Abstract

Cancer cells reorganize their metabolic pathways to fuel demanding rates of proliferation. Oftentimes, these metabolic phenotypes lie downstream of prominent oncogenes. The lipid signaling molecule phosphatidic acid (PtdOH), which is produced by the hydrolytic enzyme phospholipase D (PLD), has been identified as a critical regulatory molecule for oncogenic signaling in many cancers. In an effort to identify novel regulatory mechanisms for PtdOH, we screened various cancer cell lines, assessing whether treatment of cancer models with PLD inhibitors altered production of intracellular metabolites. Preliminary findings lead us to focus on how deoxyribonucleoside triphosphates (dNTPs) are altered upon PLD inhibitor treatment in gliomas. Using a combination of proteomics and small molecule intracellular metabolomics, we show herein that PtdOH acutely regulates the production of these pyrimidine metabolites through activation of CAD via mTOR signaling pathways independently of Akt. These changes are responsible for decreases in dNTP production after PLD inhibitor treatment. Our data identify a novel regulatory role for PLD activity in specific cancer types.
The defining characteristic of cancer is its fundamental metabolic reorganization, which allows cells to sustain abnormal rates of growth and proliferation. Otto Warburg first observed the most prominent oncogenic metabolic shift in the 1920s when he found that cancer cells consume glucose at a higher rate than that of normally differentiated tissue.1,2 A key revelation of the ‘Warburg Effect’, as it is now known, was the observation that despite their increased level of glucose consumption cells maintained a high rate of oxidative metabolism, among other metabolic disturbances. Indeed, excess lactate generated by upregulated glycolysis and decreased lactate dehydrogenase activity sustains an acidified tumor microenvironment.3 We now recognize that systemic metabolic irregularities increase glycolytic metabolites to fuel the biosynthesis of lipids, amino acids, and nucleotides: building blocks essential for cell replication and survival.4 Importantly, these altered metabolic networks observed in cancer cells are fundamentally different from those of normally differentiated tissues. Modern medicine regularly exploits increased glycolytic metabolism of cancer through the use of positron emission tomography (PET) imaging of solid tumors, whereby radiolabeled glucose is taken up more readily by solid tumors than normally differentiated tissues.5 Using these metabolic differences for a targeted cancer therapy provides the opportunity for a more specific treatment paradigm than is currently available, a central goal of drug discovery.
Often, the altered metabolic flux observed in cancer results from the dysregulation of prominent central signaling nodes. For example, hyperactivity of the serine–threonine kinase protein kinase B (Akt) is a hallmark of specific cancer types.6 Akt initiates glycolysis by activating both the glucose transporter (Glut4) and hexokinase. Together with decreased lactose dehydrogenase (LDH) activity, these central metabolic shifts are major contributors to the Warburg phenotype.7 Yet, exploiting Akt as a therapeutic target remains difficult, since it also governs metabolic processes in normally differentiated tissue. As a result, MK2206, an allosteric Akt inhibitor, displays acute, on-target side effects when used as an antitumor therapy.8 Thus, the identification of unique upstream regulators of oncogenes like Akt in cancer would result in a cancer-specific therapeutic strategy. Our lab recently identified phospholipase D2 (PLD2) as a key regulator of Akt activity in gliomas under nutrient-poor conditions.9 While directly targeting Akt to subvert oncogenic metabolism is not optimal, exploiting unique signaling nodes, like the PLD2–Akt nexus, presents a more viable strategy for a targeted, metabolic therapy.
The PLD enzymes generate phosphatidic acid (PtdOH), a lipid possessing prominent signaling roles, from membrane lipid stores through hydrolysis of the phospholipid headgroup of phosphatidylcholine.10 In this way, PLD serves as a rapid and acute source of intracellular PtdOH; PLD-generated PtdOH is thought to be highly transformative when dysregulated in cancer models.11 Indeed, a variety of cancers, including brain,12 breast,13 head and neck,14 and leukemia15 have all been shown to rely on the catalytic activity of PLD for PtdOH production and survival. The previous findings that PLD-produced PtdOH activates the oncogene Akt suggests a metabolic mechanism by which PLD sustains oncogenic proliferation. The established role of PtdOH in disease progression and newer studies suggesting its capacity to regulate cellular metabolism make PLD an ideal target through which novel metabolic regulatory check points can be determined. Thus, it was the goal of these studies to monitor whether treatment of cancer cells with PLD inhibitors would elicit changes in water-soluble metabolites essential for cell replication.
RESULTS AND DISCUSSION
dNTP Screening of PLD Inhibitor-Treated Cell Lines
Our laboratories have conducted extensive SAR studies on small molecule inhibitors of the PLD enzymes. Compound VU0364739 has been identified as a PLD2-preferring inhibitor,16,17 whereas VU0359595, as PLD1-preferring (Figure 1A).18 Both compounds are highly protein bound (>95%), dramatically reducing the free fraction of drug in culture.16 Thus, inhibitor concentrations of 5 and 10 μM (VU0364739 and VU0359595, respectively) would bring free fractions of compound in culture into subtype-selective concentration ranges, ideal for identifying downstream metabolic processes regulated by either PLD1 or PLD2.
Figure 1.
PLD inhibition by VU0359595 decreases dNTP biosynthesis in glioma models. (A) Cells were treated with two previously published PLD inhibitors, VU0359595 (PLD1 preferring) and VU0364739 (PLD2 preferring). (B) To identify novel metabolic pathways regulated by PLD, a rapid, quantitative screen of dNTPs from cell extracts was designed. UPLC-MS/MS method with 8 min run times rapidly identifies three distinct dNTP species (dATP, dCTP and TTP) using aminoallyl-UTP (AA-UTP) as an internal standard. (C) Cells were treated with either 10 μM VU0359595 or 5 μM VU0364739, dNTPs were extracted, and their molar values were totaled. Metabolomic data identified the dNTP levels of two glioma cell lines as uniquely susceptible to PLD inhibition. Screens were conducted two independent times in triplicate; experiments are representative of a larger set of additional experiments. *p < 0.05; assessed by ANOVA.
