibody, whereas corresponding uninfected cells were largely unaffected by the treatment. Similarly, reproduction of these results in uninfected Jurkat and infected J1.1 cells indicated a preferential decrease in viability of the HIV-1 infected cells upon exposure to α-BTK antibody (Figure 4B). Moreover, to confirm these observations in an inducible system, the activation of viral replication in HLM-1 cells was stimulated with the HDAC inhibitor sodium butyrate (NaB) for 72 hours prior to treatment with BTK antibody. Results in Figure 4C indicate that at 48 hours post-treatment, cells which had undergone viral induction were more susceptible to selective BTK antibody cell killing. We have previously shown that viral induction in HLM-1 cells results in virus-specific translocation of BTK to the plasma membrane (Berro et al, 2007). These data suggest a feasible correlation between BTK localization in the presence of active HIV-1 and susceptibility to therapeutic targeting of BTK.
Fig. 4. BTK antibody treatment induces cell death in HIV-1 infected, but not uninfected cells.
(A) Uninfected cells (CEM and U937) and HIV-1 infected cells (ACH2, J1.1 and U1) were treated with 5 µg/ml of IgG as a control or two concentrations of anti-BTK antibody (1 µg/ml and 5 µg /ml). Cell viability was determined by trypan blue dye exclusion cell quantitation (100 cells per sample) at 24 hours post-treatment. BTK antibody treatment decreased viability when compared to the respective uninfected controls by 36% in ACH2 cells, 59% in J1.1 cells, and by 58% in U1 cells at 5 µg /ml. (B) Jurkat (uninfected) and J1.1 chronically HIV-1 infected cells were treated with 1 µg/ml of IgG or antibody against BTK. Viability was assessed using CellTiter-Glo assay 48 hours post-treatment. Error bars show standard deviation of three independent measurements. (C) Latently HIV-1 infected HLM-1 cells were activated with 2 mM sodium butyrate (NaB) for 72 hours followed by treatment with 5 µg/ml of BTK antibody. Cells were subjected to CellTiter-Glo viability assay at 48 hours post-treatment. Results are mean ± SD of three independent preparations. (D) Uninfected monocytic control cell line U937 and latently HIV-1 infected U1 cells were subjected to differentiation with 250 nM PMA for 96 hours. The monocyte derived macrophages (U937 mΦ and U1 mΦ) were treated with 10 µg/ml of α-BTK and α-XIAP antibodies. The α-CD45 antibody was used as a positive control for receptor-mediated macrophage cell death. Cells were subjected to CellTiter-Glo viability assays at 48 hr post-treatment. Treatments were performed in triplicate and the mean plus-minus standard deviation is displayed. The double asterisks indicate p ≤ 0.01. Viability is expressed as percent of IgG control.
We have previously identified the anti-apoptotic protein X-linked inhibitor of apoptosis (XIAP) as one of the proteins enriched in the membrane fractions of latently infected cells (Berro et al, 2007). To further test the effect of BTK antibody on infected cells, we subjected U937 and U1 MDM cell models to treatment with antibodies against BTK and XIAP. Antibody treatment against CD45 was used as a positive control for macrophage-specific induced cell death (Steff et al, 2003). Six days post-treatment, we tested the effect of antibodies on cell viability by using CellTiter-Glo assay. Results in Figure 4D indicate that treatment with anti-CD45 antibody effectively induced cell death in both uninfected (92% cell death) and infected cells (96% cell death). Furthermore, BTK antibody treatment resulted in decreased viability only in the infected U1 MDM cell model (87% cell death). Interestingly, XIAP antibody treatment did not induce cell death in either U937 or U1 MDMs, suggesting that the regulatory mechanism of cell apoptosis exerted by HIV-1 via XIAP is different from that of BTK. Collectively, these results indicate that HIV-1 infected cells are more sensitive to BTK antibody treatment possibly due to the specific upregulation of BTK in infected cells.
BTK antibody treatment results in decreased HIV-1 viral replication
Therapeutic approaches that result in the killing of infected cells may affect release of immature viral particles into the extracellular space or maturation of the virions. Therefore, we next asked whether the treatment of infected cells with α-BTK antibody could modulate viral release after triggering cell death. Our rationale for these experiments came from previous findings that certain treatments that stimulate apoptosis may both decrease or increase viral replication. For instance, our earlier study showed that irradiation of chronically infected cells increases apoptosis, but also simultaneously activates cells and induces viral replication and release (Clark et al, 2000). We treated both HLM-1 cells that have undergone viral activation and the chronically infected J1.1 T cells that produce HIV-1 without additional activation with either α-BTK antibody or control IgG. Antibody treatment was performed once and supernatants were collected two days post-treatment for RT activity. Results in Figure 5A indicate that BTK antibody treatment inhibited viral replication in HLM-1 cells. Likewise, α-BTK also decreased RT activity in the supernatant from chronically infected J1.1 cells (panel B). Finally, we asked whether genomic RNA levels also decreased following antibody treatment. Experiments in panel A and B used reverse transcriptase protein as a read out of the functional viral particles in the supernatant, however unspliced RNA molecules were not assayed following antibody treatment. Experiments in panel C and D using both HLM-1 and J1-1cells showed reduced levels of genomic RNA following BTK antibody treatment. Interestingly IgG treatment showed an increase of viral RNA levels but not RT activity in the supernatants, indicating that IgG alone could increase RNA levels, but not functional virus. Taken together, these results suggest that BTK antibody treatment selectively induces apoptosis in infected cells and also decreases viral shedding from these cells.
Fig. 5. BTK antibody treatment results in decreased HIV-1 replication.
(A) Latently infected HLM-1 cells were treated with 5 µg/ml of α-BTK antibody or control IgG. Antibody treatment was performed once and supernatants were collected at 48 hr post-treatment for RT activity analysis. (B) HIV-1 infected J1.1 T cells were treated with 5 µg/ml of IgG or α-BTK antibody. Antibody treatment was performed once and supernatants were collected at 48 hr post-treatment for RT activity analysis. RT activities are expressed as percent of IgG control. Results are expressed as mean ±SD of three independent measurements. (C, D) Similar to panels A and B, except that the supernatants were assayed for presence of viral particle, using qRT/PCR for unspliced HIV-1 RNA. HLM-1 cells were treated with sodium butyrate for 72 hr to activate the latent HIV-1 provirus. Results are mean ± SD of three independent preparations. Single asterisk indicates p ≤ 0.01; double asterisks - p ≤ 0.05.
BTK antibody treatment induces cell death in HIV-1 infected PBMCs
To determine whether BTK antibody treatment had an effect on primary cells, we infected PHA/IL-2 activated PBMCs with the dual tropic HIV-1 89.6 virus and subsequently treated once with either BTK antibody or control IgG. Uninfected PBMCs were also treated in parallel as controls. On day 10, cells were stained with propidium idodide and analyzed by flow cytometry to determine whether antibody treatment resulted in alteration of the cell cycle or induction of apoptosis. Results in Figure 6A show that the treatment with BTK antibody induced substantial apoptosis (73.78%) in infected PBMCs (upper panel), while the uninfected PBMCs remained unaffected (3.97% apoptosis, lower panel). Interestingly, antibody-mediated suppression of membrane-localized BTK resulted in complete death of the cells in G0/G1 and G2/M stages of the cell cycle, whereas the cells with replicating DNA in the S phase of the cell cycle remained viable (Fig. 6B), suggesting either reduced exposure of BTK on the plasma membrane of these cells or that the BTK activity is not essential for viability of the cells in the S phase. Earlier studies of cancer cells treated with the BTK inhibitor LFM-A13 have been reported to exhibit a G2/M-arrest during cell cycle progression and lower level of cell death (Uckun, 2007), indicating that stronger pro-apoptotic effect in our experiment could be associated with HIV-1 activation. Collectively, the present data indicate that targeting BTK may result in death of HIV-1 infected primary cells. In addition, these data further suggest that BTK antibody treatment does not exhibit apparent toxicity to uninfected primary cells.
Fig. 6. BTK antibody treatment induces cell death in HIV-1 infected primary cells.
(A) Phytohemagglutinin (PHA) and IL-2 activated PBMCs from healthy donors weremaintained in culture for 48 hr prior to infection. Approximately 2.5×107 PBMCs were infected with HIV-1 89.6 virus (35,520 RT units). After 24hrs of infection, cells were subjected to one treatment with anti-BTK antibody or IgG control at 10 µg/ml. At day 10 post-infection, cells were stained with propidium iodide and analyzed for stages of cell cycles using flow cytometry. The data were acquired on a Becton Dickinson FACScalibur. On the histogram plot the dead cells are marked with blue color, the cells in G1 phase are red. (B) Samples from panel A are plotted for the presence of cells at various stages of cell cycle including G0/G1, S, and G2/M. “Sub-G1” represents cells that are in the process of cell death.
