Abstract
Rationale
An abundance of genetic and epidemiologic evidence as well as longitudinal neuroimaging data point to developmental origins for schizophrenia and other mental health disorders. Recent clinical studies indicate that microduplications of VIPR2, encoding the vasoactive intestinal peptide (VIP) receptor VPAC2, confer significant risk for schizophrenia and autism spectrum disorder. Lymphocytes from patients with these mutations exhibited higher VIPR2 gene expression and VIP responsiveness (cAMP induction), but mechanisms by which overactive VPAC2 signaling may lead to these psychiatric disorders are unknown.
Objectives
We subcutaneously administered the highly-selective VPAC2 receptor agonist Ro 25-1553 to C57BL/6 mice from postnatal day 1 (P1) to P14 to determine if overactivation of VPAC2 receptor signaling during postnatal brain maturation affects synaptogenesis and selected behaviors.
Results
Western blot analyses on P21 revealed significant reductions of synaptophysin and postsynaptic density protein 95 (PSD-95) in the prefrontal cortex, but not in the hippocampus in Ro 25-1553-treated mice. The same postnatally-restricted treatment resulted in a disruption in prepulse inhibition of the acoustic startle measured in adult mice. No effects were observed in open-field locomotor activity, sociability in the three-chamber social interaction test, or fear conditioning or extinction.
Conclusion
Overactivation of the VPAC2 receptor in the postnatal mouse results in a reduction in synaptic proteins in the prefrontal cortex and selective alterations in prepulse inhibition. These findings suggest that the VIPR2-linkage to mental health disorders may be due in part to overactive VPAC2 receptor signaling during a critical time of synaptic maturation.
Keywords: VPAC2 receptor (VIPR2), schizophrenia, prepulse inhibition, PSD-95, synaptophysin, mouse
Introduction
Two genetic studies published early in 2011 including more than 8,000 and 3,900 schizophrenia patients, respectively, provided evidence that microduplications at 7q36.3, containing VIPR2, are a risk factor for schizophrenia, with odds-ratios of 14.1 (Vacic et al. 2011) and 6.3 (Levinson et al. 2011). In one of these studies, 7q36.3 microduplications were also significantly over-represented in autism spectrum disorder (ASD), although the number of ASD patients was smaller (Vacic et al. 2011). VIPR2 encodes VPAC2, a seven transmembrane heterotrimeric G protein-coupled receptor (Gs) that binds two homologous neuropeptides with high affinity, vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating polypeptide (PACAP). Lymphocytes from patients with these microduplications exhibited higher VIPR2 gene expression and VIP responsiveness (cAMP induction) (Vacic et al. 2011), demonstrating the functional significance of the microduplications. Additionally, the blood concentration of VIP, but not PACAP, is higher in children with ASD compared to healthy control subjects (Nelson et al. 2001). Overall, the findings provided large-scale genetic evidence for a specific receptor-mediated (and potentially drug targetable) signaling pathway linked to schizophrenia and ASD that is distinct from dopaminergic, glutamatergic, and serotonergic systems.
A key question in mental health research is if alterations in the establishment of neural circuitries in the developing brain underlie diseases such as schizophrenia and ASD (Insel 2010). Key findings relevant to this question are that many schizophrenia- and ASD-linked genes have developmental functions. In this regard, the peptide ligands for the VPAC2 receptor, VIP and PACAP, are well known as pleiotropic growth factors that regulate neurogenesis, white matter development, and other aspects of brain morphogenesis (Falluel-Morel et al. 2007; Harmar et al. 1998; Nakamachi et al. 2011 Niewiadomski et al. 2013; Suj et al. 2001; Waschek 1996). The VPAC2 receptor plays a key role in circadian rhythms and mediates a variety of other neurological functions in adult mice (Harmar 2003; Itri and Colwell 2003), but its potential roles in the developmental period remain incompletely understood. Our prior studies have shown that VIPR2 gene expression in the developing mouse brain displays a pronounced peak at postnatal day (P) 12, a time of active synaptogenesis, pruning and myelination (O'Kusky et al. 2000; Paolicelli et al. 2011; Ye et al. 2002), and then declines as animals reach adulthood (Waschek et al. 1996). The peak at P12 suggested to us that the VPAC2 receptor plays an important role in the formation of synaptic circuits. Reduced levels of synaptophysin, a marker of presynaptic vesicles, and postsynaptic density protein 95 (PSD-95), a marker of excitatory postsynaptic density, were observed in cortical regions of patients with schizophrenia (Eastwood et al. 2000; Glantz and Lewis 1997; Karson et al. 1999; Kristiansen et al. 2006). Thus, in the current study, we hypothesized that overactivity of the VPAC2 receptor during the early postnatal period could result in alterations in synaptogenesis, and in behaviors associated with schizophrenia and ASD. We investigated this by systemically administering Ro 25-1553, a highly selective VPAC2 receptor agonist (Gourlet et al. 1997; Harmar et al. 2012) during P1-14, and determined the effect on PSD-95 and synaptophysin protein levels one week later, and on several behaviors relevant to schizophrenia and ASD 10 weeks after cessation of agonist administration. The results obtained provide pharmacological support for the hypothesis that VIPR2 microduplications increase the risk of schizophrenia at least partially due to overactivity of VPAC2 signaling in the brain at a critical stage of postnatal brain development.