Untargeted metabolomics is useful for identifying novel metabolic regulatory mechanisms.19,20 However, given PLD’s likely indirect role in central metabolism, we surmised that more nuanced changes in metabolite levels would likely occur upon inhibitor treatment. Therefore, alterations in specific metabolites essential for cell proliferation and survival were specifically monitored by LC-MS/MS analysis after PLD inhibitor treatment. This approach affords resolution capable of discerning subtle changes in metabolite concentrations potentially sitting downstream of PLD signaling. In addition to screening metabolites involved in both glycolysis and the Krebs cycle, various CoA species, and ATP/AMP ratios, one of our early screens focused on quantitation of dNTPs produced by various cell lines upon PLD inhibitor treatment.
For the dNTP metabolite screen, a previously published HPLC-MS/MS method used for isotopically labeled dNTPs21 was modified into a UPLC-MS/MS method with shorter run times and increased sensitivity capable of quantifying endogenous dNTP production. Individual dNTPs (dATP, dCTP, and TTP) were identified by monitoring specific MRM transitions (Figure 1B). Using standard curves and aminoallyl-UTP as an internal standard, the molar values for each dNTP were determined and the values were combined for a total cellular readout of dNTPs (see Supporting Information for method validation and standard curves). Initial experiments identified that the glioma-derived cell lines U87MG and U118MG yielded reproducible decreases in dNTPs under normal growth conditions (DMEM + 10% FBS) in response to PLD inhibitor treatment (Figure 1C). A variety of other cancer cell lines did not share this response. However, MDA-MB-231 cells did exhibit some decreases in PLD inhibition, although their response was not as consistent as in the glioma cell lines and therefore not statistically significant. Considering the specificity of this phenotype to glioma cell lines, these results were indicative of a cell-specific mechanism of action.
dNTP Biosynthesis Is Regulated by Akt-Dependent and -Independent Mechanisms
A recent report from our lab showed that PLD-produced PtdOH binds the protein kinase Akt and regulates its activity in gliomas under nutrient-poor conditions.9 Akt has two prominent phosphorylation sites, which regulate its kinase activity. The pleckstrin homology (PH) domain facilitates its association with phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) at the inner leaflet of the plasma membrane (PM).22 After PM association, Akt is phosphorylated by either the mammalian target of rapamycin complex 2 (mTORC2) at serine 473 (S473)23 or by phosphoinositide dependent kinase 1 at threonine 308 (T308).24,25 Using antibodies directed to each phosphosite discriminates which activation pathways are affected under experimental conditions after Akt associates with the PM.
Using a previous study on gliomas as a reference point for concentrations of the respective PLD inhibitors capable of eliciting changes in Akt activation,9 it was determined that treatment with 5 μM VU0364739 or 10 μM VU0359595 significantly decreased Akt activation at both S473 and T308 in parental U87MG cells under nutrient poor conditions (Figure 2A). These phosphorylation events were contrasted with those from U87MG cells stably overexpressing a constitutively activated myristoylated Akt (Myr-Akt) construct (Figure 2B) to determine whether changes in signaling were Akt-dependent. A loss of decreased Akt activation upon PLD inhibitor treatment in the U87 (Myr-Akt) cell line was observed. Consistent with previous results, PLD-mediated PtdOH activation of Akt is bypassed in these cells. Importantly, treatment of parental U87MG cells under nutrient-poor conditions with PLD inhibitors reliably decreased dNTP concentrations (both total and those of the individual metabolites) (Figure 2C and Supporting Information Figure 5), whereas an identical treatment paradigm carried in U87MG (Myr-Akt) cells resulted in a nearly complete rescue of dNTP levels (Figure 2D). Taken together with the Akt phosphorylation data, these experiments suggest that under starvation conditions dNTP production in U87MG cells is under the control of the PLD–Akt signaling axis that was previously identified.9
Figure 2.
PLD-regulated dNTP biosynthesis operates by Akt-independent mechanisms under nutrient-rich conditions. (A) Western blot analysis of Akt activation sites in starved parental U87MG cells revealed dramatic decreases in Akt activation upon PLD inhibitor treatment. (B) U87MG cells stably expressing a constitutive active myristoylated Akt construct (Myr-Akt) were resistant to changes in Akt activation upon PLD inhibitor treatment. (C) UPLC-MS/MS analysis of dNTP levels revealed that conditions decreasing Akt activation also decreased dNTP production, whereas U87MG (Myr-Akt) cells were resistant to such changes (D). (E, F) Under normal growth conditions, PDPK1-regulated T308 phosphorylation was relatively unchanged with PLD inhibitor treatment, whereas mTOR-dependent S473 phosphorylation slightly decreased in both parental U87MG cells (E) and U87MG (Myr-Akt) cells (F). These treatment conditions also corresponded to decreased dNTP production in both parental (G) and U87MG (Myr-Akt) cells (H). All experiments were conducted three independent times in triplicate; experiments are representative of a larger set of additional experiments. Statistics evaluated by ANOVA; *p < 0.01; **p < 1 × 10−4.
A parallel set of experiments in U87MG and U87MG (Myr-Akt) cells cultured under normal growth conditions (DMEM + 10% FBS) was performed. Treatment of parental U87MG cells with 10 μM VU0364739 and 10 μM VU0359595 had little effect on T308 phosphorylation, whereas S473 phosphorylation decreased slightly more (Figure 2E). Importantly, these changes in Akt activation persisted in U87MG (Myr-Akt) cells (Figure 2F), suggesting that these signaling events were operating independently of Akt activity. Measuring dNTP production with this same treatment paradigm revealed that 10 μM VU0359595 induced a robust decrease of dNTPs in parental U87MG cells (Figure 2G) and U87MG (Myr-Akt) cells (Figure 2H). Yet, 5 μM of the Akt inhibitor MK2206 behaved differently in each cell line, with MK2206 being ineffective in non-serum-starved U87MG (Myr-Akt) cells. To verify whether another signaling pathway other than Akt may be responsible for our observed decreases in dNTPs, cells under both starved and serum-containing conditions were also treated with Torin1, an mTOR-specific inhibitor. In each case, Torin1 treatment drastically reduced dNTP production in U87MG cells. Taken together, these studies suggest that dNTP biosynthesis is under a unique regulatory mechanism modulated by PLD that is Akt independent when nutrients are present.