The BTK inhibitor LFM-A13 and Ibrutinib induce cell death in HIV-1 infected, but not in uninfected cells
In addition to therapeutic effects observed by monoclonal α-BTK antibody treatment on HIV-1 infected cells, we were interested in exploring treatment effects with commercially available small molecule inhibitors such as alpha-cyano-beta-hydroxy-beta-methyl-N-(2,5-dibromophenyl)propenamide or LFM-A13. LFM-A13 is a leflunomide metabolite analog designed to target the TEC family protein tyrosine kinase BTK (Mahajan et al, 1999; Uckun et al, 2003). To determine the effect of the specific pharmacological BTK inhibitor LFM-A13 on cell viability, uninfected CEM and Jurkat and HIV-1 chronically infected T cells, alongside the uninfected monocytic U937 and infected U1 cells were subjected to LFM-A13 or vehicle for 72 hours. Results in Figure 7A show that LFM-A13 decreased cell viability in a dose-dependent manner in HIV-1 infected cells when compared to DMSO-treated cells. BTK was originally identified in B cells and it has been found more recently in myeloid cells, including monocytes, macrophages, neutrophils, and mast cells (Mohamed et al, 2009). Thus, the BJAB B cell line was used as a positive control for compound toxicity in cells that constitutively expresses the target protein. The compound did not show cell toxicity in B cells or towards the other uninfected cells, that confirm our earlier conclusions about the importance of BTK activity for the viability of HIV-1 infected, but not uninfected cells. Interestingly, we observed the strongest effect of BTK inhibition on the cell viability in the ACH2 and U1 HIV-1 infected cells, which produce minimal amount of HIV-1 particles (Clark et al, 2000), whereas the effect of LFM-A13 was minimal on the J1.1 cells that persistently produces higher amount of HIV-1 virions without activation.
Fig. 7. BTK inhibitors induce cell death in HIV-1 infected cells.
(A) A panel of matched infected (ACH2, J1.1 and U1) and uninfected (CEM, Jurkat and U937) cells was treated with 10, 50 or 100 µM of LFM-A13 for 72 hours. BJAB served as positive control. Viability was assessed by CellTiter-Glo assay as described by the manufacturer. Treatments were performed in triplicate and the mean ± SD is presented. The asterisks indicate p ≤ 0.05. Viability is expressed as percent of DMSO control. (B) Latently HIV-1 infected ACH2 cells were similarly treated with the above concentrations of LFM-A13 for 72 hours. Supernatants were collected at day 3 for RT activity analysis. Treatments were performed in triplicate and the mean ± SD of three representative experiments is provided. RT activity is expressed as percent of DMSO control. (C) Uninfected B-cells (BJAB, lanes 1–4), uninfected CEM T cells (lanes 5–8), and infected cells (ACH2, lanes 9–12), were treated with either DMSO or Ibrutinib (10, 50 or 100 nM) for 72 hr. Cell deaths following drug treatments were monitored by comparing the levels of luminescence with DMSO treatment. Results are mean ± SD of three independent measurements. Single asterisk indicates p ≤ 0.05. (D) Infected cells (ACH2), were treated with either DMSO, LFM-A13 (10, 50 or 100 µM), or Ibrutinib (10, 50 or 100 nM) for 48 hr. The supernatants were assayed for presence of viral particle, using qRT/PCR for unspliced HIV-1 RNA. Results are mean ± SD of three independent preparations. The asterisks indicate p ≤ 0.01.
To complement the effects of LFM-A13 observed on the infected cells, we explored its effect on the viral replication. LFM-A13 treatment was performed once on infected cells, and supernatants were collected at day 3 for RT analysis. Results in Figure 7B indicate that LFM-A13 triggers a dose-dependent decrease of viral replication in infected cells. These results are consistent with anti-BTK antibody treatment (Fig. 4 and 5) where BTK inhibition also induced death of HIV-1 infected cells and simultaneous decrease of viral production. We next utilized another BTK inhibitor, Ibrutinib (PCI – 32765), that was in a Phase III clinical trial for treatment of chronic lymphocytic leukemia (CLL) and recently approved by the FDA. We treated infected and uninfected cells with three concentrations of Ibrutinib for 24 and 48 hours. Results in Figure 7C indicate that HIV-1 infected cells were very sensitive to Ibrutinib as evident by the increased apoptosis. Similar to LFM-A13, Ibrutinib showed a decrease in the intracellular p24 expression (using western blot) by more than 90% in infected cells (data not shown). Finally we compared both LFM-A13 and Ibrutinib side by side in cells and looked for presence of HIV-1 genomic RNA. Results in panel D indicate that both LFM-A13 and Ibrutinib inhibited genomic RNA present in the supernatants of infected cells in a dose-dependent manner. Collectively, these data point to the use of various BTK inhibitor drugs as an alternative means of cell killing in infected cells.
Target of BTK inhibitor in infected cells
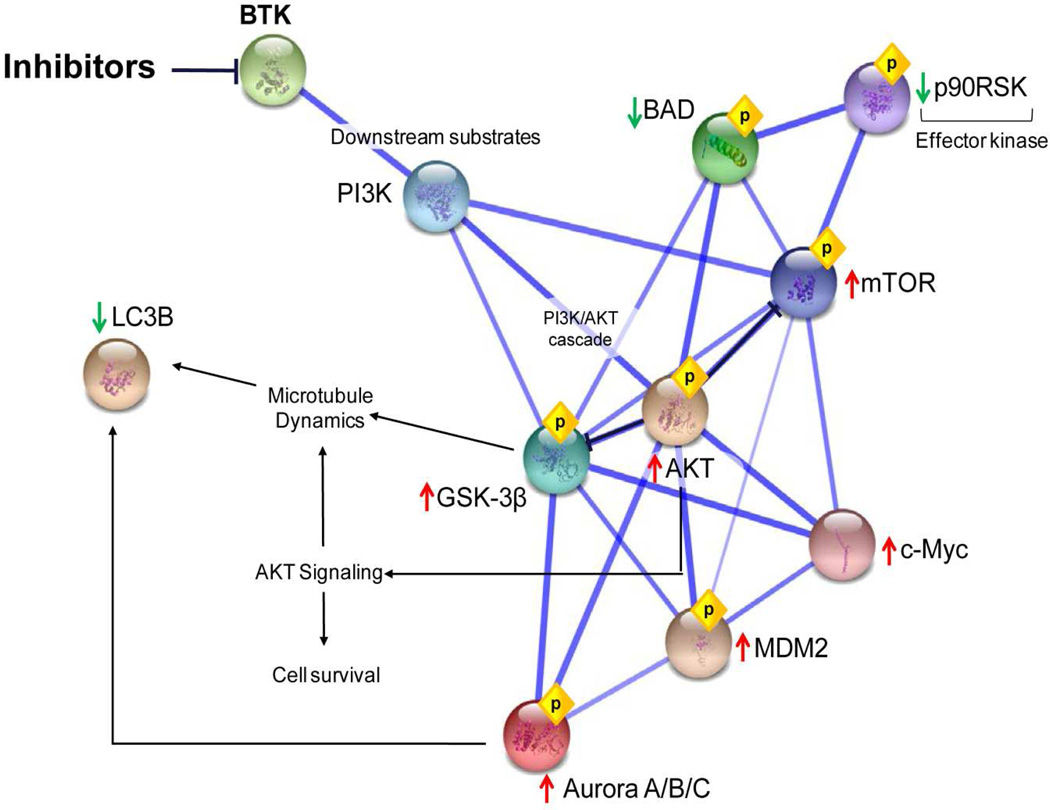
To characterize the putative mechanisms by which LFM-A13 could exert its effects on HIV-1 infected cells, we analyzed uninfected and chronically infected cells by reverse phase protein microarray (RPMA) technology. Cells were treated with LFM-A13 or DMSO for 24 hrs prior to preparation of the cell lysate. For phosphoproteomic analysis, cell lysates were immobilized by printing onto nitrocellulose membrane slides and probed with antibodies specific for phosphorylated or total forms of signaling proteins. The antibodies were selected to screen molecular networks involved in host responses believed to be affected by viral infection, specifically immune response, cell survival and apoptosis, inflammation and cell growth, and differentiation. RPMA has been extensively validated in previous studies regarding antibody specificity, sensitivity, and accuracy of phosphoprotein detection in cell lysates (Wulfkuhle et al, 2006). Moreover, RPMA has been recently used to characterize the phosphoproteome and signaling networks induced by Rift Valley Fever Virus infection (Popova et al, 2010). Table 1 shows a summary of significant changes in signal transduction upon LFM-A13 treatment of infected cells. Changes in protein expression were normalized to DMSO treated infected and LFM-A13 treated uninfected cells. To map these signaling pathways and discover important signaling pathway nodes mediated by LFM-A13, we annotated a narrow protein-protein interaction network (Fig. 8). Although PI3K was not identified as a LFM-A13 responsive node, it is functionally linked to the BTK signalosome and subsequent effector proteins. As shown in the signaling pathways, tyrosine kinases PI3K/AKT cascade, effector kinases serine/threonine GSK-3β and Aurora, p90RSK, substrates BAD, MDM2, c-Myc, mTOR, and microtubule dynamics-associated protein LC3B, were involved in the LFM-A13-mediated phosphorylation signaling pathways. Taken together, these data demonstrate that BTK inhibitor LFM-A13 induces cell death in HIV-1 infected cells suggesting that the cell death is induced largely through modulation of the PI3K/AKT signaling network.
