Abstract
Background
Out-of-hospital cardiac arrest (OHCA) remains a leading cause of death and a 2010 meta-analysis concluded that outcomes have not improved over several decades. However, guidelines have changed to emphasize CPR quality, minimization of interruptions, and standardized post-resuscitation care. We sought to evaluate whether OHCA outcomes have improved over time among agencies participating in the Resuscitation Outcomes Consortium (ROC) cardiac arrest registry (Epistry) and randomized clinical trials (RCTs).
Methods
Observational cohort study of 47,148 EMS-treated OHCA cases in Epistry from 139 EMS agencies at 10 ROC sites that participated in at least one RCT between 1/1/2006 and 12/31/2010. We reviewed patient, scene, event characteristics, and outcomes of EMS-treated OHCA over time, including subgroups with initial rhythm of pulseless ventricular tachycardia or ventricular fibrillation (VT/VF).
Results
Mean response interval, median age and male proportion remained similar over time. Unadjusted survival to discharge increased between 2006 and 2010 for treated OHCA (from 8.2% to 10.4%), as well as for subgroups of VT/VF (21.4% to 29.3%) and bystander witnessed VT/VF (23.5% to 30.3%). Compared with 2006, adjusted survival to discharge was significantly higher in 2010 for treated cases (OR = 1.72; 95% CI 1.53, 1.94), VT/VF cases (OR = 1.69; 95% CI 1.45, 1.98) and bystander witnessed VT/VF cases (OR = 1.65; 95% CI 1.36, 2.00). Tests for trend in each subgroup were significant (p < 0.001).
Conclusions
ROC-wide survival increased significantly between 2006 and 2010. Additional research efforts are warranted to identify specific factors associated with this improvement.
Keywords: Emergency Medical Services (EMS), Out-of-hospital cardiac arrest (OHCA), Resucitation
1. Introduction
Sudden out-of-hospital cardiac arrest (OHCA) remains a major public health problem with more than 420,000 EMS-assessed OHCA occurring annually in the United States.1 A 2010 meta-analysis concluded that aggregate survival (7.6%) following OHCA has not improved over a 30 year period.2 Care recommendations have changed to emphasize improvements in lay and professional rescuer cardiopulmonary resuscitation (CPR), and implementation of standardized protocols for post-resuscitation care and a call to develop regionalized systems of care.3–8 Without consistent collection of OHCA data and outcomes, the impact of these changes in care is difficult to characterize. Furthermore, prior observational studies have demonstrated inconsistent findings regarding the impact of updated care guidelines.9–12
The Resuscitation Outcomes Consortium (ROC) is an ongoing multi-center, international, research network evaluating interventions in OHCA, including registry data collection and randomized controlled clinical trials (RCTs). The ROC epidemiologic registry (Epistry) includes standardized data collection of patient, event, and EMS characteristics as well as hospital outcomes for EMS-assessed OHCA.13
Since inception, ROC has completed three large RCTs of prehospital interventions to improve OHCA outcomes, each of which has shown no significant survival differences between study arms.13–15 Despite this, it is possible that OHCA outcomes in ROC communities may have been affected by the changes in behavior inherent with ongoing observation as part of a registry, performance feedback, and participation in RCTs, or by concurrent adoption of other care interventions such as dispatch-assisted chest compressions, public access defibrillation programs, CPR quality (rate, depth, recoil) monitoring, minimization of interruptions (e.g., peri-shock pause), single versus stacked shocks, and standardized post-resuscitation protocols including controlled temperature management and early coronary angiography.6,17–24
We sought to characterize secular trends in OHCA survival to hospital discharge between 2006 and 2010 amongst EMS agencies that participated in ROC Epistry as well as at least one RCT within this period. We also assessed trends in survival among subgroups of VT/VF and bystander witnessed VT/VF.
2. Methods
2.1. Design and setting
The ROC consists of 10 North American sites, their EMS agencies, and participating hospitals, serving a population of approximately 24 million individuals.25 The ROC Epistry is a prospective database of OHCA patients for whom there is an organized EMS response. Cases are enrolled in Epistry if the patient receives chest compressions by EMS or any defibrillation; including use of an automated external defibrillator (AED).11 Epistry data collection at all ROC sites began December 1, 2005. The original dataset was developed by an interdisciplinary ROC committee using existing EMS reporting structures and OHCA templates.13 Epistry data collection was reviewed and approved by the institutional review boards (IRBs) and/or research ethics boards (REBs) at each participating site. The RCTs were IRB/REB reviewed and approved at each participating site.
Between June 2007 and November 2009, ROC sites enrolled adult OHCA patients in a multi-center RCT (Prehospital Resuscitation using an Impedance valve and Early vs. Delayed Analysis (PRIMED); www.clinicaltrials.gov NCT00394706). PRIMED was a factorial study testing two distinct randomized clinical interventions: a brief versus longer period of CPR by EMS prior to analysis and assessing the effectiveness of an active versus sham Impedance Threshold Device (ITD). Neither the CPR strategies nor the ITD was significantly associated with survival to hospital discharge or functional outcome.14,15 Concurrent with PRIMED, ROC completed a real-time CPR feedback RCT at selected EMS agencies from three sites which also showed no significant difference in outcomes between control and intervention arms.16 Regardless of trial participation, all sites maintained Epistry entry for cases not enrolled in RCTs, and trial data were later merged to create a complete OHCA dataset across the time frame for this study.
