Abstract
Lanthipeptides are members of the ribosomally synthesized and posttranslationally modified peptides (RiPPs). They are generated in two biosynthetic steps, dehydration of Ser and Thr residues to the corresponding dehydroamino acids and subsequent conjugate addition by the thiol of Cys residues to generate the characteristic lanthionine and methyllanthionine thioether-bridged structures. Typically, a lanthipeptide contains multiple thioether crosslinks. Recent studies have proposed that the final ring topology may be under thermodynamic control. If so, the Michael-type cyclization reaction would need to be reversible, but such reversibility has never been demonstrated. We show here for the class I lanthipeptide cyclase NisC and class II lanthipeptide synthetase HalM2 that indeed the conjugate addition reactions are reversible and that the enzymes can open up all thioether rings in their products. We also propose that a His residue that is conserved among the lanthipeptide cyclases acts as the acid or base that protonates or generates the enolate intermediate during thioether ring formation and opening, respectively.
Lanthipeptide biosynthesis has generated much interest recently, as the genes encoding these natural products are much more ubiquitous than previously anticipated,1 and because the high tolerance of the biosynthetic machinery offers intriguing possibilities for efficient generation of cyclic peptide libraries from genetically encoded precursors. All lanthipeptides contain lanthionine or methyllanthionine residues that are formed by the conjugate addition of cysteines to dehydroalanine (Dha) or dehydrobutyrine (Dhb) residues (Figure 1a).2 The mechanisms of Dha/Dhb generation vary in the four different classes of lanthipeptides that have been investigated thus far,3–6 but the conjugate additions are catalyzed by homologous enzymes. In class I, II, and IV lanthipeptide cyclases or cyclase domains, the Cys residue of the substrate is activated by an active site Zn2+,7–9 whereas the class III cyclase domains lack the metal ligands but do have sequence homology with the class I, II and IV cyclases.4,10,11
Figure 1. Lanthionine formation in lanthipeptide biosynthesis.
a) Formation of dehydroamino acids (Dha/Dhb) from Ser/Thr, followed by the Michael-type addition of Cys residues to Dha/Dhb to generate lanthionine/methyllanthionine residues, respectively. Performing the biosynthesis assay in H2O or D2O results in protium or deuterium labeling at the α-carbon of the thioether rings. b) The posttranslational maturation of HalA2 catalyzed by HalM2.
Most lanthipeptide synthetases act on a single substrate and turn them into a single peptide product.12 An example is the haloduracin β synthetase HalM2, which catalyzes the formation of four thioether rings in a precursor peptide (HalA2) that undergoes seven dehydrations.13 In principle, a peptide with four cysteines and seven dehydroamino acids could result in a modified peptide with 840 different ring topologies (excluding stereoisomers), yet, HalM2 specifically produces a single product (Figure 1b).
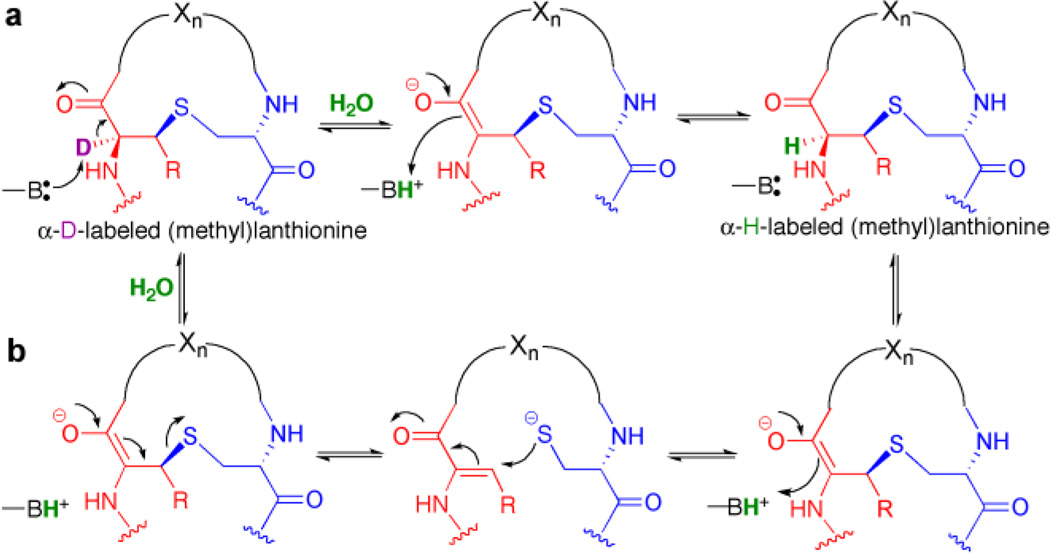