Table 1.
Effects of LFM-A13 in Signaling Pathways
| Antibody | Fold Change | p-value |
|---|---|---|
| pBAD S136 | 0.41 | 0.02 |
| pGSK-3B S9 | 1.68 | 0.02 |
| c-Myc | 1.32 | 0.00 |
| GSK-3B | 1.31 | 0.00 |
| LC3B | 0.61 | 0.00 |
| pAKTS473 | 1.46 | 0.00 |
| pAurora A/B/C T288/232/198 | 1.39 | 0.05 |
| pGSK-3a/B S21/9 | 1.43 | 0.00 |
| pMDM2 S166 | 1.67 | 0.00 |
| pmTOR S2448 | 1.26 | 0.00 |
| pp90RSK S380 | 0.59 | 0.01 |
Fig. 8. Functional categories of LFM-A13-regulated phosphoproteins and signaling networks.
Uninfected CEM and HIV-1 infected ACH2 cells were treated with DMSO or LFM-A13 for 24 hr, cell lysates were prepared and analyzed with RPMA as described in Materials and Methods. Significantly altered proteins are listed in Table 1 (two-tail t-test, α ≤ 0.05, n = 3). A protein-protein interaction network was drawn by STRING 9.0 http://string-db.org/ using proteins identified in panel A and data from SWISS-PROT function annotation as input.
Discussion
Current cART uses selective inhibitors that target only viral proteins. Novel targeted host-based therapies serve as an attractive approach for the development of new treatment alternatives as well as to advance the understanding of disease mechanisms. In this study we have further characterized BTK as a cellular factor important for HIV-1 replication and viability of infected cells. The demonstrated role of BTK in HIV-1 infection along with the undetectable effect on uninfected cells supports this host protein as a potential new target for anti-HIV interventions.
BTK was originally identified as the mutated gene product in X-linked agammaglobulinemia (Tsukada et al, 1993; Vetrie et al, 1993). BTK is a member of the Tec family of non-receptor protein tyrosine kinases (PTKs), which is encompassed within the Src superfamily, an important regulator of lymphocyte signaling (Berg et al, 2005; Mano, 1999). The enzyme has been widely characterized in B cells as an important regulator of development, differentiation, cell survival, and growth as part of a membrane associated signalosome (Kurosaki, 1997; Kurosaki, 2000; Rawlings and Witte, 1994; Uckun, 1998). Moreover, BTK was the first tyrosine kinase identified as a dual-function regulator of apoptosis in what may seem a cell-type specific function (Uckun, 1998). We have previously identified BTK as one of 17 proteins that is uniquely upregulated in the plasma membrane of HIV-1 latently infected T-cells (Berro et al, 2007). After viral activation in infected cells, there is a virus-specific redistribution of nuclear and cytoplasmic BTK to punctuated foci at the plasma membrane that does not occur in the absence of viral particle production. Of particular interest, Smith et al. (Smith et al, 1994a) originally established Tec kinase expression in hematopoietic cells with the exception of plasma and T cells, where mRNA levels of BTK were below detection levels by the Northern blot. Generally, it is widely accepted that there is a minimal BTK expression in T-lymphocytes (de Weers et al, 1993; Smith et al, 1994a; Smith et al, 1994b; Yu et al, 2008), whereas higher expression in seen in myeloid cells, including monocytes, macrophages, neutrophils, and mast cells (Mohamed et al, 2009). Thus, the observed BTK upregulation in HIV-1 infected cells sheds a new light on the involvement of Tec kinase members in infection.
To further evaluate the status of BTK expression in HIV-1 infection, we explored expression of this protein in a variety of uninfected and infected HIV-1 target cells. We have shown up-regulation of BTK expression and phosphorylation in HIV-1 chronically infected cells, as wells as in de novo infected cells and monocytic cell models. Transient RNAi selective depletion of the enzyme in latently infected cells that have undergone viral reactivation indicated that down-regulation of BTK resulted in increased detection of apoptotic markers, namely activated caspase 3 and cleaved PARP and that infected cells were more susceptible to anti-BTK antibody. Treatment of a panel of uninfected and infected cells and monocytes with LFM-A13 or Ibrutinib, reflected a similar apoptotic phenotype of BTK antibody-treated cells, with infected cells generally being more sensitive to treatment. Importantly, LFM-A13 successfully inhibited viral replication on a post-transcriptional stage of the viral replication in vivo in a humanized animal model (data not shown). Collectively, our findings indicate that BTK is specifically up-regulated in both HIV-1 infected T cells and macrophages and is associated with the plasma membrane of infected cells. We speculate that BTK is normally intracellular but may be associated with membrane (phosphorylated form) in infected cells, hence becoming accessible to antibody treatment.
The leflunomide metabolite analog LFM-A13 is a rationally-designed specific inhibitor of BTK (Mahajan et al, 1999; Robak and Robak, 2012). Recently, a number of tyrosine kinase inhibitors became available for both preclinical and clinical trials including several BTK inhibitors such as LFM-A13, GDC-0834, AVL-101, AVL-292, and PCI-32765 (Robak and Robak, 2012). Avila Therapeutics (AVL-292) and Pharmacyclics (PCI-32765) both have developed covalent BTK inhibitors that are currently in the clinical trial pipeline for treatment of various malignancies associated with BTK dysregulation. PCI-32765 or Ibrutinib, a selective and irreversible inhibitor, has gained significant ground in clinical development and was being investigated in patients with B cell malignancies, and recently FDA approved, that includes chronic lymphocytic leukemia/small lymphocytic lymphoma, mantle cell lymphoma, diffuse large B cell lymphoma, and multiple myeloma (Advani et al, 2013; Carmi et al, 2012; Harrison, 2012; Robak and Robak, 2012; Winer et al, 2012). Moreover, the advancement of these compounds in Phase I and II clinical trials could be useful towards cART complementation once their effects on HIV-1 infection have been further explored.
The exact mechanism by which BTK monoclonal antibody or small molecule inhibitor induce apoptosis in HIV-1 infected cells is not yet clear. Previously, we have identified BTK enrichment in the membrane proteome of latently infected cells, and also observed the accumulation of BTK in distinct foci of the cell membrane upon viral reactivation (Berro et al, 2007). As previously described, BTK contains a PH domain that targets it to the plasma membrane allowing it to play a pivotal role in B cell signaling (Kurosaki, 2000). Interestingly, a recent study identified a lipid raft BCR signaling tuning mechanism regulated by differential posttranslational modification (Schmidt et al, 2009), hinting towards the complex regulatory loops governing the BTK signalosome. This signalosome is believed to occur predominantly in the cholesterol and sphingolipid-rich lipid raft or caveolae environments, where additional signal transducing molecules also assemble (Fruman et al, 2000; Guo et al, 2000; Vargas et al, 2002). Once commenced, BTK signaling at the plasma membrane conveys the integration of cytoplasmic and nuclear events. For example, BTK and PLC-γ2 have been shown to play a critical role in the activation of NF-κB after lipopolysaccharide stimulation of the Toll-like receptor (TLR) or B cell receptor (BCR) engagement (Bajpai et al, 2000; Horwood et al, 2003; Petro et al, 2000), and NF-κB signaling is compromised in BTK deficiency (Yu et al, 2008). Also, it has been shown that BTK-mediated B-cell-activating factor receptor (BAFF-R)-dependent activation of NF-κB promotes B cell survival (Shinners et al, 2007). NF-κB is a canonical transcription factor that plays an essential role in innate and adaptive immunity, and given its involvement in the HIV-1 viral life cycle, it is possible that HIV-1 is exerting BTK-mediated NF-κB regulation to fine tune cell survival outcomes of the infected cell as well as viral transcription. Moreover, two functional NF-κB binding sites located at the BTK promoter have been recently described (Yu et al, 2008). These have been shown to function in a positive proteasome-dependent autoregulatory feedback mechanism that BTK uses to stimulate transcription from its own promoter. Therefore, increased BTK expression in infection may be directly linked to NF-κB availability in the presence of viral infection and T cell activation. Additionally, BTK has been shown to directly target other transcription factors including BAP-135, TFII-I, TFB, and BAM11; indicative of BTK’s role in gene expression and regulation (Hirano et al, 2004; Kikuchi et al, 2000; Novina et al, 1999; Webb et al, 2000a; Webb et al, 2000b; Yang and Desiderio, 1997).