2.2. Study population
Included were all EMS-treated adult (age ≥ 18) non-traumatic OHCA between January 1, 2006 and December 31, 2010. Post-2010 data was unavailable due to ongoing clinical trials. To reduce bias due to varying agency participation, we included only cases from agencies that participated in Epistry and at least one of the RCTs. Of the 264 agencies that had participated in Epistry prior to PRIMED, 114 were excluded because they did not qualify to participate in any component of PRIMED allowing for an unbiased analysis over time. Excluded agencies accounted for only 12% of the cases in Epistry and had not met data quality or consistency performance benchmarks as required by the ROC Study Monitoring Committee. Of the remaining 150 agencies, seven were excluded because they did not participate in Epistry after RCT participation; one was excluded for self-reported incomplete case capture; and three agencies were combined with neighboring entities. Cases from 139 agencies in the ten participating sites serving a population of nearly 21 million were ultimately available for this analysis. Agencies were not required to have participated every month between 2006 and 2010 but were required to have contributed at least 6 months of data in Epistry and PRIMED. To avoid bias due to convenient sampling, we examined whether agencies had consistent case capture and entry into the ROC database during all months of participation. We first calculated average monthly enrollment counts for each agency. Assuming the monthly enrollment counts had a Poisson distribution, we then calculated a 95% lower bound for each agency. If an agency’s enrollment during any month was lower than this bound, we assumed incomplete case capture and excluded the agency’s cases for that month.
2.3. Outcome measures
The primary outcome was survival to hospital discharge as this was consistently captured across all cases. We considered changes in survival by time, as well as by site. Secondary outcomes included proportion of cases with available CPR process data as well as survival in OHCA subgroups by first recorded rhythm (grouped as with or without VT/VF), and bystander witnessed VT/VF.
2.4. Statistical analysis
To assess the adjusted association with survival to hospital discharge, we used multi-level mixed-effects logistic regression with a hierarchical random effects structure (individual patients nested in geographic region nested in site) using the xtmelogit function in STATA. We used independent covariance structure with random intercepts. The models provide the OR [95% CI] for study period after adjustment for the following a priori key covariates: year (factor – 2006, 2007, 2008, 2009, 2010); age (continuous); male sex (yes/no); first agency arrival time ≥6 min (yes/no); witnessed status (factor – EMS, bystander, none, unknown); bystander CPR (factor – yes, no, unknown); public location (yes/no); and first recorded rhythm (factor – VT/VF, PEA, Asystole, No-Shock No Strip, Cannot Determine). The models were run for all treated cases as well as for cases with presumed cardiac etiology only. Data management was performed in S-PLUS version 6.2.1 (Insightful Corporation, Seattle, WA) while regression analyses were performed in Stata Statistical Software: Release 11 (StataCorp LP, College Station, TX).
3. Results
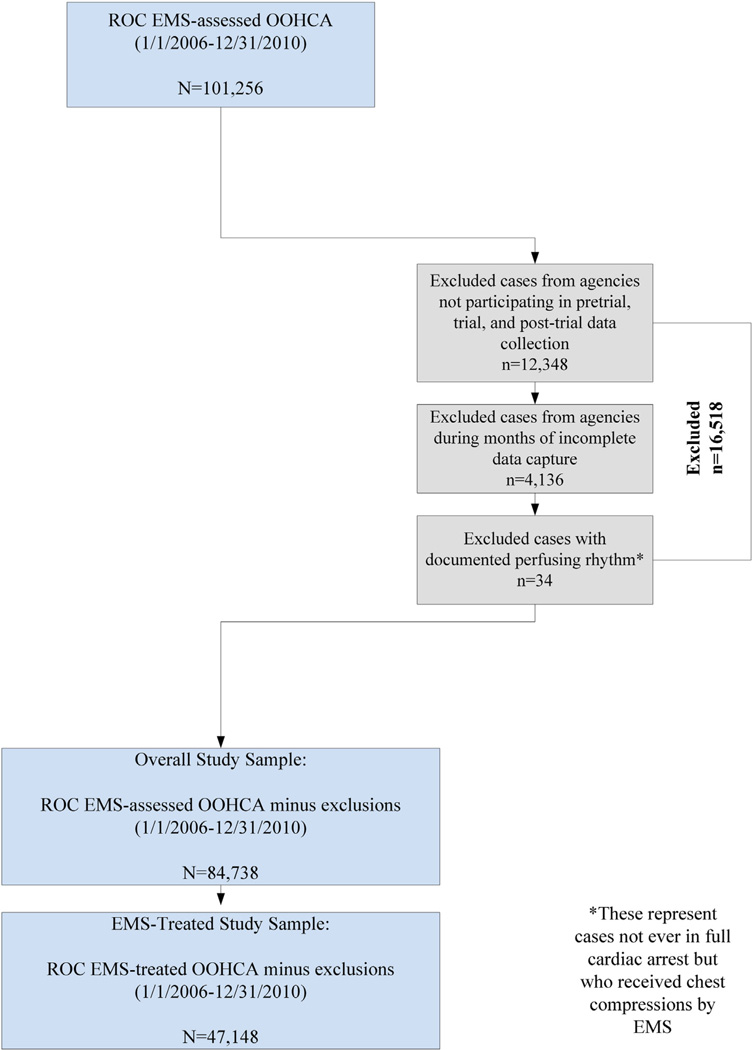
There were 84,738 OHCA patients assessed by EMS throughout the study period, with 47,148 receiving treatment (Fig. 1). Table 1 shows the proportion of treated episodes remained similar in each year (range: 54.4–56.9%), but there was an absolute increase in the count of treated cases. Mean patient age and male proportion were similar over time, as was the percentage of OHCA witnessed by bystanders. The proportion of OHCA in public locations decreased over time, while EMS-witnessed events and proportion with bystander CPR increased. The proportion of OHCA where an AED was applied prior to EMS arrival increased minimally, although AED defibrillation remained stable. Mean EMS response interval remained consistent at or near 6.0 min. Initial cardiac arrest rhythms differed, however, with the proportion of cases with a first recorded rhythm of VT/VF gradually decreasing over time (24.1% in 2006, 21.5% in 2010) while the proportion of cases with PEA and asystole increased. The proportion of cases determined to have a non-cardiac etiology were highest in 2006 (10.1%), but decreased to 4.7% in 2010. Of note, etiology determination was based solely on prehospital records and not validated through additional records review. The availability of EMS cardiac monitor files from resuscitations improved during the study period, with 41.9% of treated cases in 2007 having an available .file compared to 73.9% in 2010. Of available files, those with usable data increased as well, from 36.7% to 62.8%. Similar descriptive analyses were performed on a sample with no case exclusions (RCT participation or excluded months of data) to address potential selection bias. Findings remained consistent with our primary sample.