More recently, a novel lanthipeptide synthetase (ProcM) was discovered that acts on as many as 30 different peptides with very different sequences, turning them into a library of peptides with different ring topologies.14–16 How one enzyme could possibly process so many different peptides has been an unanswered question. One possibility that has been offered is that the peptide sequence, and not the enzyme, determines the outcome of the cyclization process.17 A possible mechanism would be thermodynamic control of product formation. Thermodynamic control would require the cyclization processes to be reversible, but this has never been demonstrated for lanthipeptides. A recent study showed that ProcM could exchange the α-proton of methyllanthionine residues with protons derived from solvent in some but not all of its products.18 However, exchange does not necessarily indicate a retro-Michael addition and could simply result from a deprotonation-reprotonation sequence (Scheme 1).
Scheme 1. Two mechanisms for α-proton exchange of deuteri-um-labeled (methyl)lanthionines by lanthipeptide synthetases.
a) Exchange by deprotonation-reprotonation. b) Exchange by retro-Michael reaction followed by Michael addition. During the step labeled H2O, the deuteron that is initially on the active site base exchanges with protons from solvent.
In this study we demonstrate that HalM2 not only is able to exchange the α-proton of all four thioether rings in its product with protons from solvent, but that the enzyme also catalyzes retro-Michael type additions that open up the ring structures. In addition, ring opening was also observed for the class I lanthipeptide cyclase NisC.
RESULTS AND DISCUSSION
Exchange of the α-proton of (methyl)lanthionine
To test the ability of HalM2 to deprotonate at the α-position of the (methyl)lanthionine residues in its product, a prerequisite for a retro-Michael process (Scheme 1), HalA2 was modified by HalM2 in buffer made in D2O. The reaction was analyzed by matrix-assisted laser-desorption time-of-flight mass spectrometry (MALDI-TOF MS). Seven dehydrations and incorporation of four deuterium atoms were observed (Figure 2a), as expected for a peptide cyclized by four Michael-type additions in D2O. The product peptide was then treated with N-ethylmaleimide (NEM), which alkylates free thiols. As anticipated, no NEM adducts were observed indicating that all four thioether ring structures were formed (Figure 4a).
Figure 2. Time-dependent loss of deuterium atoms from mHa-lA2.
MALDI-TOF MS spectra demonstrating step-wise replace-ment of deuterium atoms with protium when deuterium-incorporated mHalA2 (20 µM) was re-incubated with HalM2 (10 µM) in H2O. The blue dashed lines show the exchange of one to four deuterons with protons (labeled as 1–4 ex) after a) 0 min, b) 3 min, c) 15 min, d) 3 h, and e) 8 h. f) No exchange was observed after incubation with HalM2-H791A for 16 h.
Figure 4. MALDI-TOF MS data demonstrating the interception of free cysteine residues formed by the retro-Michael reaction.
Shown are spectra of a) deuterium-labeled mHalA2 incubated with NEM for 16 h in the absence of HalM2; b) deuter-ium-labeled mHalA2 incubated with HalM2 for 1 h, followed by HalM2 inactivation, and treatment of the assay with NEM for 2 h. c) deuterium-labeled mHalA2 incubated with HalM2 for 1 h, followed by addition of NEM, and incubation of the assay for 2 h and d) for 24 h. e) Deuterium-labeled mHalA2 was incubated with HalM2-H791A for 1 h, and the assay solution was treated with NEM for 16 h.