Materials and methods
Animals and treatment
All animal studies were approved by the Animal Research Committee at University of California, Los Angeles (UCLA). All experimental procedures were conducted in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals (National Research Council 1996). Pregnant C57BL/6 females were from our breeding colony maintained at UCLA and monitored for the parturition date, which was taken as postnatal day 0 (P0). For behavioral experiments, all litters were randomly divided into Ro 25-1553 (GL Biochem (Shanghai) Ltd., Shanghai, China) and phosphate-buffered saline (PBS)-treated groups. From P1 until P7 or P14, mice were injected subcutaneously (s.c.) once daily with Ro 25-1553 at dose of 0.25 μg/g (= 0.07 nmol/g) body weight. The dose regimen was based on a previous study in which PACAP was administered to postnatal rats and produced rather profound neurodevelopmental alterations (Reglödi et al. 2003). Mice treated with PBS from P1 to P14 were used as control. The time needed for the injection in each mouse was less than 1 min, which minimized the influence of separation of neonates from the mothers on maternal behaviors directed towards the pups. Animals were weaned at P21, and divided by gender at P28. We used male mice exclusively to minimize any potential variability due to sex-specific effects in behavioral performance. For example, reproductive state in female mice affects prepulse inhibition (PPI), latency of the acoustic startle response, and cognitive behaviors (Charitidi et al. 2012; Walf et al. 2009). All groups were derived from at least 4 different litters to preclude possible differences in individual maternal behavior as a mitigating factor in any subsequent long-lasting changes induced in the offspring (Table 1). Behavioral analyses of mice were carried out at 3–4 months of age. Experimenters were blinded to the treatment during testing. All behavioral tests were performed in the UCLA behavioral test core.
Table 1.
Number of pups in each treatment group.
| Litter No. | Number of pups |
||
|---|---|---|---|
| PBS | Ro 25-1553 (P1-7) | Ro 25-1553 (P1-14) | |
| 1 | 3 | 0 | 2 |
| 2 | 1 | 2 | 1 |
| 3 | 1 | 2 | 1 |
| 4 | 1 | 2 | 1 |
| 5 | 1 | 0 | 2 |
| 6 | 1 | 2 | 1 |
Western blot analysis
Western blot analysis was performed as previously reported (Ghiani and Gallo 2001). Mice were injected s.c. once daily with PBS or Ro 25-1553 (0.07 nmol/g) from P1 until P14. One week after the last injection, each mouse was anesthetized with isoflurane and the brain was rapidly removed. The hippocampus and prefrontal cortex were dissected on ice, frozen on dry ice and stored at –80 °C until assay. Tissue samples were homogenized at 4 °C in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% NP-40, 0.25% sodium deoxycholate, 1 mM Na3VO4 and 1 mM NaF) supplemented with a protease inhibitor cocktail (Sigma, St. Louis, MO). The homogenate was incubated at 4 °C for 30 min and then centrifuged at 14,000g for 10 min at 4 °C, and the resulting supernatant was collected. Adequate amounts of protein were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred electrophoretically to a hydrophobic polyvinylidene fluoride membrane. The blotted membranes were blocked for 1 h at room temperature in 5% nonfat dry milk/TBS-T (20 mM Tris-HCl, 137 mM NaCl and 0.1% Tween-20; pH 7.4), and then incubated with primary antibodies: mouse monoclonal anti-PSD-95 (1:1000, overnight at 4 °C; UC Davis/NIH NeuroMab Facility), rabbit polyclonal anti-synaptophysin (1:1000, overnight at 4 °C; Cell Signaling Technology, Danvers, MA) or mouse monoclonal anti-β-actin (1:5000, 45 min at room temperature; Sigma). Membranes were then incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (1:5000, 1 h at room temperature; Cell Signaling Technology) for synaptophysin or with HRP-conjugated anti-mouse IgG (1:5000, 1 h at room temperature; Sigma) for PSD-95 and β-actin. The immune complexes were visualized using ECL2 Western Blotting Detection Reagents (Thermo Fisher Scientific, Rockford, IL). The densitometric analysis was carried out using an ImageJ 1.47 software package (NIH, Bethesda, MD). Expression levels were normalized to β-actin and are presented as fold change relative to control PBS results.