SILAC Phosphoproteomics Maps Pathways Regulated by PLD
The biosynthesis of dNTPs is a complex process, drawing on a network of metabolic enzymes for either de novo metabolism or salvage pathways (Figure 3A).26 Deoxyribose sugars, shared by all dNTP molecules, are derived through the import of glucose and glycolytic metabolites via the pentose phosphate pathway and subsequent reduction at the 2′-position.4 Meanwhile, distinct metabolic pathways utilize aspartic acid, glutamine, and glycine to assemble either the purine and pyrimidine ring structures by which guanine, adenine, cytidine, or thymidine are defined.26 In parallel, in order to establish Akt-independent candidate pathways regulating dNTP biosynthesis, global alterations in phosphorylation sites in U87MG cells using a SILAC-based phosphoproteomics screen were mapped.
Figure 3.
SILAC phosphoproteomics offers a platform to deconvolute signaling and metabolic pathways regulated by PLD. (A) The sugar portion of dNTPs is derived from ribose generated through the pentose phosphate pathway (PPP) and subsequent reduction. The heterocyclic portion of the dNTPs is synthesized independently using glycine, aspartic acid, and glutamine through a number of metabolic steps. (B) SILAC phosphoproteomics offers an unbiased strategy to map changes in signaling pathways between treated and untreated cells. After labeling cells with 15N- and 13C-labeled lysine and arginine (heavy media) or 14N- and 12C- lysine and arginine (light media), cells were treated with either 10 μM VU0359595 or DMSO. After treatment, cells were lysed and digested with trypsin, and phosphopeptides were enriched over TiO2 beads. After MudPIT analysis, changes in the ratios of heavy/light peptides were indicative of altered phosphorylation events between treatment conditions. (C) Network analysis identified significant changes in phosphopeptides of proteins in mTOR signaling pathways. mTOR pathway components were confirmed by fragmentation analysis of their corresponding peptide tandem mass spectra. Positively identified phosphorylation sites include 4E-BP1 Ser 65, RAPTOR Ser 877 and 884 and CAD Ser 1859. Asterisks indicate phosphosites validated upon peptide fragmentation.
Stable isotope labeling by amino acids in cell culture (SILAC) offers a platform to quantify changes in protein expression patterns or post-translational modifications across two experimental conditions using stable isotope labeling and quantitative mass spectrometry.27 The method utilizes two cell populations, one grown in heavy (13C, 15N Arg and Lys containing) media and one grown in light (12C, 14N Arg and Lys) media. The labeled amino acids are efficiently (98–99%) incorporated after 6–7 passages. Measurement of the ratios of heavy/light-labeled peptides by mass spectrometry after specific treatments reflects their relative abundance as a result of that treatment. For this experiment, parental U87MG cells were cultured in media containing stable isotope-labeled heavy arginine and lysine and treated with 10 μM VU0359595 for 16 h, whereas cells cultured in media containing light arginine and lysine (light media) were treated in parallel with vehicle control (DMSO). Cells were then lysed, lysates were mixed in equal amounts (by weight), proteins were trypsin-digested, and phosphopeptide enrichment was performed using TiO2 beads.28 Following MudPIT LC-MS/MS analysis, database searching and quantitative analysis of SILAC-labeled peptides were performed. Normally, heavy-to-light ratios of peptides represent differences in protein expression between treatment groups. However, because our experiment involved enrichment of phosphopeptides, the heavy-to-light ratios reported here specifically represent changes in protein kinase-mediated signaling events (Figure 3B).
After statistical analysis (see Supporting Information), phosphopeptides significantly altered upon 10 μM VU0359595 treatment were analyzed using Ingenuity Pathway Analysis (IPA) software. This level of analysis revealed that the most prominent kinase signaling network decreased upon inhibitor treatment were among peptides involved in mTOR signaling. Phosphopeptides from proteins involved in mTOR signaling identified by IPA analysis are shown in Figure 3C.
Focusing on this set of altered phosphopeptides, the most striking changes we observed in our data set corresponded to a prominent phosphopeptide from eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1), an important regulator of ribosomal protein translation. When phosphorylated in an mTOR-dependent fashion at Ser 65, 4E-BP1 releases from eIF4B initiating translation.29,30 Treatment with VU0359595 produced a heavy-to-light-ratio (H/L) of 0.4 for the 4E-BP1 peptide phosphorylated at Ser 65, indicating a substantial decrease in mTOR activation. Consistent with these changes, we also identified a H/L of 0.6 for a polyphosphorylated peptide from RAPTOR. A recent study identified that phosphorylation events in this region of RAPTOR, the regulatory subunit of mTORC1, corresponded to increased mTORC1 kinase activity.31 Taking these results together, our quantitative phosphoproteomic analysis strongly suggests that overall mTOR signaling is decreased in response to the loss of PLD catalytic activity, supporting our earlier findings implying Akt-independent response under serum growth conditions.
After identifying changes in the mTOR-signaling network, we worked to identify the link between mTOR signaling and reduced dNTP levels in these cells upon PLD inhibitor treatment. Two recent independent reports describing mTOR signaling specifically regulating pyrimidine biosynthesis32,33 identified that upon rapamycin treatment decreases in phosphorylation of Ser 1859 on the multifunctional enzyme CAD were observed. CAD is a large, multidomain enzyme that performs the first three catalytic steps in pyrimidine biosynthesis (carbamoyl aspartate synthase, aspartate transcarbamylase, and dihydro-orotase).34 Carefully examining our SILAC data set, we found that a phosphopeptide containing Ser 1859 from CAD was identified with H/L ratios of 0.7 to 0.8. These data, along with the observed mTOR signaling changes, suggested that upon inhibition of PLD catalytic activity with 10 μM VU0359595 mTOR signaling is decreased, limiting CAD catalytic activity and thus dNTP biosynthesis in U87MG cells.