The dehydrated and cyclized HalA2 product (termed mHa-lA2 for modified HalA2)1 with four deuterium atoms incorporated was purified by high-performance liquid chromatography (HPLC) and incubated with HalM2 in buffer made in H2O. The assay mixture was analyzed by MALDI-TOF MS at various time points, revealing a time-dependent replacement of the four deuterium atoms with protium (Figure 2a–e). NEM-treatment of the intermediates after inactivating HalM2 at acidic pH19 showed that the MS intensity of NEM-adduct species was very low, suggesting that the peptides remained mostly cyclized.
The exchange process occurs stepwise with several inter-mediates in which one to four deuteriums have been exchanged (Figure 2). To determine if the exchange process displays directionality, the intermediates were analyzed by MALDI-TOF tandem mass spectrometry. It is important to point out that the fragmentation patterns of all the exchanged intermediates remain almost identical (Figure S1), indicating that the peptides did not undergo changes in the ring topology during the entire process. Closer analysis of each set of fragment ions revealed that the first two exchanges occur at the two C-terminal rings (rings C and D, Figure 3b), as illustrated by the y9 ion. The close proximity of these two rings prevented formation of fragment ions that could distinguish whether the first exchange occurs in the C- or D-ring. However, the third exchange clearly occurs at the B-ring as deduced by comparing the y9 ions with the y14/y18 ions of three-fold exchanged peptide (Figure 3b). The b6/b7/b8 ions did not have sufficient resolution to localize the last exchanged position, and the b1/b2 ions have a Dhb at position 1 and therefore also do not report on exchange.20 However, the final exchange does not occur C-terminal to Dhb7 as shown by the y18 ion of four-fold exchanged peptide (Figure 3b). It also does not take place N-terminal to Dhb1 as shown by the b-1 ions (Figure 3b). Hence, by process of elimination, the fourth and last exchange must take place at the A-ring. Therefore the observed order of exchange is the opposite of the order of cyclization,21 implying that the exchange process reflects the reversal of the cyclization steps.
Figure 3. Tandem MS analysis of intermediates of the HalM2-mediated exchange process of deuterium-labeled mHalA2.
a) Tandem MALDI-TOF mass spectrum of mHalA2. b) Representa-tive fragment ion sets (y9, y14, y18, and b−1) of intermediates present during the exchange process. The predominant number of exchanged deuterons in each peptide mixture is shown on the left. * represents a minor ion resulting from fragmentation in the A ring. Such fragmentation inside a ring when it contains a dehydroamino acid has been observed previously.20,22
If the exchange process involves a retro-Michael reaction, rather than simply deprotonation and reprotonation of the eno-late (Scheme 1), and if the exchange process indeed reflects the reverse process of cyclization, then the observation of four exchanges implies that the B-D rings must all be open in order for the fourth and last exchange to take place in the A-ring. This hypothesis could explain why it took 3–5 h to complete each of the last two exchanges whereas the first two exchanges were completed within 15 min (Figure 2). However, as mentioned above, NEM-treatment of the exchange intermediates after HalM2 inactivation indicated very little ring-opened structures at any given time (e.g. Figure 4b). This observation is not unexpected since thio-Michael addition is an exergonic process and therefore the ring-closed forms are favored over the open forms.23
Interception of ring-opened intermediates
We next attempted to trap the ring-opened intermediates by adding aliquots of the exchange reaction with deuterium labeled mHalA2 into an NEM alkylation solution without inactivating HalM2, with the aim of intercepting any free thiols that may be formed in the exchange process. Indeed, several alkylation products were observed that contained one to three NEM adducts, and a peptide with four NEM adducts was also observed under prolonged incubation time with HalM2 and NEM (Figure 4c, d). Closer examination of the mass of each ion confirmed that the α-deuterium removal occurred along-side the NEM alkylation, and that the peptide containing four NEM adducts had all four deuteriums removed (Figure S2). No such products were observed in control experiments in which HalM2 was omitted (Figure 4a). The ability to alkylate free thiols in the presence of HalM2 and NEM is consistent with the presence of a very small amount of ring opened product at any given time such that trapping of the thiol by NEM can drive the equilibrium towards the ring-opened forms. Collectively, these results indicate that the exchange process occurs via a HalM2 catalyzed retro-Michael addition.