In this study, we have demonstrated using antibody, siRNA or other inhibitor molecule that BTK positively regulates productive HIV-1 infection either by activating the infected cells or enhancing the release of virus from the infected cells. Nevertheless, other members of this family have been reported to also have a direct effect on HIV-1 replication (Prins et al, 2012; Readinger et al, 2008; Schiralli Lester et al, 2013). For example, ITK (inducible T cell kinase), another member of the Tec family tyrosine kinase, induces HIV-1 replication by regulating multiple steps of viral life cycle such as T cell activation, actin polymerization/polarization, virion assembly and release. Specifically, ITK has been shown to co-localize with HIV-1 Gag on plasma membrane during virus release (Schiralli Lester et al, 2013). ITK has also been shown to be involved in inducing activation signals during the formation of virological synapse that enhances cell to cell spread of HIV-1 (Prins et al, 2012). Future studies will focus on elucidating the mechanistic details regarding the importance of BTK and other Tec family members for HIV-1 infection and how targeting the overexpressed Tec tyrosine kinases could eliminate HIV-1 infection especially in the latently infected cells.
Materials and methods
Cell cultures and reagents
HLM-1 cells obtained from the AIDS Research and Reference Reagents Program (NIH, Bethesda, MD, USA) were grown in DMEM supplemented with 10% heat-inactivated FBS, 1% L-glutamine, and 1% streptomycin/penicillin (Gibco/BRL, Gaithersburg, MD, USA). HLM-1 cells are HeLa-T4+ cells containing one integrated copy of HIV-1 genome with a Tat-defective mutation at the first AUG of Tat gene. In the absence of stimulation, HLM-1 is completely negative for virus particle production, and viral transcripts (unspliced) are completely absent (Berro et al, 2006; Guendel et al, 2013). HLM-1 cells were stimulated with 2 mM sodium butyrate for viral induction. The HIV-1 infected human cells J1.1, ACH2 and their uninfected counterparts Jurkat and CEM cells, as well as the infected monocytic U1 cells and the corresponding uninfected U937 cells, were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated FBS, 1% L-glutamine, and 1% streptomycin/penicillin(Guendel et al, 2013). The uninfected B-cells (BJAB) were also grown in the same media. The J1.1 cell line is a Jurkat E6.1 derivative chronically infected with HIV-1 (LAI strain) (Perez et al, 1991). ACH2 T cells are also infected with HIV-1 LAI strain. ACH2 and J1.1 cells contain a single integrated copy of HIV-1 genome (Clouse et al, 1989), whereas U1 cells harbor two copies (one wild type and one mutant) of the viral genome (LAV-1 strain) (Folks et al, 1987; Guendel et al, 2013). Monocytic U937 and U1 cells were induced to differentiate into monocytes derived macrophages (MDM) with 250 nM of phorbol-12-myristate-13-acetate (PMA) for 96 hrs. All cells were incubated at 37°C in the presence of 5% CO2.
Therapeutic treatments
Antibodies against BTK (611116) and XIAP (610762) were purchased from BD Transduction Laboratories (San Diego, CA, USA) and anti-CD45 (H-130) antibody was purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, USA). Mouse monoclonal anti-BTK IgG antibody (clone 53/BTK from BD Biosciences) recognizes human BTK, but does not cross react with ITK (Tec kinase family member). This antibody is also specific for human BTK as neither wildtype nor BTK deficient chicken B cells show any reactivity with this antibody (Douhan Iii et al, 2007). Inhibitions studies were always performed with fresh antibodies. Fifty thousand cells were plated per well in a 96-well plate and treated the next day with the appropriate antibody dilution to a final concentration of 1, 5 or 10 µg/ml. Antibodies against β-actin (C-11), caspase 3 (H-277), and PARP-1/2 (H-250) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The commercially available BTK inhibitor, LFM-A13 or α-Cyano-β-hydroxy-β-methyl-N-(2,5-dibromophenyl)propenamide, was obtained from Sigma Saint Louis, MO, USA) and prepared as a 10 mM stock solution dissolved in DMSO. Ibrutinib was purchased from Selleckchem, Catalog #S2680.
Cell viability assay
Viability was assessed by the trypan blue exclusion method using a Neubauer counting chamber. Fifty thousand cells were plated per well in a 96-well plate and treated the next day with 1 and 5 µg of antibody against BTK. After 48 hrs, CellTiter-Glo (Promega, Madison, WI, USA) was used to measure viability following the manufacturer’s recommendations. CellTiter-Glo is a luminescent assay used to measure cell viability by analyzing the ATP levels. The reagent was added to the wells (1:1 reagent:media) and incubated at room temperature for 10 min protected from light. The luminescence signal was recorded using the GloMax-Multi Detection System (Promega)(Guendel et al, 2013). The viable cell number was normalized with control group and the results were expressed as relative cell viability
Protein extracts and immunoblotting
Cells were washed with PBS and lysed in a buffer containing Tris–HCl pH 7.5, 120 mM NaCl, 5 mM EDTA, 0.5% NP-40, 50 mM NaF, 0.2 mM Na3VO4, 1 mM DTT and one tablet of complete protease inhibitor cocktail per 50 ml. Cell lysis was performed on ice for 30 min and before centrifugation (at 14,000 rpm) for 5 minutes at 4°C (Van Duyne et al, 2011). For separating cytoplasmic and nuclear proteins a differential salt extraction method was employed. First, cells were collected and washed twice with PBS w/out Ca2+ and Mg2+. Cytosolic extracts were then prepared by resuspending cells in 80 µl Buffer A (10 mM KCl, 10 mM MgCl2, 10 mM HEPES, 1 mM EDTA, 1 mM DTT, 0.5% NP-40, 0.1% PMSF and one EDTA-free complete protease inhibitor tablet per 50 mL) and incubating for 10 min on ice. Samples were spun at 5,000 g for 5 min at 4°C and the supernatants saved as cytosolic extract (CE). The pelleted nuclei were washed with 200 µl of Buffer A, and spun at 5,000 g for 5 min at 4°C. The supernatants were removed and nuclei resuspended in 80 µl of Buffer B (450 mM NaCl, 1.5 mM MgCl2, 20 mM HEPES, 0.5 mM EDTA, 1 mM DTT, 0.5% NP-40, 0.1% PMSF and one EDTA-free complete protease inhibitor tablet per 50 mL) and incubated for 10 min on ice (Popova et al, 2010). Samples were spun at 20,000 g for 10 min at 4°C and supernatants saved as nuclear extract (NE). The protein concentration in the supernatant was determined with a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). For separating cytoplasmic versus nuclear proteins a differential salt extraction method was employed. Cell extracts were resolved by SDS PAGE on a 4–20% tris-glycine gel (Invitrogen, Carlsbad, CA, USA) and analyzed by WB using specific antibodies.
Reverse transcriptase activity assay
For RT assays, supernatants from infected cells (10 µl) were incubated in a 96-well plate with RT reaction mixture containing 1× RT buffer (50 mM Tris–HCl, 1 mM DTT, 5 mM MgCl2, 20 mM KCl), 0.1% Triton, poly(A) (10–2 U), poly(dT) (10–2 U) and [3H]TTP. The mixture was incubated overnight at 37°C and 5 µl of the reaction mix was spotted on a DEAE Filter mat paper (PerkinElmer, Shelton, CT, USA) washed four times with 5% Na2HPO4, three times with water, and then dried completely (Van Duyne et al, 2011). RT activity was measured in a Betaplate counter (Wallac, Gaithersburg, MD).
RNA extraction and RT-PCR
Humanized mice were treated with LFM-A13 post- and pre-infection with dual-tropic HIV-1 89.6 virus. Total RNA was isolated from the infected mouse PMBCs using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's protocol. Five hundred ng of total RNA was used to generate cDNA with the iScript cDNA Synthesis kit (Bio-Rad) using oligo-dT reverse primers (Guendel et al, 2013). The HIV-1 LTR and env genes were amplified by RT-PCR using primers specific for LTR and env as previously described (Narayanan et al, 2013; Zhou et al, 2004).