Verification of mTOR Signaling Changes
We first sought to confirm changes in mTOR signaling identified in our SILAC phosphoproteomics screen. We obtained an antibody raised against phosphorylated Ser 65 on 4E-BP1 and asked whether concentrations of PLD inhibitors that decreased dNTPs also led to decreased phosphorylation at this mTOR-specific site. Our initial experiments with U87MG cells found that PLD inhibition reliably reduced Ser 65 phosphorylation, albeit not as completely as that from treatment with an mTOR-specific inhibitor, Torin1 (Figure 4A). Moreover, changes we observed were most prominent with 10 μM doses of VU0364739 and VU0359595, concentrations that inhibited the PLD1 isoform. Repeating these same conditions in U87MG (Myr-Akt) cells (Figure 4B), we found that 4E-BP1 Ser 65 phosphorylation was also reduced upon PLD inhibition in these cells, suggesting that changes in mTOR activity in response to 10 μM VU0359595 operate independently of Akt, mirroring our U87MG parental results. Closer examination of our data, specifically those obtained with 10 μM treatments of VU0359595, revealed that upon blot quantitation of U87MG (parental cells; Figure 4C) and U87MG (Myr-Akt cells; Figure 4D) the magnitude of signaling changes in both cell lines is strikingly similar. These results further support our earlier findings that these changes are an Akt-independent phenotype. Indeed, only higher (10 μM) doses of MK2206 were able to elicit changes in mTOR signaling in U87MG (Myr-Akt) cells.
Figure 4.
PLD inhibitor treatment decreases mTOR signaling events. (A, B) Phosphorylation at serine 65 phosphosite on 4E-BP1 after treatment of U87MG cells (A) and U87MG (Myr-Akt) (B) with different inhibitors. Treatment with 10 μM VU0359595 decreased phosphorylation at this site, validating the findings of the SILAC phosphoproteomics screen. (C, D) Magnitude of decrease in phosphorylation was similar in U87 parental (C) and U87Myr-Akt (D) cells, confirming the Akt independence of the changes in mTOR signaling induced by the PLD inhibitor. (E, F) siRNA knockdown of the PLD enzymes confirmed that PLD1 knockdown specifically decreased phosphorylation of serine 65 on 4E-BP1. Statistics evaluated by ANOVA; *p < 0.05; **p < 0.01.
To confirm the PLD1 specificity of mTOR signaling, we asked whether siRNA knockdown of either PLD1 or PLD2 affected mTOR signaling, as determined by 4E-BP1 Ser 65 phosphorylation. Blot quantification verified that 4E-BP1 Ser 65 phosphorylation decreased in response to PLD1 knockdown and not PLD2 knockdown, in good agreement with our observations using the PLD1-preferring VU0359595 (Figures 4E,F). Importantly, neither PLD1 nor PLD2 siRNA treatment resulted in decreased concentrations of dNTPs (data not shown). Technical differences mandate distinct time courses for acute pharmacological inhibition and RNA interference-mediated knockdown of the targeted message. Some concern is prudent in the use of small molecule inhibitors as to potential off-target effects and other targets in the signaling pathways; therefore, different chemical scaffolds should be used to validate findings. Since dNTP biosynthesis is central to cellular proliferation and survival, CAD activity is known to be regulated by other signaling mechanisms in addition to mTOR, namely, MAP kinase.35 Therefore, the presence of an observable change in metabolite levels and signaling events after an acute 16 h treatment of VU0359595 but not a more prolonged 64 h siRNA treatment is not unexpected due to dynamic responses by the cells.
CAD Activity Decreases upon PLD-Inhibitor Treatment
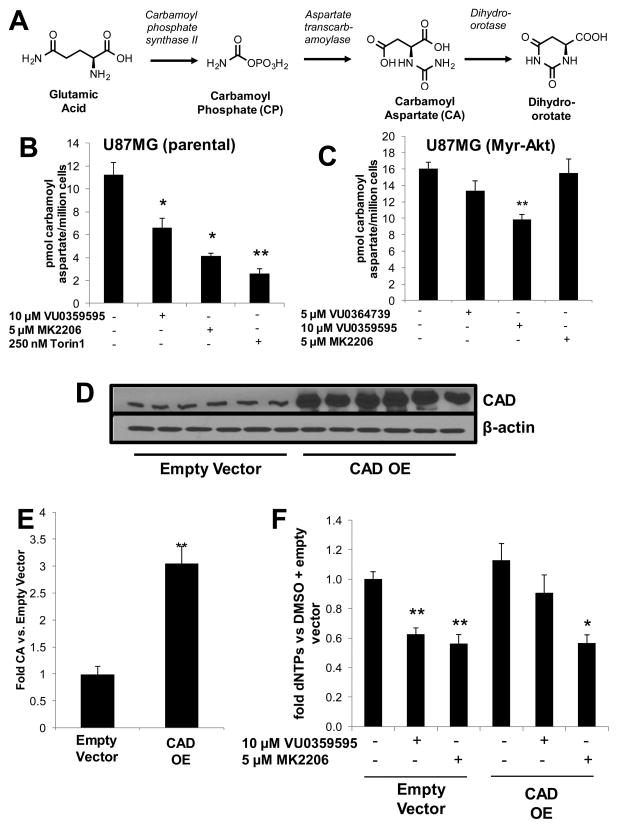
To establish CAD as the relevant biosynthetic enzyme responsible for our observed dNTP phenotype, we developed a targeted metabolomic UPLC-MS/MS method capable of quantifying CAD metabolites (Figure 5A). Given the similarities of pyrimidine metabolites to TCA cycle metabolites, we rapidly adapted a previously reported chromatographic method to our UPLC-MS/MS platform for pyrimidine metabolite quantitation.36 This method can reliably quantify all CAD metabolites within a 15 min run time and maintains linearity over a wide range of analyte concentrations. However, given the rapid decomposition of carbamoyl phosphate upon aqueous extraction, we focused on quantitation of carbamoyl aspartate from cells as a marker of CAD activity (see method validation for carbamoyl aspartate analysis in the Supporting Information).
Figure 5.
CAD catalytic activity regulates dNTP levels downstream of PLD catalytic activity. (A) CAD performs the first three steps of pyrimidine biosynthesis producing carbamoyl phosphate, carbamoyl aspartate and dihydroorotate. (B, C) Quantification of carbamoyl aspartate from parental U87MG (B) and U87MG (Myr-Akt) (C) revealed similar patterns to those observed in dNTP metabolites. (D) Human CAD cDNA was transfected into parental U87MG cells. (E) Increased enzyme expression correlated nicely with large increases in carbamoyl aspartate production. (F) Treatment of cells overexpressing CAD with 10 μM VU0359595 and 5 μM MK2206 showed that increased CAD activity rescued decreases in VU0359595-induced dNTP biosynthesis. Statistics evaluated by ANOVA; *p < 0.5; **p < 0.001.