We also observed very similar alkylated intermediates when we incubated HalM2 in the presence of NEM with mHalA2 containing protiums at the α-positions of the four thioether rings (Figure S3). Moreover, the exchange and ring-opening process of HalM2 did not require ATP, consistent with previous observations that the cyclization reaction of class II lanthionine synthetases is not ATP-dependent.24 However, the leader peptide appears to be required since no ring opening could be detected when haloduracin β was incubated with HalM2 in the presence of NEM (Figure S4). Overall, the data strongly suggest that HalM2 catalyzes thioether ring foration/opening in a reversible manner.
Given the observed products of retro-Michael reaction catalyzed by HalM2, we next performed the alkylation assay with the class I lanthipeptide cyclase NisC. We first generated the dehydrated and cyclized peptide mNisA by coexpression of NisA, NisB, and NisC in Escherichia coli.20a Following incubation of NisC with mNisA that has five thioether rings (Figure 5a), we observed the formation of ring-opened intermediates over time represented by peptides with one to five NEM adducts (Figure 5c). We analyzed the species with one, two and three NEM adducts by tandem mass spectrometry (Figure 5d and Figure S5). The data show that the Cys in the C-ring is alkylated first followed by the Cys of the E-ring, and the Cys forming the D-ring. We note that important caveats exist with respect to using the sites of NEM alkylation as readout for the order of ring opening. Most importantly, non-enzymatic NEM alkylation is in competition with enzymatic re-formation of the ring. Hence, a Cys for which this competition is more favorable towards NEM alkylation might be observed as the first-captured alkylation site even if it is not the first ring opened by the enzyme.
Figure 5. NisC catalyzed retro-Michael reaction.
a) The post-translational maturation of NisA catalyzed by the dehydratase NisB and the cyclase NisC. b) MALDI-TOF MS spectrum of mNisA treated with NEM for 16 h in the absence of NisC. c) MALDI-TOF mass spectrum of mNisA incubated with NisC for 1 h, and then treated with NEM for 12 h. (* represents NisA pep-tides that were incomplete dehydrated;20a ° represents the leader peptide of mNisA). d) Tandem MALDI-TOF MS analysis of the ring-opened intermediate with three NEM adducts.
A conserved His is likely responsible for enolate protonation
With the establishment that exchange of the α-protons of (methyl)lanthionine residues is associated with ring opening, we were able to investigate the role of His791, which is present in the cyclization domain of HalM2, and is highly conserved among class I and class II synthetases (Figure S6). Mutation of His791 to Ala significantly decreased the cyclization activity of HalM2 with linear HalA2 (Figure S7). Previous studies based on the crystal structure of NisC have proposed that this residue could either serve as the active site acid that protonates the enolate intermediate during ring formation, or the base that deprotonates the Cys prior to the Michael addition (Scheme 1).25 However, it is unlikely that the residue is responsible for both proton transfers, since the anti-addition mechanism of the Michael-type addition requires the acid/base to be placed on opposite faces of the dehydroamino acid. If His791 acts as the base to deprotonate the Cys, and hence as acid to protonate the Cys in a retro-Michael process, we anticipated that the ring-opening process with mHalA2 might be hampered by mutating this residue, while α-proton exchange would be unaffected. Incubation of deuterium-labeled mHalA2 with HalM2-H791A completely abolished both the exchange and ring-opening activities (Figures 2f and 4e). These observations suggest that His791 serves as the active site acid that protonates the enolate intermediate during ring formation and deprotonates the α-proton of the thioether rings during ring opening.
In summary, we provide the first direct evidence for reversible Michael-type addition during lanthipeptide biosynthesis. HalM2 and NisC are shown to be capable of opening up multiple rings under the conditions of catalysis, even though at equilibrium only the ring-closed peptide is observed. We were also able to provide support for the role of the conserved His residue in the active site of class I and class II synthetases, which we propose protonates the enolate intermediate during ring formation. These findings suggest that thermodynamic control over the ring topology cannot be ruled out, and may help provide further insights towards the role of the peptide sequence in determining the cyclization pattern.