Fig. 1.
Study sample flowchart.
Table 1.
Overall patient, event, and EMS characteristics over time.
| 2006 | 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|---|
| Treated episodes, n | 7659 | 8275 | 9524 | 10,475 | 11,215 |
| Median age (IQR) | 67(26) | 68(27) | 67(26) | 67(27) | 66(27) |
| Male, % | 63.8 | 63.1 | 63.9 | 63.9 | 63.4 |
| Public location, % | 16.1 | 16.2 | 15.8 | 14.4 | 13.7 |
| AED applied, % | 2.5 | 2.6 | 3.5 | 3.3 | 3.2 |
| AED shock, % | 1.4 | 1.4 | 2.0 | 1.6 | 1.7 |
| Witness status | |||||
| EMS, % | 9.2 | 9.2 | 9.9 | 11.1 | 11.7 |
| Bystander, % | 39.7 | 37.8 | 37.7 | 37.8 | 37.6 |
| None, % | 42.1 | 46.0 | 48.2 | 46.6 | 47.7 |
| Unknown, % | 9.1 | 7.0 | 3.9 | 4.2 | 3.0 |
| Bystander CPR, % | 33.8 | 35.5 | 36.0 | 39.2 | 40.1 |
| Mean arrival time in minutes (sd) | 6.0 (9.3) | 6.1 (3.6) | 6.1 (3.4) | 5.9 (4.6) | 5.9 (3.1) |
| First EMS rhythm | |||||
| VT/VF, % | 24.1 | 22.7 | 22.2 | 22.3 | 21.5 |
| PEA, % | 20.3 | 20.4 | 20.9 | 22.8 | 21.8 |
| Asystole, % | 39.3 | 41.6 | 44.0 | 43.1 | 44.2 |
| No shock, no strip, % | 7.8 | 8.0 | 1.3 | 2.5 | 9.6 |
| Cannot determine, % | 6.9 | 70 | 10.7 | 7.9 | 2.6 |
| Transported, % | 60.0 | 58.9 | 60.2 | 61.2 | 62.8 |
| Non-cardiac etiology, % | 10.1 | 7.0 | 5.4 | 4.9 | 4.7 |
| Available ECG, % | n/a | 41.9 | 64.3 | 73.3 | 73.9 |
| ECG with data, % | n/a | 36.7 | 55.5 | 61.6 | 62.8 |
3.1. Survival to hospital discharge
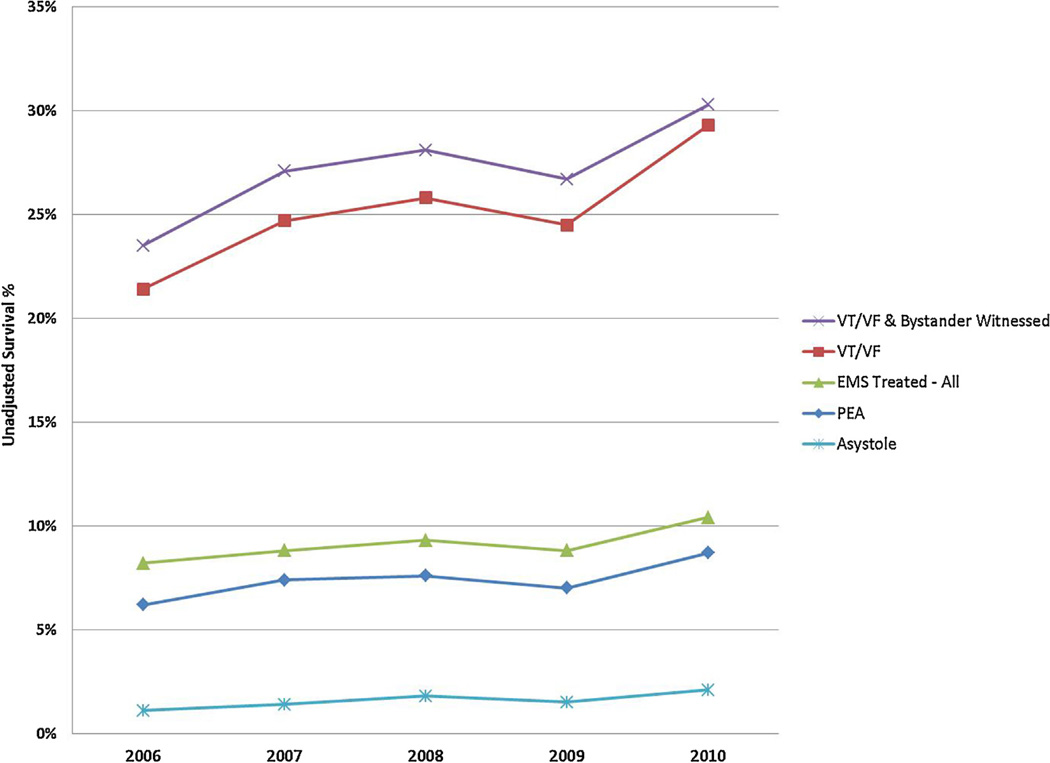
Overall, survival to discharge improved from 8.2% to 10.4% among EMS-treated OHCA in the study period (absolute difference 2.2% (95% CI 1.3%, 3.0%)) (Table 2). Survival after initial VT/VF increased from 21.4% to 29.3% (absolute difference 7.9% (95% CI 5.3%, 10.5%)). Survival in the subgroup of bystander witnessed VT/VF increased from 23.5% to 30.3% (absolute difference 6.8% (95% CI 3.4%, 10.2%)). Survival also increased for those presenting in PEA (absolute difference: 2.5% (95% CI 0.9%, 4.2%) or asystole (absolute difference: 1.0% (95% CI 0.4%, 1.6%), although these increases were of smaller magnitude (Fig. 2).