Using this method under experimental conditions previously shown to decrease dNTPs, we found that carbamoyl aspartate was robustly decreased with 10 μM treatment of VU0359595 (Figure 5B). Similar to our dNTP studies, a 5 μM MK2206 treatment and 250 nM Torin1 treatment also substantially decreased carbamoyl aspartate production. Carbamoyl aspartate levels in U87MG (Myr-Akt) cells decreased with 10 μM VU0359595 treatment but not 5 μM MK2206 treatment, suggesting that changes in CAD-regulated pyrimidine biosynthesis are independent of Akt activity under normal growth conditions (Figure 5C). These results are identical to those observed in our previous experiments and offer mechanistic insight into the Akt-independence of dNTP decreases upon PLD-inhibitor administration.
Our results suggest that PLD inhibition sits upstream of mTOR signaling and CAD catalytic activity. To verify whether changes in CAD catalytic activity were responsible for the observed dNTP phenotype, transfection of CAD cDNA into parental U87MG cells was tested for its ability to rescue changes in dNTP production upon PLD inhibitor treatment. Transfection of CAD cDNA was verified using a CAD-specific antibody (Figure 5D). Increased CAD expression resulted in a large increase in carbamoyl aspartate levels, as assessed by UPLC-MS/MS method (Figure 5E). Finally, after transfection, U87MG cells were found to be resistant to 10 μM VU0359595-induced decreases in dNTP production, but not those elicited by 5 μM MK2206 treatment, when compared to those cells transfected with an empty expression vector (Figure 5F). These data strongly support our hypothesis that changes in CAD catalytic activity upon PLD inhibition are responsible for the decreases in dNTP production.
Other PLD Inhibitor Scaffolds Also Elicit Decreases in Cellular dNTP and Carbamoyl Aspartate Production
Studies by our laboratories and others have confirmed that specific selective estrogen receptor modulator (SERM) scaffolds act as inhibitors of PLD catalytic activity.37 Most notable among this structural class is raloxifene, which inhibits the catalytic activity of the PLD enzymes with an IC50 value of approximately 4 μM.38 To address whether changes in carbamoyl aspartate and dNTP metabolites as well as CAD and mTOR signaling events were mTOR specific, raloxifene, a PLD inhibitor derived from a different structural scaffold than VU0359595, was tested to determine whether it produced similar phenotypes.
Previous studies have shown that a 20 μM treatment of raloxifene ablates PLD catalytic activity in U87MG cells and was selected for use in these experiments (unpublished data). It has been reported that prolonged doses of raloxifene in estrogen receptor positive breast cancer models have no direct effect on mTOR signaling.39 However, using UPLC-MS/MS methods for both dNTPs and carbamoyl aspartate detection, we found that a 20 μM dose of raloxifene in the U87MG system was sufficient to dramatically reduce cellular abundance of both metabolites (Figure 6A,B). Importantly, these changes in metabolite concentrations corresponded to decreases in 4E-BP1 Ser 65 phosphorylation. Taken together, these results further support the hypothesis that a unique PLD and mTOR signaling axis exists in a malignant glioma model system.
Figure 6.
Raloxifene treatment induces a similar response as that of VU0359595 in parental U87MG cells. Previous studies have identified that 20 μM raloxifene ablates cellular PLD activity. These concentrations of raloxifene decreased both dNTP (A) and carbamoyl aspartate levels (B) in U87MG cells. (C) Decreased 4EBP1 (Ser 65) phosphorylation was also observed upon raloxifene treatment. Statistics evaluated by ANOVA; **p < 0.001.
According to our data, CAD catalytic activity is responsible for PLD inhibitor-induced decreases in dNTP production. These changes correspond to upstream decreases in mTOR activation, as observed by RAPTOR phosphorylation in the SILAC proteomics data set as well as catalytic activity, indicated by 4E-BP1 Ser 65 phosphorylation. The persistence of these phenotypes in cells containing a stably overexpressed Myr-Akt construct suggests that the observed phenotypes are operating independently of an Akt–mTOR signaling axis.
Over the past decade, a growing body of evidence suggests that PtdOH plays an important role in directly activating mTOR. Studies have found that PLD-produced PtdOH mechanically activates mTOR40 and that this interaction is responsible for key physiological processes, such as myoblast differentiation.41 Interestingly, myoblast differentiation was found to be specifically dependent upon a PLD1–mTOR signaling axis. Taken together, these data support the hypothesis that, in U87MG cells, PLD1-produced PtdOH bypasses Akt to directly regulate mTOR activity and pyrimidine biosynthesis. This further elaborates our understanding of the already quite complex cell signaling pathways that modulate both basal and receptor-stimulated PLD activity in mammalian cells.9,10,42
mTOR is critical for cell metabolism, often cited as a master regulator of metabolic flux in response to nutrient availability.43 Our studies suggest that PLD regulation of Akt and mTOR changes is based on nutrient availability, in keeping with mTOR’s role as a nutrient-sensitive signaling pathway. However, much like the direct inhibition of Akt by MK2206 elicits undesirable on-target effects, direct inhibitors of mTOR activity, like Torin1, have similar shortcomings since mTOR activity is central to all cell types.44,45 Thus, targeting cancer-specific upstream regulators of mTOR catalytic activity offers an alternative mechanism to disrupt this pathway.