METHODS
Materials
All chemicals were purchased from Fisher Scientific or Aldrich unless noted otherwise. Peptide Calibration Standard II for MALDI-TOF MS was purchased from Bruker. mNisA, NisC, HalA2 and HalM2 were produced in Escherichia coli as previously described.13,20a Haloduracin β was purified from Bacillus halodurans C-125, as described previously.26
Preparation of deuterium labeled mHalA2
The in vitro modification reaction contained 40 µM His6-tagged HalA2, 1 µM HalM2, 1 mM ATP, 1 mM MgCl2, and 0.5 mM TCEP in 25 mM HEPES at pD=8.2 in D2O. The reaction was incubated at room temperature overnight, and mHalA2 was purified by reversed-phase HPLC using a C18 column. mHalA2 peptide was digested with endoproteinase Glu-C to generate the core peptide with a 13 amino acid residue overhang on the N-terminus. The complete incorporation of four deuterium atoms into the core peptide was confirmed by MALDI-TOF MS.
Procedure for D-H exchange assay
The exchange assay contained 20 µM deuterium-labeled mHalA2, 10 µM HalM2, 0.5 mM TCEP, 25 mM HEPES at pH=7.8 in H2O. The reaction was incubated at room temperature. At different time points, aliquots of the reaction were subjected to Glu-C digestion and MALDI-TOF MS to monitor the extent of exchange.
NEM alkylation assay
An aliquot of the above assay was diluted into twice the volume of NEM alkylation buffer containing 500 mM HEPES, 3 mM NEM, pH=6.5. The reaction was incubated at 37 °C. At different time points, an aliquot of the reaction was desalted with a C4 Solid Phase Extraction (SPE) cartridge The samples were eluted from the SPE column by 50% acetonitrile with 0.1% TFA and the solvent removed by centrifugal evaporation and lyophilization. For the ring-opening assay on mNisA, 20 µM mNisA was incu-bated with 5 µM NisC and 0.5 mM TCEP in 25 mM HEPES buffer (pH=6.9) at room temperature for 1 h, followed by the same NEM alkylation procedure as described above. The resulting peptide was subjected to endoproteinase Glu-C (for mHalA2) or Arg-C (for mNisA) digestion and MALDI-TOF MS to determine the alkylation state of the core peptide. The NEM alkylation assay with haloduracin β was performed under the same conditions as with the full-length mHalA2 peptide.
MALDI-TOF mass spectrometry
After desalting using a zip-tip (C18), samples were analyzed by MALDI-TOF mass spectrometry on a Bruker UltrafleXtreme spectrometer using a matrix solution containing 35 mg/mL 2,5-dihydroxybenzoic acid (DHB) in 3:2 MeCN/H2O with 0.1% TFA. Peptide Calibration Standard II was used as external standard.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the US National Institutes of Health (GM 58822 to W.A.v.d.D.). Mass spectra were recorded in part on an instrument purchased with grant S10RR027109-01 from the US National Institutes of Health.