Table 2.
Treatment, transport and survival from OHCA over time: all sites.
| 2006 | 2007 | 2008 | 2009 | 2010 | |
|---|---|---|---|---|---|
| EMS-assessed episodes, n | 13,920 | 14,617 | 16,736 | 18,848 | 20,617 |
| Treated episodes, n | 7659 | 8275 | 9524 | 10,475 | 11,215 |
| Treated, % | 55.0 | 56.6 | 56.9 | 55.6 | 54.4 |
| Pronounced in .eld, n (% of treated) | 40.0 | 41.0 | 39.7 | 38.7 | 37.1 |
| Transported to ED, n (% of treated) | 60.0 | 58.9 | 60.2 | 61.2 | 62.8 |
| Survival to hospital discharge by subgroup | |||||
| EMS treated, % | 8.2 | 8.8 | 9.3 | 8.8 | 10.4 |
| Presenting rhythm VT/VF, % | 21.4 | 24.7 | 25.8 | 24.5 | 29.3 |
| Presenting rhythm VT/VF and Bystander Witnessed, % | 23.3 | 27.1 | 28.1 | 26.7 | 30.3 |
| Presenting rhythm PEA, % | 6.2 | 7.4 | 7.6 | 7.0 | 8.7 |
| Presenting rhythm asystole, % | 1.1 | 1.4 | 1.8 | 1.5 | 2.1 |
Fig. 2.
Out of hospital cardiac arrest survival over time – all sites and rhythm groups.
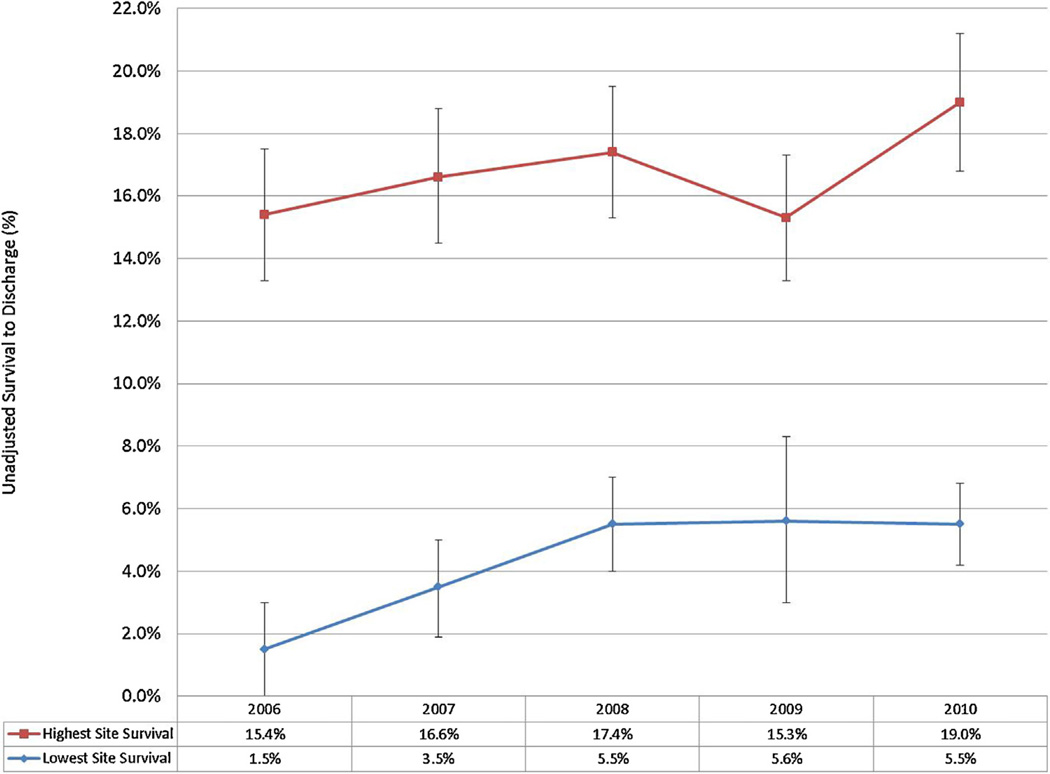
Changes in survival amongst the ten participating sites were also identified (Online Appendix). The range of survival among sites varied both at baseline and over time from 1.5–15.4% in 2006 to 5.5–19.0% in 2010. Sites with relatively low and high baseline survival demonstrated improvements over time (Fig. 3). For example, the site with a 1.5% survival proportion in 2006 experienced a more than four-fold increase to 6.6% in 2010 and the site with 15.4% survival in 2006 increased to 19.0% in 2010. Site-level variation was also evident in VT/VF cases, with baseline (2006) survival spanning a ten-fold difference; 3.1% survival for the lowest site to 37.5% for the highest. By 2010, the lowest reported site-level survival had increased to 14.8% while highest reported survival was 41.4%, indicating a much larger proportional increase for the initially lower-performing sites. These trends remained true for bystander-witnessed VT/VF cases, with baseline survival ranging from 0% to 45%. By 2010, the lowest-reported bystander-witnessed VT/VF survival was 19.4% and the highest was 46.7%.