Previous studies have identified cell-specific roles for PtdOH regulation of mTOR.40,41 Our findings support an additional mechanism at work in gliomas whereby PLD-produced PtdOH regulates dNTP biosynthesis under basal growth conditions through the catalytic activity of CAD. In addition, our data also demonstrate how the identification of specific signaling nodes, like the PLD–mTOR–CAD axis, provide a nuanced means to disrupt metabolic pathways in specific cancer types. While these data suggests that PLD inhibition offers a modest approach by which to alter mTOR signaling and dNTP production, it does so in a nuanced way that is not accessible to many forms of cancer or normally differentiated tissues. Targeting such unique, cell-specific pathways offers a platform from which to target diseases with potentially fewer side effects. Indeed, recent studies in our lab confirmed that no direct toxicity is associated with PLD inhibition.46 The recent development of PLD1 and PLD2 isoenzyme preferring inhibitors has helped to clarify PLD function in cells and fostered an appreciation for PLD as a pharmacological target in human diseases.10,17,38,47 The use of complementary approaches to assess specificity, including the development of PLD inhibitors with differential chemical scaffolds, will further advance our understanding of the therapeutic value of this target.37,48 Future experiments will focus on addressing whether similar mechanisms are at work in other relevant disease models.
METHODS
Cell Culture
U87MG cells were obtained from ATCC and maintained in DMEM containing 10% FBS (Atlanta Biologicals) and 1% penicillin/streptomycin (Invitrogen). U87MG (Myr-Akt) cells were generated from parental U87MG cells in a previous study9 and maintained in DMEM with 10% tetracycline-free FBS (Atlanta Biologicals) and 1% penicillin/streptomycin (Invitrogen). Cells were maintained at 37 °C with 5% CO2 in a humidified incubator.
Cell Treatment Conditions
For metabolite quantification experiments, cells were plated on a 10 cm plate and allowed to grow under normal conditions for 24 h; U87MG (Myr-Akt) cells were cultured for 24 h in media containing 0.1 μg/mL tetracycline to induce Myr-Akt expression. After 24 h, the media was removed and replaced with treatment media containing the relevant concentrations of inhibitors; treatment media for U87MG (Myr-Akt) also contained 0.1 μg/mL tetracycline. Cells were treated for a total of 16 h. Cells used for western blot analysis were cultured and treated identically to those used for metabolite analysis except cells that they were cultured in 6-well plates.
Transfection and siRNA Treatment
Cells were transfected were CAD cDNA obtained from Open Biosystems with Fugene6 according to the manufacturer’s protocol. siRNA was obtained from Dharmacon as a pool of four oligonucleotide-targeting sequences. The manufacturer’s protocol was used to transfect siRNA into cells using the Dharmafect reagent with 100 nM siRNA.
Water-Soluble Metabolite Extraction
After treatment, cells were scraped in 5 mL of ice-cold PBS and pelleted at 600g. After centrifugation, the supernatant was aspirated and the pellet was resuspended in 500 μL of a 70% methanol/water mixture at −20 °C. Suspensions were transferred to bullet tubes and agitated for 4 min at 4 °C; extracts were then incubated at −20 °C for 1 h. After incubation, internal standards were added to the extracts; for dNTPs, 4 nmols of aminoallyl-UTP was added to extracts, whereas 5 nmols of citrate-d4 was added to carbamoyl aspartate extracts. Extracts were agitated again at 4 °C for 1 min and centrifuged at 18 000g for 10 min at 4 °C. The supernatant was collected and placed in a separate bullet tube, and the solvent was evaporated under vacuum. Immediately prior to analysis, extracts were reconstituted in 100 μL of a 2 mM ammonium acetate and 3 mM hexylamine solution in water (pH 9.2), which is solvent A in the dNTP chromatographic method.
UPLC-MS/MS Quantification of dNTPs
Metabolites were chromatographically resolved on a Waters (Milford, MA) Acquity I-class UPLC. An MDS SCIEX 4000QTRAP hybrid triple quadrupole/linear ion trap mass spectrometer (Applied Biosystems) was used for detection. A binary solvent gradient was used to resolve dNTPs and adapted from a previous study21 using a Waters Acquity BEH C18 column (2.1 × 50 mm, 1.7 μ) with a 10 μL sample injection. Solvent A contained 2 mM ammonium acetate and 3 mM hexylamine in water (pH 9.2); solvent B was 100% acetonitrile. Flow rate of 0.6 mL/min was maintained with a linear gradient as follows: 0 min, 9% B; 2 min 16% B; 5 min, 16% B; 5.5 min, 100% B; 6.5 min, 100% B; 7 min, 9% B; 8 min, 9% B.
For dNTP analysis, the mass spectrometer was operated in negative MRM mode; the following mass transitions were observed: dATP, 490/159; dCTP, 466/159; and TTP, 481/159. dGTP could not be reliably quantified with this method since its molecular fragmentation pattern and retention time were identical to that of ATP.
UPLC-MS/MS Quantification of Carbamoyl Aspartate
To resolve carbamoyl aspartate from cell extracts, the Acquity I-Class UPLC system was used. An MDS SCIEX 4000QTRAP hybrid triple quadrupole/linear ion trap mass spectrometer (Applied Biosystems) was used for detection. A linear gradient separation was performed on an Acquity HSS T3 column (2.1 × 100 mm, 1.8 μ). Mobile phase A was composed of 100% water containing 5 mM hexylamine (pH 6.3 adjusted with glacial acetic acid). Mobile phase B was composed of 90% methanol and 10% 10 mM ammonium acetate (pH 8.5 adjusted with ammonium hydroxide). Flow rate of 0.4 mL/min was maintained; the gradient was linearly increased as follows: 0 min, 0% B; 1 min, 0% B; 12 min, 65% B; 12.5 min, 100% B; 13.5 min, 100% B; 14 min, 0% B; 15 min, 0% B. Pyrimidine metabolites were quantified by operating the mass spectrometer in negative MRM mode, and the following mass transitions were monitored: 155/111, orotic acid; 175/132, carbamoyl aspartate; and 195/113, citrate-d4.
SILAC Phosphoproteomics and Statistical Analysis
Detailed description of the SILAC experiment can be found in the Supporting Information.