Footnotes
ASSOCIATED CONTENT
Supporting Information. Supporting figures with descriptions of procedures and interpretation. This information is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, Cotter PD, Craik DJ, Dawson M, Dittmann E, Donadio S, Dorrestein PC, Entian K-D, Fischbach MA, Garavelli JS, Göransson U, Gruber CW, Haft DH, Hemscheidt TK, Hertweck C, Hill C, Horswill AR, Jaspars M, Kelly WL, Klinman JP, Kuipers OP, Link AJ, Liu W, Marahiel MA, Mitchell DA, Moll GN, Moore BS, Müller R, Nair SK, Nes IF, Norris GE, Olivera BM, Onaka H, Patchett ML, Piel J, Reaney MJT, Rebuffat S, Ross RP, Sahl H-G, Schmidt EW, Selsted ME, Severinov K, Shen B, Sivonen K, Smith L, Stein T, Süssmuth RE, Tagg JR, Tang G-L, Truman AW, Vederas JC, Walsh CT, Walton JD, Wenzel SC, Willey JM, van der Donk WA. Ribosomally Synthesized and Post-translationally Modified Peptide Natural Products: Overview and Recommendations for a Universal Nomenclature. Nat. Prod. Rep. 2013;30:108–160. doi: 10.1039/c2np20085f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knerr PJ, van der Donk WA. Discovery, biosynthesis, and engineering of lantipeptides. Annu. Rev. Biochem. 2012;81:479–505. doi: 10.1146/annurev-biochem-060110-113521. [DOI] [PubMed] [Google Scholar]
- 3.Xie L, Miller LM, Chatterjee C, Averin O, Kelleher NL, van der Donk WA. Lacticin 481: in vitro reconstitution of lantibiotic synthetase activity. Science. 2004;303:679–681. doi: 10.1126/science.1092600. [DOI] [PubMed] [Google Scholar]
- 4.Müller WM, Schmiederer T, Ensle P, Süssmuth RD. In vitro biosynthesis of the prepeptide of type-III lantibiotic labyrinthopeptin A2 including formation of a C-C bond as a post-translational modification. Angew. Chem. Int. Ed. 2010;49:2436–2440. doi: 10.1002/anie.200905909. [DOI] [PubMed] [Google Scholar]
- 5.Goto Y, Li B, Claesen J, Shi Y, Bibb MJ, van der Donk WA. Discovery of unique lanthionine synthetases reveals new mechanistic and evolutionary insights. PLoS Biol. 2010;8:e1000339. doi: 10.1371/journal.pbio.1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortega MA, Hao Y, Zhang Q, Walker MC, van der Donk WA, Nair SK. Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature. 2014 doi: 10.1038/nature13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li B, Yu JP, Brunzelle JS, Moll GN, van der Donk WA, Nair SK. Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis. Science. 2006;311:1464–1467. doi: 10.1126/science.1121422. [DOI] [PubMed] [Google Scholar]
- 8.Paul M, Patton GC, van der Donk WA. Mutants of the zinc ligands of lacticin 481 synthetase retain dehydration activity but have impaired cyclization activity. Biochemistry. 2007;46:6268–6276. doi: 10.1021/bi7000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goto Y, Ökesli A, van der Donk WA. Mechanistic studies of Ser/Thr dehydration catalyzed by a member of the LanL lanthionine synthetase family. Biochemistry. 2011;50:891–898. doi: 10.1021/bi101750r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodani S, Hudson ME, Durrant MC, Buttner MJ, Nodwell JR, Willey JM. The SapB morphogen is a lantibiotic-like peptide derived from the product of the developmental gene ramS in Streptomyces coelicolor. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11448–11453. doi: 10.1073/pnas.0404220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, van der Donk WA. Biosynthesis of the Class III Lantipeptide Catenulipeptin. ACS Chem. Biol. 2012;7:1529–1535. doi: 10.1021/cb3002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Y, Zhang Q, van der Donk WA. Insights into the evolution of lanthipeptide biosynthesis. Protein Sci. 2013;22:1478–1489. doi: 10.1002/pro.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClerren AL, Cooper LE, Quan C, Thomas PM, Kelleher NL, van der Donk WA. Discovery and in vitro biosynthesis of haloduracin, a two-component lantibiotic. Proc. Natl. Acad. Sci. U. S. A. 2006;103:17243–17248. doi: 10.1073/pnas.0606088103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li B, Sher D, Kelly L, Shi Y, Huang K, Knerr PJ, Joewono I, Rusch D, Chisholm SW, van der Donk WA. Catalytic promiscuity in the biosynthesis of cyclic peptide secondary metabolites in planktonic marine cyanobacteria. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10430–10435. doi: 10.1073/pnas.0913677107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang W, van der Donk WA. Structural characterization of four prochlorosins: a novel class of lantipeptides produced by planktonic marine cyanobacteria. Biochemistry. 2012;51:4271–4279. doi: 10.1021/bi300255s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, Yang X, Wang H, van der Donk WA. High divergence of the precursor peptides in combinatorial lanthipeptide biosynthesis. ACS Chem. Biol. 2014;9:2686–2694. doi: 10.1021/cb500622c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang X, van der Donk WA. Ribosomally Synthesized and Post-Translationally Modified Peptide Natural Products: New Insights into the Role of Leader and Core Peptides during Biosynthesis. Chem. Eur. J. 2013;19:7662–7677. doi: 10.1002/chem.201300401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukherjee S, van der Donk WA. Mechanistic Studies on the Substrate-Tolerant Lanthipeptide Synthetase ProcM. J. Am. Chem. Soc. 2014;136:10450–10459. doi: 10.1021/ja504692v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thibodeaux CJ, Ha T, van der Donk WA. A Price To Pay for Relaxed Substrate Specificity: A Comparative Kinetic Analysis of the Class II Lanthipeptide Synthetases ProcM and HalM2. J. Am. Chem. Soc. 2014;136:17513–17529. doi: 10.1021/ja5089452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Dhb in the b1 and b2 ions is formed in the mass spectrometer and not by the enzyme, since the fragment is also observed in mHalA2 that has not been exposed to HalM2 (Figure S1, top spectrum). Although the mechanism of fragmentation is not known, similar fragmentation for (methyl)lanthionine rings that contain Dha/Dhb is observed for other lanthipeptides, e.g. Shi Y, Yang X, Garg N, van der Donk WA. Production of lantipeptides in Escherichia coli. J. Am. Chem. Soc. 2011;133:2338–2341. doi: 10.1021/ja109044r. Garg N, Tang W, Goto Y, van der Donk WA. Geobacillins: lantibiotics from Geobacillus thermodenitrificans. Proc. Natl. Acad. Sci. U. S. A. 2012;109:5241–5246. doi: 10.1073/pnas.1116815109. Tang W, van der Donk WA. The sequence of the enterococcal cytolysin imparts unusual lanthionine stereochemistry. Nat. Chem. Biol. 2013;9:157–159. doi: 10.1038/nchembio.1162.
- 21.Lee MV, Ihnken LA, You YO, McClerren AL, van der Donk WA, Kelleher NL. Distributive and directional behavior of lantibiotic synthetases revealed by high-resolution tandem mass spectrometry. J. Am. Chem. Soc. 2009;131:12258–12264. doi: 10.1021/ja9033507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Ortega M, Shi Y, Wang H, Melby JO, Tang W, Mitchell DA, van der Donk WA. Structural investigation of ribosomally synthesized natural products by hypothetical structure enumeration and evaluation using tandem MS. Proc. Natl. Acad. Sci. U. S. A. 2014;111:12031–12036. doi: 10.1073/pnas.1406418111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krenske EH, Petter RC, Zhu Z, Houk KN. Transition states and energetics of nucleophilic additions of thiols to substituted alpha,beta-unsaturated ketones: substituent effects involve enone stabilization, product branching, and solvation. J. Org. Chem. 2011;76:5074–5081. doi: 10.1021/jo200761w. [DOI] [PubMed] [Google Scholar]
- 24.Chatterjee C, Miller LM, Leung YL, Xie L, Yi M, Kelleher NL, van der Donk WA. Lacticin 481 synthetase phosphorylates its substrate during lantibiotic production. J. Am. Chem. Soc. 2005;127:15332–15333. doi: 10.1021/ja0543043. [DOI] [PubMed] [Google Scholar]
- 25.Li B, van der Donk WA. Identification of essential catalytic residues of the cyclase NisC involved in the biosynthesis of nisin. J. Biol. Chem. 2007;282:21169–21175. doi: 10.1074/jbc.M701802200. [DOI] [PubMed] [Google Scholar]
- 26.Oman TJ, van der Donk WA. Insights into the Mode of Action of the Two-Peptide Lantibiotic Haloduracin. ACS Chem. Biol. 2009;4:865–874. doi: 10.1021/cb900194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.