Fig. 3.
Out of hospital cardiac arrest site-level EMS-treated OHCA over time (lowest and highest survival by site by year, with 95% CIs).
To examine site-level variation in more detail, we reviewed patient, event, and scene characteristics for each site in each year (data not shown). At the site level, age, proportion treated, patient sex, and arrival time were largely consistent over time. Sites with the greatest increases in survival over time exhibited increases in EMS-witnessed OHCA, increased frequency of bystander CPR, and increased AED use. Sites that demonstrated decreased or fluctuating survival over time reported a lower proportion of VT/VF cases over time.
After adjusting for key covariates, survival to hospital discharge increased among all EMS-treated OHCA, with each subsequent year demonstrating significantly higher odds of survival compared to 2006 (Table 3). The largest difference occurred when comparing 2010 with 2006 (OR = 1.72; 95% CI 1.53, 1.94). This survival trend was also evident among patients with an initial rhythm of VT/VF (OR = 1.69; 95% CI 1.45, 1.98) and among bystander-witnessed VT/VF (OR = 1.65; 95% CI 1.36, 2.00). Tests for trend in each subgroup were significant (p < 0.001). A second model excluding cases with non-cardiac etiology is also presented in Table 3 and demonstrates virtually identical findings.
Table 3.
Logistic regression results.
| Model 1: All treated cases n = 44,666 | Model 2: Presumed cardiac etiology cases n = 41,950 | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| 2006 | Reference | Reference | ||
| 2007 | 1.29 | (1.14, 1.46) | 1.25 | (1.10, 1.43) |
| 2008 | 1.39 | (1.23, 1.58) | 1.40 | (1.22, 1.59) |
| 2009 | 1.29 | (1.14, 1.47) | 1.29 | (1.13, 1.48) |
| 2010 | 1.72 | (1.53, 1.94) | 1.73 | (1.53, 1.96) |
| Age < 40 | Reference | Reference | ||
| Age 40–60 | 0.76 | (0.67, 0.86) | 0.76 | (0.66, 0.88) |
| Age > 60 | 0.47 | (0.41, 0.53) | 0.47 | (0.41, 0.54) |
| Female | Reference | Reference | ||
| Male | 0.89 | (0.83, 0.96) | 0.90 | (0.84, 0.98) |
| Arrival time <6 min | Reference | Reference | ||
| Arrival time ≥ 6 min | 0.73 | (0.67, 0.79) | 0.71 | (0.66, 0.77) |
| Not witnessed | Reference | Reference | ||
| EMS witnessed | 5.48 | (4.85, 6.21) | 5.88 | (5.17, 6.69) |
| Bystander witnessed | 2.49 | (2.27, 2.74) | 2.44 | (2.21, 2.70) |
| No bystander CPR | Reference | Reference | ||
| Bystander CPR | 1.20 | (1.10, 1.30) | 1.24 | (1.13, 1.35) |
| Public location | 1.85 | (1.70, 2.01) | 1.86 | (1.71, 2.03) |
| Initial rhythm: asystole | Reference | Reference | ||
| Initial rhythm: VT/VF | 13.73 | (12.10, 15.57) | 13.30 | (11.70, 15.20) |
| Initial rhythm: PEA | 3.07 | (2.67, 3.54) | 2.81 | (2.42, 3.26) |
| Presumed cardiac etiology | Reference | N/A | N/A | |
| Non-cardiac etiology | 1.47 | (1.25, 1.72) | N/A | N/A |
4. Discussion
Cross-site survival after OHCA significantly increased over time for patients treated by EMS agencies participating in ROC Epistry and at least one RCT. The greatest survival increase was seen in the cohorts with VT/VF. Survival also increased in subgroups with initial PEA and asystole. These results have important public health implications as OHCA is a leading cause of death in the United States and reported survival over the last thirty years has not changed significantly.2 While this study demonstrated site-level variation, overall survival increased 1.7-fold between 2006 and 2010. If the increase noted here was replicated on a national level in the United States, an additional almost 6500 premature deaths could have been prevented in 2010 as compared to 2006.
These results were observed despite changes over time in patient, event, and EMS characteristics known to be associated with a favorable prognosis in this population.26–29 For example, although bystander CPR rates and EMS-witnessed events increased, the presence of VT/VF as an initial rhythm and the proportion of events occurring in public locations decreased. Importantly, the survival increases persisted after adjusting for these known predictors of survival.