Cell Lysis and Western Blot Analysis
All cells were lysed in 10 mM Tris buffer (pH 7.4) containing 10% SDS. Pellets were resuspended in lysis buffer and boiled twice until viscosity was reduced. Samples were then resolved by SDS-PAGE on gels composed of 6% (CAD), 10% (Akt, PLD1, and PLD2), or 15% (4EBP1) acrylamide. Gels were transferred to nitrocellulose membranes overnight and blocked with 5% milk (non-phospho-specific antibodies) or 5% BSA (phospho-specific antibodies). Blots were incubated in primary antibodies at 4 °C for 16 h followed by either mouse- or rabbit-specific antibodies conjugated to horse radish peroxidase at RT for 1 h. Blots were developed using Pierce ECL western blotting substrate (Thermo Scientific) according to the manufacturer’s specifications. Antibodies were obtained from Cell Signaling Technologies (Danvers, MA) and Bethyl (Montgomery, TX).
Supplementary Material
Acknowledgments
This work was supported in part by a training grant through the National Institute of Mental Health, grant no. 5T32MH93366-02 (support for T.P.M.); the National Institutes of Health, grant nos. 1U54MH084659 (to C.W.L.) and PO1-ES013125 and U54 069338 (to H.A.B.), and the Voices Against Brain Cancer. Thanks to R. Weichselbaum, for his generous gift of the SQ20B head and neck carcinoma cell line.
ABBREVIATIONS
- dATP
deoxy-adenosine triphosphate
- dCTP
deoxy-cytidine triphosphate
- TTP
thymidine triphosphate
- AA-UTP
aminoallyl uridine triphosphate
- dNTP
deoxy-ribonucleotide triphosphate
Footnotes
The authors declare no competing financial interest.
Standard curves for methods used in this study to quantify dNTPs and pyrimidine metabolic precursors. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Racker E. Bioenergetics and the problem of tumor growth. Am Sci. 1972;60:56–63. [PubMed] [Google Scholar]
- 3.Yamagata M, Hasuda K, Stamato T, Tannock IF. The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br J Cancer. 1998;77:1726–1731. doi: 10.1038/bjc.1998.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeBerardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith TA. The rate-limiting step for tumor [18F]fluoro-2-deoxy-D-glucose (FDG) incorporation. Nucl Med Biol. 2001;28:1–4. doi: 10.1016/s0969-8051(00)00177-3. [DOI] [PubMed] [Google Scholar]
- 6.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 7.Robey RB, Hay N. Is Akt the “Warburg kinase”? —Akt–energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yap TA, Yan L, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Baird RD, Delgado L, Taylor A, Lupinacci L, Riisnaes R, Pope LL, Heaton SP, Thomas G, Garrett MD, Sullivan DM, de Bono JS, Tolcher AW. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. doi: 10.1200/JCO.2011.35.5263. [DOI] [PubMed] [Google Scholar]
- 9.Bruntz RC, Taylor HE, Lindsley CW, Brown HA. Phospholipase D2 mediates survival signaling through direct regulation of Akt in glioblastoma cells. J Biol Chem. 2014;289:600–616. doi: 10.1074/jbc.M113.532978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvy PE, Lavieri RR, Lindsley CW, Brown HA. Phospholipase D: enzymology, functionality, and chemical modulation. Chem Rev. 2011;111:6064–6119. doi: 10.1021/cr200296t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchanan FG, McReynolds M, Couvillon A, Kam Y, Holla VR, Dubois RN, Exton JH. Requirement of phospholipase D1 activity in H-RasV12-induced transformation. Proc Natl Acad Sci USA. 2005;102:1638–1642. doi: 10.1073/pnas.0406698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park M, Ahn B, Hong Y, Min D. Overexpression of phospholipase D enhances matrix metalloproteinase-2 expression and glioma cell invasion via protein kinase C and protein kinase A/NF-κB/Sp1-mediated signaling pathways. Carcinogenesis. 2008;30:356–365. doi: 10.1093/carcin/bgn287. [DOI] [PubMed] [Google Scholar]
- 13.Toschi A, Lee E, Thompson S, Gadir N, Yellen P, Drain CM, Ohh M, Foster D. Phospholipase D–mTOR requirement for the Warburg effect in human cancer cells. Cancer Lett. 2010;299:72–79. doi: 10.1016/j.canlet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avila MA, Otero G, Cansado J, Dritschilo A, Velasco JA, Notario V. Activation of phospholipase D participates in signal transduction pathways responsive to gamma-radiation. Cancer Res. 1993;53:4474–4476. [PubMed] [Google Scholar]
- 15.Di Fulvio M, Frondorf K, Henkels KM, Grunwald WC, Cool D, Gomez-Cambronero J. Phospholipase D2 (PLD2) shortens the time required for myeloid leukemic cell differentiation: mechanism of action. J Biol Chem. 2012;287:393–407. doi: 10.1074/jbc.M111.259465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavieri RR, Scott SA, Selvy PE, Kim K, Jadhav S, Morrison RD, Daniels JS, Brown HA, Lindsley CW. Design, synthesis, and biological evaluation of halogenated N-(2-(4-oxo-1-phenyl-1,3,8-triazaspiro[4.5]decan-8-yl)ethyl)benzamides: discovery of an isoform-selective small molecule phospholipase D2 inhibitor. J Med Chem. 2010;53:6706–6719. doi: 10.1021/jm100814g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, Thomas AL, Armstrong MD, Arteaga CL, Lindsley CW, Brown HA. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat Chem Biol. 2009;5:108–117. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis JA, Scott SA, Lavieri R, Buck JR, Selvy PE, Stoops SL, Armstrong MD, Brown HA, Lindsley CW. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part I: Impact of alternative halogenated privileged structures for PLD1 specificity. Bioorg Med Chem Lett. 2009;19:1916–1920. doi: 10.1016/j.bmcl.2009.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long JZ, Cisar JS, Milliken D, Niessen S, Wang C, Trauger SA, Siuzdak G, Cravatt BF. Metabolomics annotates ABHD3 as a physiologic regulator of medium-chain phospholipids. Nat Chem Biol. 2011;7:1–3. doi: 10.1038/nchembio.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dang L, White DW, Gross S, Bennett BD, Bittinger MA, Driggers EM, Fantin VR, Jang HG, Jin S, Keenan MC, Marks KM, Prins RM, Ward PS, Yen KE, Liau LM, Rabinowitz JD, Cantley LC, Thompson CB, Vander Heiden MG, Su SM. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2010;465:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fromentin E, Gavegnano C, Obikhod A, Schinazi RF. Simultaneous quantification of intracellular natural and antiretroviral nucleosides and nucleotides by liquid chromatography-tandem mass spectrometry. Anal Chem. 2010;82:1982–1989. doi: 10.1021/ac902737j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas CC, Deak M, Alessi DR, van Aalten DM. High-resolution structure of the pleckstrin homology domain of protein kinase B/Akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr Biol. 2002;12:1256–1262. doi: 10.1016/s0960-9822(02)00972-7. [DOI] [PubMed] [Google Scholar]