RT reaction and Quantitative PCR
For quantitative analysis of HIV-1 RNA, total RNA was isolated from culture supernatants of HIV-1 infected cells at different time points. RNA was purified using Trizol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. A total of 0.5µg of RNA from the RNA fraction was treated with 0.25mg/ml DNase I RNase-free (Roche, Mannheim, Germany) for 60 minutes in the presence of 5mM MgCl2, followed by heat inactivation at 65° C for 15 minutes. A 200–500 ng aliquot of total RNA was used to generate cDNA with the GoScript Reverse Transcription System (Promega, Madison, WI) using oligo-dT reverse primer. Subsequent quantitative real-time PCR analysis was performed with 2µl of undiluted and 10−1, and 10−2 diluted aliquots of RT reaction mixes. The iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) was used with the primers specific for HIV-1 gag gene: Gag1483-F (5’-AAGGGGAAGTGACATAGCAG-3’) and Gag1625-R (5’-GCTGGTAGGGCTATACATTCTTAC-3’) amplified 143 nt. fragment of HIV-1 gag gene. Serial dilutions of DNA from 8E5 cells (CEM cell line containing a single copy of HIV-1 LAV provirus per cell) were used as the quantitative standards. Real-time PCR reactions were carried out at least in triplicate using the CFX96 Real-Time PCR System (BioRad) and SFX Manager Software ver. 2.0.
Transfection assays
For siRNA-mediated knockdown of BTK, HLM-1 cells were transfected with 10 nM of double-stranded duplex HsBTK2 (BTK #1) or HsBTK3 (BTK #2) FlexiTube siRNA (SI00001029, SI00001036) HP Validated siRNA (Qiagen, Valencia, CA, USA) or luciferase (Dharmacon, Lafayette, CO, USA) using HiPerfect reagent according to the manufacturer's recommendations (Qiagen) in 24-well plates. HIV-1 proviral DNA construct pNL4.3 (5 µg) was also transfected in U937 and Jurkat cells using Effectene (Qiagen) according to the manufacturer's instructions (Jaworski et al, 2014).
Size-exclusion chromatography
Both uninfected U937 and infected U1 monocytic cells were washed with PBS without Mg2+ and Ca2+ and resuspended in lysis buffer (50 mM Tris–HCl pH 7.5, 120 mM NaCl, 5 mM EDTA, 0.5% NP-40, 50 mM NaF, 0.2 mM Na3VO4, 1 mM DTT, and one complete protease cocktail tablet per 50 ml) and incubated on ice for 20 minutes, with gentle vortexing. Lysates were then centrifuged at 4°C at 10,000 rpm for 10 min, and protein concentration in the supernatants was determined using the Bradford protein assay (Bio-Rad, Hercules, CA, USA). For each cell line, 2.5 mg of protein was brought up to 1 ml total volume using chromatography running buffer (0.2 M Tris-HCl pH 7.5, 0.5 M NaCl, and 5% glycerol). The lysates were run on a Superose 6 10/300 size-exclusion chromatography column (GE Healthcare Bio-Sciences, Uppsala, Sweden) using the ÄKTA Purifier system (GE Healthcare Bio-Sciences, Piscataway, NJ, USA)(Guendel et al, 2013). A quarter inch gap was introduced to the top of the Superose 6 column to better separate small molecular weight complexes from fractions eluting off the far right side of the chromatogram. After sample injection (using 1 ml loop), running buffer was set at a flow rate of 0.3 ml/minute, and 0.5 ml fractions of the flow-through were collected at 4°C for a total of approximately 60 fractions. Every 5th fraction was acetone precipitated (~250 µl) using 4 volumes of ice-cold 100% acetone, incubating for 15 minutes on ice. Precipitates were centrifuged at 4°C f or 10 min at 14,000 rpm, supernatants were removed, and the pellets were dried for about 10 min at 95°C (Guendel et al, 2013). The pellets were resuspended in Laemmli buffer and analyzed by immunoblotting with BTK (BD Biosciences) and actin antibodies (Santa Cruz).
Cell cycle analysis and apoptosis
Cells were washed with PBS and fixed with 70% ethanol. Following rehydration in PBS, cells were stained in PBS containing 25 µg/ml propidium iodide (Sigma, Saint Louis, MO, USA), 10 µg/ml RNase A (Sigma) and 0.1% NP-40. Cells were analyzed on a BD FacsCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Acquisition was done with CELLQuest software, and cell cycle analysis was performed with ModFit LT software (Verity Software House, Topsham, ME, USA). Aggregates and debris were excluded by gating on the FL2W and FL2A parameters. The gates were at channel 200 for FL2 width versus FL2 area, with a doublet discriminator. Apoptosis was considered to be the population of cells localized in sub-G1 area.
Surface Staining of BTK and FACS Analysis
For each cell line analyzed, 5 mL of culture (5 × 105 cells/mL starting density) were incubated in T25 flasks at 37°C in 5% CO2. For the U937 and U1 cell lines, two cultures were maintained either with or without 50 nM PMA stimulation. After 48 hours of incubation, cells were counted and then 5 × 105 cells from each cell line were pelleted. Next, cells were resuspended in 100 µL of PBS + 0.1% BSA plus α-BTK mouse monoclonal antibodies (equal parts BD Transduction (Cat# 611116) and Santa Cruz Biotechnology (Cat# sc-28387)) and incubated on ice for 30 minutes. Two cell pellets, one Jurkat and one U937, were incubated with PBS + 0.1% BSA alone to serve as negative controls for non-specific secondary antibody binding. Cells were then washed with 2 mL of cold PBS + 0.1% BSA and resuspended in 100 µL of PBS + 0.1% BSA plus FITC-labeled α-mouse secondary antibody and incubated on ice for 30 minutes in the dark. Cells were washed again, and resuspended in 100 µL of PBS + 0.1% BSA plus 100 µL of 2% paraformaldehyde. FACS analysis was then carried out using a FACSCalibur (BD Biosciences) and data analyzed using CellQuest software (BD Biosciences).
Reverse Phase Microarray
Approximately 30 nL of each sample (equivalent to 40 lysed cells) were arrayed onto nitrocellulose slides (Whatman, MA) by direct contact printing using a high-resolution 2470 arrayer (Aushon Biosystems, Billerica, MA). Samples were printed as duplicates of an 8.2-fold dilution series (Popova et al, 2010). The linear range of each dilution curve was then used for quantitative comparisons between samples. Slides were stored at −20°C prior to detection. To estimate the total protein amount in each spot, several randomly selected slides were stained with Sypro Ruby Protein Blot Stain (Molecular Probes, Eugene, OR) and visualized on a Fluorchem imaging system (Alpha Innotech, San Leandro, CA). Slides were stained on a Dako Autostainer with specific antibodies using a biotin-linked peroxidase-catalyzed signal amplification (Dako, CSA kit). Specifically, the arrayed slides were placed into Re-Blot solution (Chemicon, Temecula, CA) for 15 min, washed twice in PBS 5 min each, placed into I-Block solution (Applied Biosystems, Foster City, CA) in PBS with 0.1% Tween-20 for at least 2 h, and then immunostained using an automatic slide stainer (Autostainer, Dako Cytomation) and the manufacturer-supplied reagents (Popova et al, 2010). Briefly, the slides were incubated for 5 min with hydrogen peroxide, rinsed with high-salt Tris-buffered saline (CSA Buffer, Dako) supplemented with 0.1% Tween-20, blocked with avidin block solution for 10 min, rinsed with CSA buffer, and then incubated with biotin block solution for 10 min. After another CSA buffer rinse, 5 min incubation with protein block solution was followed by air drying (Popova et al, 2010). The slides were incubated with either a specific primary antibody diluted in Dako antibody diluent or with only diluent as a control, for 30 min. Every antibody underwent extensive validation ensuring a specific detection of the antigenic protein as a single band on the Western blot of cell lysate. The slides were washed with CSA buffer and incubated with a secondary biotinylated goat anti-rabbit IgG antibody (1:7500) (Vector Labs, Burlingame, CA) for 15 min. Slides were incubated with diaminobenzidine chromogen diluted in Dako diaminobenzidine diluent for 5 min, washed in deionized water and imaged using a Umax PowerLook III scanner (Umax, Dallas, TX) at 600 dpi. The images were analyzed with software AlphaAse FC (Alpha Innotech)(Popova et al, 2010). For each antibody, the average pixel intensity value for negative control (staining with second antibody only) was subtracted from the average pixel intensity value for specific antibody and then normalized by the corresponding average value of the total protein intensity. Each sample dilution curve in each experiment was fitted with a nonlinear approximation equation and statistically evaluated using GraphPad Prism ver5 software (Graphpad Software, CA)(Popova et al, 2010). All measurements were performed in triplicate. Data are represented as mean values with standard deviation (two-tail t-test, α = 0.05, n = 3).
Data analysis and bioinformatics
Quantitative data were analyzed by two-way ANOVA (OriginPro v. 8.0) and Student’s t test (Microsoft Excel). Standard deviation was calculated in all quantitative experiments for at least three independent preparations. The difference was considered to be statistically significant when P ≤ 0.05 (Iordanskaia and Nawshad, 2011). The function of the RPMA identified proteins was elucidated by SWISS-PROT database http://www.expasy.org and the interaction between the differentially expressed/modified proteins. A protein-protein interaction network was drawn by STRING 9.0 http://string-db.org/ using proteins identified in this work and data from SWISSPROT function annotation as input (Popova et al, 2010).