Several factors may be responsible for these findings. First, public health campaigns have emphasized lay use of chest compressions without ventilations;5 dispatchers have begun to provide instructions in lay use of chest compressions without ventilations;27,28 EMS instruction, real time and post-event review have emphasized use of optimal compression rates, deeper chest compressions, fewer interruptions, briefer peri-shock pauses, and single rather than stacked shocks; standardized post-resuscitation care protocols, with some communities implementing regionalized systems of care.6–8,17–24,30–34 We cannot attribute which, if any, of these interventions was most responsible for improved outcomes. Extrapolating from the care of patients with ST-elevation myocardial infarction, the most important factor may be a change in culture to recognize that OHCA is a treatable condition rather than changes in the process of care.35
Second, uniform data collection and quality improvement reporting may have helped EMS agencies identify weaknesses in the chain of survival within their systems.29 All ROC EMS agencies were required to participate in Epistry in order to enroll patients in RCTs. This encouraged sites to develop and/or maintain strong relationships with EMS agencies.
Third, though clinical trials did not show significant differences between study arms, the phenomena of altered performance as a result of being part of a study, the so called Hawthorne effect, could account for some of the observed changes.36 Evidence suggesting benefit from RCT participation is weak and primarily reported in the setting of successful interventions.37 Campbell et al. showed that the Hawthorne effect exists in EMS and does not necessarily require direct observation or feedback, but only a perceived demand for improved performance.36 Provider hand-washing behavior among ICU workers changed notably when subjects were aware of being observed, with compliance increasing from 29% to 45%.38 In a systematic review, Braunholtz et al. concluded that it is likely that RCTs have a positive rather than negative outcome on patients especially when non-trial patients receive protocol-driven care.37 In this regard, it is important to note that EMS training for the PRIMED study emphasized the importance of high quality CPR.6 Emphasis was placed on the need for correct rate and depth of chest compression, complete chest wall recoil, avoidance of hyperventilation and limited hands-off time.6 Performance was verified in part through analysis of CPR fraction, an important covariate in the PRIMED trial.14,15 Consistent post-resuscitation care in receiving hospitals was also encouraged through the dissemination of best practice guidelines at all sites to enhance a systems of care approach.
Finally, implementation of the AHA resuscitation guidelines released in December 2005 could also account in part for the observed findings.3 The 2005 guideline changes emphasized a 30:2 compression ventilation ratio and single shocks to reduce no-flow time during CPR.3 Although some studies reported improved survival following implementation of the 2005 guidelines, others, including one from ROC, failed to demonstrate this effect.9–12
5. Limitations
This study has several limitations. The number of reported OHCA cases increased over time. This increase was in part due to the exclusion of data from one site in 2006 and 2007 related to self-reported incomplete case capture. Additional possible explanations include better case ascertainment, population growth, or an increase in risk. A few sites also added EMS agencies over time, resulting in more cases overall. We attempted to control for any incomplete ascertainment by eliminating months where case identification was lower than a calculated monthly boundary. Moreover, the proportion of treated cases remained consistent over time. We did see a notable decrease in non-cardiac etiology cases over time. We believe this is due to changes in coding and case identification at the site level. Regardless of circumstance, we believe it is unlikely that the change in case number accounted for a temporal selection bias that would improve survival over time since fixed characteristics such as VT/VF incidence and public setting arrests became less favorable over time.
We are unable to adjust for changes in post-resuscitation care processes in particular the use of targeted temperature management and early coronary artery angiography that may have occurred over time as these hospital treatment variables were not captured in the first version of Epistry.25,39 Neurologic outcome at survival is not available for all patients in the study time frame, so that information has been excluded. Similarly, knowledge translation and behavior change occur at variable rates and it is impossible to adjust for these changes. We also excluded EMS agencies that did not meet pre-defined performance benchmarks before the clinical trials, which represents a potential selection bias. But a post hoc analysis of all Epistry cases with no exclusions due to etiology or case capture demonstrated similar improvements in survival as the primary analysis, which suggests that any selection bias is small at best.
Though geographically diverse, the ROC sites may not be representative of all EMS agencies across North America limiting the potential generalizability of these findings. Furthermore, we restricted this analysis to only agencies that participated in Epistry and at least one RCT. However, the baseline survival (8.2%) across all ROC sites in this study was similar to the average survival rate (7.6%) reported in the 30 year systematic review by Sasson et al.,2 Finally, as with any registry-level data, there is the possibility for residual confounding since traditional Utstein factors thought to predict survival accounted incompletely for variation in survival between ROC sites.40 Important strengths of Epistry, however, include its independent assessment of complete case ascertainment, use of range and logic checks to enhance data quality, as well as independent periodic audit of data collection and abstraction procedures at each site.12,41 Although the availability of CPR process files with data was included in this study, actual CPR quality measures were not analyzed or adjusted for, due to the limited quantity of files at baseline.
6. Conclusions
We found significant and important increases in survival from EMS-treated OHCA over time among ROC communities geographically dispersed throughout North America. The survival increases demonstrate that OHCA is a condition whose treatment warrants ongoing investment of limited health care resources to achieve further improvements. Further research is required to identify the specific factors associated with this improvement.
Supplementary Material
Acknowledgements
We wish to acknowledge and thank all of the participating EMS personnel, agencies and medical directors, as well as the hospitals that collected and contributed data for this project.