- 23.Sarbassov D. Phosphorylation and regulation of Akt/PKB by the RICTOR–mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 24.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 25.Stephens L. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 26.Christopherson RI, Lyons SD, Wilson PK. Inhibitors of de novo nucleotide biosynthesis as drugs. Acc Chem Res. 2002;35:961–971. doi: 10.1021/ar0000509. [DOI] [PubMed] [Google Scholar]
- 27.Ong SE, Mann M. A practical recipe for stable isotope labeling by amino acids in cell culture (SILAC) Nat Protoc. 2006;1:2650–2660. doi: 10.1038/nprot.2006.427. [DOI] [PubMed] [Google Scholar]
- 28.Chaturvedi R, Asim M, Piazuelo MB, Yan F, Barry DP, Sierra JC, Delgado AG, Hill S, Jr, RAC, Bravo LE, Dominguez RL, Correa P, Polk DB, Washington MK, Rose KL, Schey KL, Morgan DR, Jr, RMP, Wilson KT. Activation of EGFR and ERBB2 by Helicobacter pylori results in survival of gastric epithelial cells with DNA damage. Gastroenterology. 2014;146:1739–1751. e1714. doi: 10.1053/j.gastro.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gingras A. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 31.Foster K, Acosta-Jaquez H, Romeo Y, Ekim B, Soliman G, Carriere A, Roux P, Ballif B, Fingar D. Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. J Biol Chem. 2010;285:80–94. doi: 10.1074/jbc.M109.029637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ben-Sahra I, Howell JJ, Asara JM, Manning BD. Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science. 2013;339:1323–1328. doi: 10.1126/science.1228792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robitaille AM, Christen S, Shimobayashi M, Cornu M, Fava LL, Moes S, Prescianotto-Baschong C, Sauer U, Jenoe P, Hall MN. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;339:1320–1323. doi: 10.1126/science.1228771. [DOI] [PubMed] [Google Scholar]
- 34.Grande-García A, Lallous N, Díaz-Tejada C, Ramón-Maiques S. Structure, functional characterization, and evolution of the dihydroorotase domain of human CAD. Structure. 2014;22:185–198. doi: 10.1016/j.str.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Graves LM, Guy HI, Kozlowski P, Huang M, Lazarowski E, Pope RM, Collins MA, Dahlstrand EN, Earp HS, Evans DR. Regulation of carbamoyl phosphate synthetase by MAP kinase. Nature. 2000;403:328–332. doi: 10.1038/35002111. [DOI] [PubMed] [Google Scholar]
- 36.Lu W, Clasquin MF, Melamud E, Amador-Noguez D, Caudy AA, Rabinowitz JD. Metabolomic analysis via reversed-phase ion-pairing liquid chromatography coupled to a stand alone orbitrap mass spectrometer. Anal Chem. 2010;82:3212–3221. doi: 10.1021/ac902837x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisen SF, Brown HA. Selective estrogen receptor (ER) modulators differentially regulate phospholipase D catalytic activity in ER-negative breast cancer cells. Mol Pharmacol. 2002;62:911–920. doi: 10.1124/mol.62.4.911. [DOI] [PubMed] [Google Scholar]
- 38.Scott SA, Mathews TP, Ivanova PT, Lindsley CW, Brown HA. Chemical modulation of glycerolipid signaling and metabolic pathways. Biochim Biophys Acta, Mol Cell Biol Lipids. 2014;1841:1060–1084. doi: 10.1016/j.bbalip.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuart EC, Jarvis RM, Rosengren RJ. In vitro mechanism of action for the cytotoxicity elicited by the combination of epigallocatechin gallate and raloxifene in MDA-MB-231 cells. Oncol Rep. 2010;24:779–785. doi: 10.3892/or_00000921. [DOI] [PubMed] [Google Scholar]
- 40.Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S. The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. Proc Natl Acad Sci USA. 2006;103:4741–4746. doi: 10.1073/pnas.0600678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon MS, Chen J. PLD regulates myoblast differentiation through the mTOR-IGF2 pathway. J Cell Sci. 2008;121:282–289. doi: 10.1242/jcs.022566. [DOI] [PubMed] [Google Scholar]
- 42.Scott S, Xiang Y, Mathews T, Cho H, Myers D, Armstrong M, Tallman K, O’Reilly M, Lindsley C, Brown H. Regulation of phospholipase D activity and phosphatidic acid production after purinergic (P2Y6) receptor stimulation. J Biol Chem. 2013;288:20477–20487. doi: 10.1074/jbc.M113.451708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, Reichling LJ, Sim T, Sabatini DM, Gray NS. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaytseva YY, Valentino JD, Gulhati P, Evers BM. mTOR inhibitors in cancer therapy. Cancer Lett. 2012;319:1–7. doi: 10.1016/j.canlet.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Oguin TH, Sharma S, Stuart AD, Duan S, Scott SA, Jones CK, Daniels JS, Lindsley CW, Thomas PG, Brown HA. Phospholipase D facilitates efficient entry of influenza virus allowing escape from innate immune inhibition. J Biol Chem. 2014;289:25405–25417. doi: 10.1074/jbc.M114.558817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruntz RC, Lindsley CW, Brown HA. Phospholipase D signaling pathways and phosphatidic acid as therapeutic targets in cancer. Pharmacol Rev. 2014;66:1033–1079. doi: 10.1124/pr.114.009217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scott SA, Spencer CT, O’Reilly MC, Brown KA, Lavieri RL, Cho CH, Jung DI, Larock RC, Brown HA, Lindsley CW. Discovery of desketoraloxifene analogues as inhibitors of mammalian, Pseudomonas aeruginosa, and NAPE phospholipase D enzymes. ACS Chem Biol. 2014 doi: 10.1021/cb500828m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.