Acknowledgments
We would like to thank the members of the Kashanchi lab for experiments and assistance with the manuscript, and the NIH AIDS Research and Reference Reagent Program for the contribution of the important reagents. This work was supported by National Institutes of Health grant AI070740, AI043894, AI11340, and AI114490 to FK and a grant from Virginia’s Commonwealth Health Research Board to KK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
References
- (UNAIDS Report 2012) UNAIDS Report on the Global AIDS Epidemic 2012 UNAIDS Corporate Publication. http://wwwunaidsorg/en/media/unaids/contentassets/documents/document/2011/JC2215GlobalAIDSResposeProgressReportpdf. [Google Scholar]
- Advani RH, Buggy JJ, Sharman JP, Smith SM, Boyd TE, Grant B, Kolibaba KS, Furman RR, Rodriguez S, Chang BY, Sukbuntherng J, Izumi R, Hamdy A, Hedrick E, Fowler NH. Bruton Tyrosine Kinase Inhibitor Ibrutinib (PCI-32765) Has Significant Activity in Patients With Relapsed/Refractory B-Cell Malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O. A unified concept of HIV latency. Expert Opin Biol Ther. 2006;6:1135–1149. doi: 10.1517/14712598.6.11.1135. [DOI] [PubMed] [Google Scholar]
- Bajpai UD, Zhang K, Teutsch M, Sen R, Wortis HH. Bruton's tyrosine kinase links the B cell receptor to nuclear factor kappaB activation. J Exp Med. 2000;191:1735–1744. doi: 10.1084/jem.191.10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. Tec family kinases in T lymphocyte development and function. Annu Rev Immunol. 2005;23:549–600. doi: 10.1146/annurev.immunol.22.012703.104743. [DOI] [PubMed] [Google Scholar]
- Berro R, de la Fuente C, Klase Z, Kehn K, Parvin L, Pumfery A, Agbottah E, Vertes A, Nekhai S, Kashanchi F. Identifying the membrane proteome of HIV-1 latently infected cells. J Biol Chem. 2007;282:8207–8218. doi: 10.1074/jbc.M606324200. [DOI] [PubMed] [Google Scholar]
- Berro R, Kehn K, de la Fuente C, Pumfery A, Adair R, Wade J, Colberg-Poley AM, Hiscott J, Kashanchi F. Acetylated Tat regulates human immunodeficiency virus type 1 splicing through its interaction with the splicing regulator p32. J Virol. 2006;80:3189–3204. doi: 10.1128/JVI.80.7.3189-3204.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmi C, Mor M, Petronini PG, Alfieri RR. Clinical perspectives for irreversible tyrosine kinase inhibitors in cancer. Biochem Pharmacol. 2012 doi: 10.1016/j.bcp.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell Jt, Bixby D, Savona MR, Collins KL. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16:446–451. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagna A, Biswas P, Beretta A, Lazzarin A. The appealing story of HIV entry inhibitors : from discovery of biological mechanisms to drug development. Drugs. 2005;65:879–904. doi: 10.2165/00003495-200565070-00001. [DOI] [PubMed] [Google Scholar]
- Chun TW, Davey RT, Jr, Engel D, Lane HC, Fauci AS. Re-emergence of HIV after stopping therapy. Nature. 1999;401:874–875. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- Clark E, Santiago F, Deng L, Chong S, de La Fuente C, Wang L, Fu P, Stein D, Denny T, Lanka V, Mozafari F, Okamoto T, Kashanchi F. Loss of G(1)/S checkpoint in human immunodeficiency virus type 1-infected cells is associated with a lack of cyclin-dependent kinase inhibitor p21/Waf1. J Virol. 2000;74:5040–5052. doi: 10.1128/jvi.74.11.5040-5052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse KA, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci AS, Folks TM. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- Cobos-Jimenez V, Booiman T, Hamann J, Kootstra NA. Macrophages and HIV-1. Curr Opin HIV AIDS. 2011;6:385–390. doi: 10.1097/COH.0b013e3283497203. [DOI] [PubMed] [Google Scholar]
- Cohen P. Come out. come out. A recent study suggests a novel treatment might flush out latent copies of HIV hiding in the body--and re-ignites discussion over the challenges of eradicating HIV infection. IAVI Rep. 2005;9:1, 6–9. [PubMed] [Google Scholar]
- Contreras CM, Halcomb KE, Randle L, Hinman RM, Gutierrez T, Clarke SH, Satterthwaite AB. Btk regulates multiple stages in the development and survival of B-1 cells. Mol Immunol. 2007;44:2719–2728. doi: 10.1016/j.molimm.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weers M, Verschuren MC, Kraakman ME, Mensink RG, Schuurman RK, van Dongen JJ, Hendriks RW. The Bruton's tyrosine kinase gene is expressed throughout B cell differentiation, from early precursor B cell stages preceding immunoglobulin gene rearrangement up to mature B cell stages. Eur J Immunol. 1993;23:3109–3114. doi: 10.1002/eji.1830231210. [DOI] [PubMed] [Google Scholar]
- Douhan Iii J, Miyashiro JS, Zhou X, Cole DC, Wu PW, Collins M, Dunussi-Joannopoulos K. A FLIPR-Based Assay to Assess Potency and Selectivity of Inhibitors of the TEC Family Kinases Btk and Itk. Assay Drug Dev Technol. 2007 doi: 10.1089/adt.2007.9982. [DOI] [PubMed] [Google Scholar]
- Evans VA, Kumar N, Filali A, Procopio FA, Yegorov O, Goulet JP, Saleh S, Haddad EK, da Fonseca Pereira C, Ellenberg PC, Sekaly RP, Cameron PU, Lewin SR. Myeloid dendritic cells induce HIV-1 latency in non-proliferating CD4+ T cells. PLoS Pathog. 2013;9:e1003799. doi: 10.1371/journal.ppat.1003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluckiger AC, Li Z, Kato RM, Wahl MI, Ochs HD, Longnecker R, Kinet JP, Witte ON, Scharenberg AM, Rawlings DJ. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks TM, Justement J, Kinter A, Dinarello CA, Fauci AS. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987;238:800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Fruman DA, Satterthwaite AB, Witte ON. Xid-like phenotypes: a B cell signalosome takes shape. Immunity. 2000;13:1–3. doi: 10.1016/s1074-7613(00)00002-9. [DOI] [PubMed] [Google Scholar]
- Gougeon ML, Melki MT, Saidi H. HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells. Cell Death Differ. 2012;19:96–106. doi: 10.1038/cdd.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guendel I, Iordanskiy S, Van Duyne R, Kehn-Hall K, Saifuddin M, Das R, Jaworski E, Sampey GC, Senina S, Shultz L, Narayanan A, Chen H, Lepene B, Zeng C, Kashanchi F. Novel Neuroprotective GSK-3beta Inhibitor Restricts Tat-Mediated HIV-1 Replication. J Virol. 2013 doi: 10.1128/JVI.01940-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Kato RM, Garcia-Lloret M, Wahl MI, Rawlings DJ. Engagement of the human pre-B cell receptor generates a lipid raft-dependent calcium signaling complex. Immunity. 2000;13:243–253. doi: 10.1016/s1074-7613(00)00024-8. [DOI] [PubMed] [Google Scholar]
- Gustafsson MO, Hussain A, Mohammad DK, Mohamed AJ, Nguyen V, Metalnikov P, Colwill K, Pawson T, Smith CI, Nore BF. Regulation of nucleocytoplasmic shuttling of Bruton's tyrosine kinase (Btk) through a novel SH3-dependent interaction with ankyrin repeat domain 54 (ANKRD54) Mol Cell Biol. 2012;32:2440–2453. doi: 10.1128/MCB.06620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C. Trial watch: BTK inhibitor shows positive results in B cell malignancies. Nat Rev Drug Discov. 2012;11:96. doi: 10.1038/nrd3656. [DOI] [PubMed] [Google Scholar]
- Hirano M, Kikuchi Y, Nisitani S, Yamaguchi A, Satoh A, Ito T, Iba H, Takatsu K. Bruton's tyrosine kinase (Btk) enhances transcriptional co-activation activity of BAM11, a Btk-associated molecule of a subunit of SWI/SNF complexes. International Immunology. 2004;16:747–757. doi: 10.1093/intimm/dxh076. [DOI] [PubMed] [Google Scholar]