Funding
The ROC is supported by a series of cooperative agreements to nine regional clinical centers and one Data Coordinating Center (5U01 HL077863 – University of Washington Data Coordinating Center, HL077866 – Medical College of Wisconsin, HL077867 – University of Washington, HL077871 – University of Pittsburgh, HL077872 – St. Michael’s Hospital, HL077873 – Oregon Health and Science University, HL077881 – University of Alabama at Birmingham, HL077885 – Ottawa Hospital Research Institute, HL077887 – University of Texas SW Medical Center/Dallas, HL077908 –University of California San Diego) from the National Heart, Lung and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) – Institute of Circulatory and Respiratory Health, Defence Research and Development Canada and the Heart, Stroke Foundation of Canada and the American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.resuscitation. 2015.02.003.
Footnotes
A Spanish translated version of the summary of this article appears as Appendix in the final online version at http://dx.doi.org/10.1016/j.resuscitation.2015.02.003.
Conflicts of interest statement
None.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation. 2014;128 doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Sasson C, Rogers MA, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 3.Hazinski MF, Nadkarni VM, Hickey RW, et al. Major changes in the 2005 AHA Guidelines for CPR and ECC: reaching the tipping point for change. Circulation. 2005;112:IV206–IV211. doi: 10.1161/CIRCULATIONAHA.105.170809. [DOI] [PubMed] [Google Scholar]
- 4.Hazinski MF, Nolan JP, Billi JE, et al. Part 1: Executive summary: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122:S250–S275. doi: 10.1161/CIRCULATIONAHA.110.970897. [DOI] [PubMed] [Google Scholar]
- 5.Sayre MR, Berg RA, Cave DM, et al. Hands-only (compression-only) cardiopulmonary resuscitation: a call to action for bystander response to adults who experience out-of-hospital sudden cardiac arrest: a science advisory for the public from the American Heart Association Emergency Cardiovascular Care Committee. Circulation. 2008;117:2162–2167. doi: 10.1161/CIRCULATIONAHA.107.189380. [DOI] [PubMed] [Google Scholar]
- 6.Meaney PA, Bobrow BJ, Mancini ME, et al. Cardiopulmonary resuscitation quality: improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417–423. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 7.Nichol G, Aufderheide TP, Eigel B, et al. Regional systems of care for out-of-hospital cardiac arrest: a policy statement from the American Heart Association. Circulation. 2010;121:709–729. doi: 10.1161/CIR.0b013e3181cdb7db. [DOI] [PubMed] [Google Scholar]
- 8.Spaite DW, Bobrow BJ, Stolz U, et al. Statewide regionalization of postarrest care for out-of-hospital cardiac arrest: association with survival and neurologic outcome. Ann Emerg Med. 2014;64:496–506. doi: 10.1016/j.annemergmed.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Steinmetz J, Barnung S, Nielsen SL, Risom M, Rasmussen LS. Improved survival after an out-of-hospital cardiac arrest using new guidelines. Acta Anaesthesiol Scand. 2008;52:908–913. doi: 10.1111/j.1399-6576.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- 10.Sayre MR, Cantrell SA, White LJ, Hiestand BC, Keseg DP, Koser S. Impact of the 2005 American Heart Association cardiopulmonary resuscitation and emergency cardiovascular care guidelines on out-of-hospital cardiac arrest survival. Prehosp Emerg Care. 2009;13:469–477. doi: 10.1080/10903120903144965. [DOI] [PubMed] [Google Scholar]
- 11.Bigham BL, Koprowicz K, Rea T, et al. Cardiac arrest survival did not increase in the Resuscitation Outcomes Consortium after implementation of the 2005 AHACPR and ECC guidelines. Resuscitation. 2011;82:979–983. doi: 10.1016/j.resuscitation.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deasy C, Bray JE, Smith K, et al. Cardiac arrest outcomes before and after the 2005resuscitation guidelines implementation: evidence of improvement? Resuscitation. 2011;82:984–988. doi: 10.1016/j.resuscitation.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Morrison LJ, Nichol G, Rea TD, et al. Rationale, development and implementation of the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Resuscitation. 2008;78:161–169. doi: 10.1016/j.resuscitation.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stiell IG, Nichol G, Leroux BG, et al. Early versus later rhythm analysis in patients with out-of-hospital cardiac arrest. N Engl J Med. 2011;365:787–797. doi: 10.1056/NEJMoa1010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aufderheide TP, Nichol G, Rea TD, et al. A trial of an impedance threshold device in out-of-hospital cardiac arrest. N Engl J Med. 2011;365:798–806. doi: 10.1056/NEJMoa1010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hostler D, Everson-Stewart S, Rea TD, et al. Effect of real-time feedback during cardiopulmonary resuscitation outside hospital: prospective, cluster-randomised trial. Br Med J. 2011;342:d512. doi: 10.1136/bmj.d512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rea TD, Helbock M, Perry S, et al. Increasing use of cardiopulmonary resuscitation during out-of-hospital ventricular fibrillation arrest: survival implications of guideline changes. Circulation. 2006;114:2760–2765. doi: 10.1161/CIRCULATIONAHA.106.654715. [DOI] [PubMed] [Google Scholar]