- Horwood NJ, Mahon T, McDaid JP, Campbell J, Mano H, Brennan FM, Webster D, Foxwell BM. Bruton's tyrosine kinase is required for lipopolysaccharide-induced tumor necrosis factor alpha production. J Exp Med. 2003;197:1603–1611. doi: 10.1084/jem.20021845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz B, Frank HG, Kaufmann P. The apoptosis cascade--morphological and immunohistochemical methods for its visualization. Anat Embryol (Berl) 1999;200:1–18. doi: 10.1007/s004290050254. [DOI] [PubMed] [Google Scholar]
- Iordanskaia T, Nawshad A. Mechanisms of transforming growth factor beta induced cell cycle arrest in palate development. J Cell Physiol. 2011;226:1415–1424. doi: 10.1002/jcp.22477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam TC, Smith CI. The cellular phenotype conditions Btk for cell survival or apoptosis signaling. Immunol Rev. 2000;178:49–63. doi: 10.1034/j.1600-065x.2000.17811.x. [DOI] [PubMed] [Google Scholar]
- Jaworski E, Saifuddin M, Sampey G, Shafagati N, Van Duyne R, Iordanskiy S, Kehn-Hall K, Liotta L, Petricoin E, 3rd, Young M, Lepene B, Kashanchi F. The use of Nanotrap particles technology in capturing HIV-1 virions and viral proteins from infected cells. PLoS ONE. 2014;9:e96778. doi: 10.1371/journal.pone.0096778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehn-Hall K, Guendel I, Carpio L, Skaltsounis L, Meijer L, Al-Harthi L, Steiner JP, Nath A, Kutsch O, Kashanchi F. Inhibition of Tat-mediated HIV-1 replication and neurotoxicity by novel GSK3-beta inhibitors. Virology. 2011;415:56–68. doi: 10.1016/j.virol.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y, Hirano M, Seto M, Takatsu K. Identification and characterization of a molecule, BAM11, that associates with the pleckstrin homology domain of mouse Btk. Int Immunol. 2000;12:1397–1408. doi: 10.1093/intimm/12.10.1397. [DOI] [PubMed] [Google Scholar]
- Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–318. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- Kurosaki T. Functional dissection of BCR signaling pathways. Curr Opin Immunol. 2000;12:276–281. doi: 10.1016/s0952-7915(00)00087-x. [DOI] [PubMed] [Google Scholar]
- Lederman MM, Penn-Nicholson A, Cho M, Mosier D. Biology of CCR5 and its role in HIV infection and treatment. Jama. 2006;296:815–826. doi: 10.1001/jama.296.7.815. [DOI] [PubMed] [Google Scholar]
- Lindvall JM, Blomberg KE, Valiaho J, Vargas L, Heinonen JE, Berglof A, Mohamed AJ, Nore BF, Vihinen M, Smith CI. Bruton's tyrosine kinase: cell biology, sequence conservation, mutation spectrum, siRNA modifications, and expression profiling. Immunol Rev. 2005;203:200–215. doi: 10.1111/j.0105-2896.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Miller H, Hui KL, Grooman B, Bolland S, Upadhyaya A, Song W. A balance of Bruton's tyrosine kinase and SHIP activation regulates B cell receptor cluster formation by controlling actin remodeling. J Immunol. 2011;187:230–239. doi: 10.4049/jimmunol.1100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DL, Pereira DS, Zhu Z, Hicklin DJ, Bohlen P. Monoclonal antibody therapeutics and apoptosis. Oncogene. 2003;22:9097–9106. doi: 10.1038/sj.onc.1207104. [DOI] [PubMed] [Google Scholar]
- Mahajan S, Ghosh S, Sudbeck EA, Zheng Y, Downs S, Hupke M, Uckun FM. Rational design and synthesis of a novel anti-leukemic agent targeting Bruton's tyrosine kinase (BTK), LFM-A13 [alpha-cyano-beta-hydroxy-beta-methyl-N-(2, 5-dibromophenyl)propenamide] J Biol Chem. 1999;274:9587–9599. doi: 10.1074/jbc.274.14.9587. [DOI] [PubMed] [Google Scholar]
- Mano H. Tec family of protein-tyrosine kinases: an overview of their structure and function. Cytokine Growth Factor Rev. 1999;10:267–280. doi: 10.1016/s1359-6101(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Mikami Y, Ohtani M, Fujiwara M, Hirata Y, Minowa A, Terauchi Y, Kadowaki T, Koyasu S. Critical role of class IA PI3K for c-Rel expression in B lymphocytes. Blood. 2009;113:1037–1044. doi: 10.1182/blood-2008-06-163725. [DOI] [PubMed] [Google Scholar]
- Mohamed AJ, Nore BF, Christensson B, Smith CI. Signalling of Bruton's tyrosine kinase, Btk. Scand J Immunol. 1999;49:113–118. doi: 10.1046/j.1365-3083.1999.00504.x. [DOI] [PubMed] [Google Scholar]
- Mohamed AJ, Vargas L, Nore BF, Backesjo CM, Christensson B, Smith CI. Nucleocytoplasmic shuttling of Bruton's tyrosine kinase. J Biol Chem. 2000;275:40614–40619. doi: 10.1074/jbc.M006952200. [DOI] [PubMed] [Google Scholar]
- Mohamed AJ, Yu L, Backesjo CM, Vargas L, Faryal R, Aints A, Christensson B, Berglof A, Vihinen M, Nore BF, Smith CI. Bruton's tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol Rev. 2009;228:58–73. doi: 10.1111/j.1600-065X.2008.00741.x. [DOI] [PubMed] [Google Scholar]
- Narayanan A, Iordanskiy S, Das R, Van Duyne R, Santos S, Jaworski E, Guendel I, Sampey G, Dalby E, Iglesias-Ussel M, Popratiloff A, Hakami R, Kehn-Hall K, Young M, Subra C, Gilbert C, Bailey C, Romerio F, Kashanchi F. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem. 2013;288:20014–20033. doi: 10.1074/jbc.M112.438895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A, Sampey G, Van Duyne R, Guendel I, Kehn-Hall K, Roman J, Currer R, Galons H, Oumata N, Joseph B, Meijer L, Caputi M, Nekhai S, Kashanchi F. Use of ATP analogs to inhibit HIV-1 transcription. Virology. 2012;432:219–231. doi: 10.1016/j.virol.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novina CD, Kumar S, Bajpai U, Cheriyath V, Zhang K, Pillai S, Wortis HH, Roy AL. Regulation of nuclear localization and transcriptional activity of TFII-I by Bruton's tyrosine kinase. Mol Cell Biol. 1999;19:5014–5024. doi: 10.1128/mcb.19.7.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez VL, Rowe T, Justement JS, Butera ST, June CH, Folks TM. An HIV-1-infected T cell clone defective in IL-2 production and Ca2+ mobilization after CD3 stimulation. J Immunol. 1991;147:3145–3148. [PubMed] [Google Scholar]
- Petro JB, Rahman SM, Ballard DW, Khan WN. Bruton's tyrosine kinase is required for activation of IkappaB kinase and nuclear factor kappaB in response to B cell receptor engagement. J Exp Med. 2000;191:1745–1754. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova TG, Turell MJ, Espina V, Kehn-Hall K, Kidd J, Narayanan A, Liotta L, Petricoin EF, 3rd, Kashanchi F, Bailey C, Popov SG. Reverse-phase phosphoproteome analysis of signaling pathways induced by Rift valley fever virus in human small airway epithelial cells. PLoS ONE. 2010;5:e13805. doi: 10.1371/journal.pone.0013805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins KC, Vasiliver-Shamis G, Cammer M, Depoil D, Dustin ML, Hioe CE. Imaging of HIV-1 envelope-induced virological synapse and signaling on synthetic lipid bilayers. J Vis Exp. 2012 doi: 10.3791/3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings DJ, Witte ON. Bruton's tyrosine kinase is a key regulator in B-cell development. Immunol Rev. 1994;138:105–119. doi: 10.1111/j.1600-065x.1994.tb00849.x. [DOI] [PubMed] [Google Scholar]
- Readinger JA, Schiralli GM, Jiang JK, Thomas CJ, August A, Henderson AJ, Schwartzberg PL. Selective targeting of ITK blocks multiple steps of HIV replication. Proc Natl Acad Sci U S A. 2008;105:6684–6689. doi: 10.1073/pnas.0709659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Robak T, Robak E. Tyrosine kinase inhibitors as potential drugs for B-cell lymphoid malignancies and autoimmune disorders. Expert Opin Investig Drugs. 2012;21:921–947. doi: 10.1517/13543784.2012.685650. [DOI] [PubMed] [Google Scholar]