- 18.Bobrow BJ, Clark LL, Ewy GA, et al. Minimally interrupted cardiac resuscitation by emergency medical services for out-of-hospital cardiac arrest. JAMA. 2008;299:1158–1165. doi: 10.1001/jama.299.10.1158. [DOI] [PubMed] [Google Scholar]
- 19.Garza AG, Gratton MC, Salomone JA, Lindholm D, McElroy J, Archer R. Improved patient survival using a modified resuscitation protocol for out-of-hospital cardiac arrest. Circulation. 2009;119:2597–2660. doi: 10.1161/CIRCULATIONAHA.108.815621. [DOI] [PubMed] [Google Scholar]
- 20.Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Hallstrom AP, Ornato JP, Weisfeldt M, et al. Public-access defibrillation and survival after out-of-hospital cardiac arrest. N Engl J Med. 2004;351:637–646. doi: 10.1056/NEJMoa040566. [DOI] [PubMed] [Google Scholar]
- 22.Bobrow BJ, Panczyk M, Subido C. Dispatch-assisted cardiopulmonary resuscitation: the anchor link in the chain of survival. Curr Opin Crit Care. 2012;18:228–233. doi: 10.1097/MCC.0b013e328351736b. [DOI] [PubMed] [Google Scholar]
- 23.Cheskes S, Schmicker RH, Christenson J, et al. Perishock pause: an independent predictor of survival from out-of-hospital shockable cardiac arrest. Circulation. 2011;124:58–66. doi: 10.1161/CIRCULATIONAHA.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perman SM, Goyal M, Neumar RW, et al. Clinical applications of targeted temperature management. Chest. 2014;145:386–393. doi: 10.1378/chest.12-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis DP, Garberson LA, Andrusiek DL, et al. A descriptive analysis of Emergency Medical Service Systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehosp Emerg Care. 2007;11:369–382. doi: 10.1080/10903120701537147. [DOI] [PubMed] [Google Scholar]
- 26.Larsen MP, Eisenberg MS, Cummins RO, Hallstrom AP. Predicting survival from out-of-hospital cardiac arrest: a graphic model. Ann Emerg Med. 1993;22:1652–1658. doi: 10.1016/s0196-0644(05)81302-2. [DOI] [PubMed] [Google Scholar]
- 27.Hollenberg J, Herlitz J, Lindqvist J, et al. Improved survival after out-of-hospital cardiac arrest is associated with an increased in proportion of emergency crew-witnessed cases and bystander cardiopulmonary resuscitation. Circulation. 2008;118:389–396. doi: 10.1161/CIRCULATIONAHA.107.734137. [DOI] [PubMed] [Google Scholar]
- 28.Stromsoe A, Afzelius S, Axelsson C, et al. Improvement in logistics could increase survival after out-of-hospital cardiac arrest in Sweden. J Intern Med. 2013;273:622–627. doi: 10.1111/joim.12041. [DOI] [PubMed] [Google Scholar]
- 29.Eisenberg M. Resuscitate! How your community can improve survival from sudden cardiac arrest. Second edition. Seattle: University of Washington Press; 2013. Print. [Google Scholar]
- 30.Svensson L, Bohm K, Castrèn M, et al. Compression-only CPR or standard CPR in out-of-hospital cardiac arrest. N Engl J Med. 2010;363:434–442. doi: 10.1056/NEJMoa0908991. [DOI] [PubMed] [Google Scholar]
- 31.Rea TD, Fahrenbruch C, Culley L, et al. CPR with chest compression alone or with rescue breathing. N Engl J Med. 2010;363:423–433. doi: 10.1056/NEJMoa0908993. [DOI] [PubMed] [Google Scholar]
- 32.Idris AH, Guffey D, Aufderheide TP, et al. Relationship between chest compression rates and outcomes from cardiac arrest. Circulation. 2012;125:3004–3012. doi: 10.1161/CIRCULATIONAHA.111.059535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vadeboncoeur T, Stolz U, Panchal A, et al. Chest compression depth and survival in out-of-hospital cardiac arrest. Resuscitation. 2014;85:182–188. doi: 10.1016/j.resuscitation.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Cheskes S, Schmicker RH, Verbeek PR, et al. The impact of perishock pause on survival from out-of-hospital shockable cardiac arrest during the Resuscitation Outcomes Consortium PRIMED trial. Resuscitation. 2014;85:336–342. doi: 10.1016/j.resuscitation.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curry LA, Spatz E, Cherlin E, et al. What distinguishes top-performing hospitals in acute myocardial infarction mortality rates? A qualitative study. Ann Intern Med. 2011;154:384–390. doi: 10.7326/0003-4819-154-6-201103150-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell JP, Maxey VA, Watson WA. Hawthorne effect: implications for pre-hospital research. Ann Emerg Med. 1995;26:590–594. doi: 10.1016/s0196-0644(95)70009-9. [DOI] [PubMed] [Google Scholar]
- 37.Braunholtz DA, Edwards SJL, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a trial effect. J Clin Epidemiol. 2001;54:217–224. doi: 10.1016/s0895-4356(00)00305-x. [DOI] [PubMed] [Google Scholar]
- 38.Eckmanns T, Bessert J, Behnke M, Gastmeier P, Ruden H. Compliance with anti-septic hand rub use in intensive care units: the Hawthorne effect. Infect Control Hosp Epidemiol. 2006;27:931–934. doi: 10.1086/507294. [DOI] [PubMed] [Google Scholar]
- 39.Callaway CW, Schmicker RH, Brown SP, et al. Early coronary angiography and induced hypothermia are associated with survival and functional recovery after out-of-hospital cardiac arrest. Resuscitation. 2014;85:657–663. doi: 10.1016/j.resuscitation.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rea TD, Cook AJ, Stiell IG, et al. Predicting survival after out-of-hospital cardiac arrest: role of the Utstein data elements. Ann Emerg Med. 2010;55:249–257. doi: 10.1016/j.annemergmed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 41.Goldberger ZD, Nichol G. Registries to measure and improve outcomes after cardiac arrest. Curr Opin Crit Care. 2013;19:208–213. doi: 10.1097/MCC.0b013e328360ad06. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.