- Sadaie MR, Tschachler E, Valerie K, Rosenberg M, Felber BK, Pavlakis GN, Klotman ME, Wong-Staal F. Activation of tat-defective human immunodeficiency virus by ultraviolet light. New Biol. 1990;2:479–486. [PubMed] [Google Scholar]
- Saito K, Tolias KF, Saci A, Koon HB, Humphries LA, Scharenberg A, Rawlings DJ, Kinet JP, Carpenter CL. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19:669–678. doi: 10.1016/s1074-7613(03)00297-8. [DOI] [PubMed] [Google Scholar]
- Salim K, Bottomley MJ, Querfurth E, Zvelebil MJ, Gout I, Scaife R, Margolis RL, Gigg R, Smith CI, Driscoll PC, Waterfield MD, Panayotou G. Distinct specificity in the recognition of phosphoinositides by the pleckstrin homology domains of dynamin and Bruton's tyrosine kinase. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite AB, Li Z, Witte ON. Btk function in B cell development and response. Semin Immunol. 1998;10:309–316. doi: 10.1006/smim.1998.0123. [DOI] [PubMed] [Google Scholar]
- Scharenberg AM, Kinet JP. PtdIns-3,4,5-P3: a regulatory nexus between tyrosine kinases and sustained calcium signals. Cell. 1998;94:5–8. doi: 10.1016/s0092-8674(00)81214-3. [DOI] [PubMed] [Google Scholar]
- Schiralli Lester GM, Akiyama H, Evans E, Singh J, Gummuluru S, Henderson AJ. Interleukin 2-inducible T cell kinase (ITK) facilitates efficient egress of HIV-1 by coordinating Gag distribution and actin organization. Virology. 2013;436:235–243. doi: 10.1016/j.virol.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Kim D, Ippolito GC, Naqvi HR, Probst L, Mathur S, Rosas-Acosta G, Wilson VG, Oldham AL, Poenie M, Webb CF, Tucker PW. Signalling of the BCR is regulated by a lipid rafts-localised transcription factor, Bright. EMBO J. 2009;28:711–724. doi: 10.1038/emboj.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinners NP, Carlesso G, Castro I, Hoek KL, Corn RA, Woodland RT, Scott ML, Wang D, Khan WN. Bruton's tyrosine kinase mediates NF-kappa B activation and B cell survival by B cell-activating factor receptor of the TNF-R family. J Immunol. 2007;179:3872–3880. doi: 10.4049/jimmunol.179.6.3872. [DOI] [PubMed] [Google Scholar]
- Smith CI, Baskin B, Humire-Greiff P, Zhou JN, Olsson PG, Maniar HS, Kjellen P, Lambris JD, Christensson B, Hammarstrom L, et al. Expression of Bruton's agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J Immunol. 1994a;152:557–565. [PubMed] [Google Scholar]
- Smith CI, Islam KB, Vorechovsky I, Olerup O, Wallin E, Rabbani H, Baskin B, Hammarstrom L. X-linked agammaglobulinemia and other immunoglobulin deficiencies. Immunol Rev. 1994b;138:159–183. doi: 10.1111/j.1600-065x.1994.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Steff AM, Fortin M, Philippoussis F, Lesage S, Arguin C, Johnson P, Hugo P. A cell death pathway induced by antibody-mediated cross-linking of CD45 on lymphocytes. Crit Rev Immunol. 2003;23:421–440. doi: 10.1615/critrevimmunol.v23.i56.40. [DOI] [PubMed] [Google Scholar]
- Tsukada S, Saffran DC, Rawlings DJ, Parolini O, Allen RC, Klisak I, Sparkes RS, Kubagawa H, Mohandas T, Quan S, et al. Deficient expression of a B cell cytoplasmic tyrosine kinase in human X-linked agammaglobulinemia. Cell. 1993;72:279–290. doi: 10.1016/0092-8674(93)90667-f. [DOI] [PubMed] [Google Scholar]
- Uckun FM. Bruton's tyrosine kinase (BTK) as a dual-function regulator of apoptosis. Biochem Pharmacol. 1998;56:683–691. doi: 10.1016/s0006-2952(98)00122-1. [DOI] [PubMed] [Google Scholar]
- Uckun FM. Chemosensitizing anti-cancer activity of LFM-A13, a leflunomide metabolite analog targeting polo-like kinases. Cell Cycle. 2007;6:3021–3026. doi: 10.4161/cc.6.24.5096. [DOI] [PubMed] [Google Scholar]
- Uckun FM, Vassilev A, Bartell S, Zheng Y, Mahajan S, Tibbles HE. The anti-leukemic Bruton's tyrosine kinase inhibitor alpha-cyano-beta-hydroxy-beta-methyl-N-(2,5-dibromophenyl) propenamide (LFM-A13) prevents fatal thromboembolism. Leuk Lymphoma. 2003;44:1569–1577. doi: 10.3109/10428190309178781. [DOI] [PubMed] [Google Scholar]
- Van Duyne R, Guendel I, Narayanan A, Gregg E, Shafagati N, Tyagi M, Easley R, Klase Z, Nekhai S, Kehn-Hall K, Kashanchi F. Varying modulation of HIV-1 LTR activity by Baf complexes. J Mol Biol. 2011;411:581–596. doi: 10.1016/j.jmb.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas L, Nore BF, Berglof A, Heinonen JE, Mattsson PT, Smith CI, Mohamed AJ. Functional interaction of caveolin-1 with Bruton's tyrosine kinase and Bmx. J Biol Chem. 2002;277:9351–9357. doi: 10.1074/jbc.M108537200. [DOI] [PubMed] [Google Scholar]
- Varnai P, Rother KI, Balla T. Phosphatidylinositol 3-kinase-dependent membrane association of the Bruton's tyrosine kinase pleckstrin homology domain visualized in single living cells. J Biol Chem. 1999;274:10983–10989. doi: 10.1074/jbc.274.16.10983. [DOI] [PubMed] [Google Scholar]
- Vetrie D, Vorechovsky I, Sideras P, Holland J, Davies A, Flinter F, Hammarstrom L, Kinnon C, Levinsky R, Bobrow M, et al. The gene involved in X-linked agammaglobulinaemia is a member of the src family of protein-tyrosine kinases. Nature. 1993;361:226–233. doi: 10.1038/361226a0. [DOI] [PubMed] [Google Scholar]
- Webb CF, Nixon N, Ayers N, Evetts S, Paulin Y. Expression of the transcription factor bright in human tonsil germinal centers and description of a new bright isoform. Faseb Journal. 2000a;14:A1036–A1036. [Google Scholar]
- Webb CF, Yamashita Y, Ayers N, Evetts S, Paulin Y, Conley ME, Smith EA. The transcription factor Bright associates with Bruton's tyrosine kinase, the defective protein in immunodeficiency disease. Journal of Immunology. 2000b;165:6956–6965. doi: 10.4049/jimmunol.165.12.6956. [DOI] [PubMed] [Google Scholar]
- Wightman F, Solomon A, Khoury G, Green JA, Gray L, Gorry PR, Ho YS, Saksena NK, Hoy J, Crowe SM, Cameron PU, Lewin SR. Both CD31(+) and CD31(-) naive CD4(+) T cells are persistent HIV type 1-infected reservoirs in individuals receiving antiretroviral therapy. J Infect Dis. 2010;202:1738–1748. doi: 10.1086/656721. [DOI] [PubMed] [Google Scholar]
- Winer ES, Ingham RR, Castillo JJ. PCI-32765: a novel Bruton's tyrosine kinase inhibitor for the treatment of lymphoid malignancies. Expert Opin Investig Drugs. 2012;21:355–361. doi: 10.1517/13543784.2012.656199. [DOI] [PubMed] [Google Scholar]
- Wulfkuhle JD, Edmiston KH, Liotta LA, Petricoin EF., 3rd Technology insight: pharmacoproteomics for cancer--promises of patient-tailored medicine using protein microarrays. Nat Clin Pract Oncol. 2006;3:256–268. doi: 10.1038/ncponc0485. [DOI] [PubMed] [Google Scholar]
- Yang W, Desiderio S. BAP-135, a target for Bruton's tyrosine kinase in response to B cell receptor engagement. Proc Natl Acad Sci U S A. 1997;94:604–609. doi: 10.1073/pnas.94.2.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Mohamed AJ, Simonson OE, Vargas L, Blomberg KE, Bjorkstrand B, Arteaga HJ, Nore BF, Smith CI. Proteasome-dependent autoregulation of Bruton tyrosine kinase (Btk) promoter via NF-kappaB. Blood. 2008;111:4617–4626. doi: 10.1182/blood-2007-10-121137. [DOI] [PubMed] [Google Scholar]
- Zhou M, Deng L, Lacoste V, Park HU, Pumfery A, Kashanchi F, Brady JN, Kumar A. Coordination of transcription factor phosphorylation and histone methylation by the P-TEFb kinase during human immunodeficiency virus type 1 transcription. J Virol. 2004;78:13522–13533. doi: 10.1128/JVI.78.24.13522-13533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]