Behavioral analysis
Open-field test
Each mouse was placed inside a clear Plexiglas chamber (27.5 cm × 27.5 cm × 20.5 cm) under an illumination of 30 lx and video-recorded for 20 min using TopScan (CleverSys Inc., VA). A central area, occupying 60% of the total area, was superimposed over the arena profile and the travelling distance of mouse was scored for the periphery and center of the arena. The time spent in the center of the arena was also determined.
Acoustic startle and prepulse inhibition (PPI)
Acoustic startle responses were measured in a startle chamber (SR-LAB®; San Diego Instruments, San Diego, CA) as previously reported (Gale et al. 2009). Briefly, after a background noise of 65 dB had been presented for the 5-min acclimation period, each subject was presented with a total of 52 trials. The test session consisted of startle trials (40 ms burst of 120 dB white noise) and PPI trials [a prepulse (20 ms burst of white noise at 70, 75 or 80 dB intensity) preceded the 120 dB startle pulse (40 ms) by 100 ms]. Trials were pseudo-randomly presented with an inter-trial interval between 9 and 24 s (average: 15 s). The startle response was recorded every 1 ms for 100 ms after onset of a startle stimulus and the maximum startle amplitude was used as the dependent variable. Baseline startle responses were calculated as the average response to the pulse-alone trials. PPI was calculated as a percentage score for each prepulse trial type: PPI (%) = (1–[(startle response for pulse with prepulse)/(startle response for pulse alone)])×100.
Three-chamber social interaction test
Mice were tested for social interactions using a three-chambered social interaction apparatus (Nadler et al. 2004). In brief, after habituation, a mouse was placed in the central chamber of a clear Plexiglas box (41 cm × 60 cm × 23.5 cm) divided into three interconnected chambers under an illumination of 5 lx and was given the choice to interact with either an empty wire cup (located in one side chamber) or a similar wire cup with an unfamiliar mouse (a sex- and age-matched C57BL/6) inside (located in the opposite chamber). The amount of time spent in each of the three chambers was recorded for the test mouse in a 10-min trial using TopScan (CleverSys Inc.).
Fear conditioning test
All behavioral testing was performed using four identical fear conditioning chambers (30 cm × 24 cm × 21 cm, Med-Associates, Inc. St. Albans, VT), equipped with a MedAssociates VideoFreeze system as previously reported (Jacobs et al. 2010; Zelikowsky et al. 2013). Tone conditioning on Day 1 in the conditioning chamber (Context A) consisted of a 150-s baseline period followed by five tones (30 s, 2.8 kHz, 80 dB) paired with electric footshock (2 s, 0.5 mA) that began immediately after the offset of each tone presentation, with a 90-s inter-trial interval in between the termination of each shock and the onset of the following tone. Freezing was recorded during both the baseline period and each tone period. Twenty-four hours later (Day 2), mice were placed in the same conditioning chamber (Context A) for an 8-min context fear test. No stimuli were presented during this period. Freezing was recorded throughout the 8-min test. In the cued fear test on Day 3, mice were placed in the chamber with a dark roof-like triangular ceiling and grid floor covering (Context B) and allowed to explore the novel environment for 150 s, and then five tones (30 s, 2.8 kHz, 80 dB) were presented, each spaced by a 90-s inter-trial interval. Freezing was recorded during both the baseline period and each tone period. The extinction phase started in Context B 24 h following the cued fear test. After 3-min of exploration, the mice were exposed to twenty tones (30 s, 2.8 kHz, 80 dB) with a 5-s inter-trial interval each day for 2 consecutive days (Day 4 and 5). Mice were removed from the chamber 3 min after the final cue presentation. Freezing was recorded during both the baseline period and each tone period. Data are expressed as 4 bins from the average for every 5 tones.
Data analysis
All data are expressed as the mean ± standard error of the mean (SEM). For fear conditioning test, analyses were made using two-way analysis of variance (ANOVA) with drug treatment as the intersubject factor and repeated measures with tone exposure as the intrasubject factor, followed by the Tukey-Kramer post-hoc test. Data for the contextual test and startle amplitude were analyzed using one-way ANOVA. For other behavioral experiments, analyses were made using two-way ANOVA for treatment as the intersubject factor and repeated measures with time (open-field test), chamber (social interaction test) or prepulse intensity (startle test) as the intrasubject factor, followed by the Tukey-Kramer post-hoc test. Data for Western Blot were analyzed by Student's t-test. Statistical analyses were made using a software package StatView® 5.0 for Windows (SAS Institute, Cary, NC). A value of P < 0.05 was considered statistically significant.
Results
Effects of the VPAC2 receptor activation during early postnatal development on subsequent synaptic marker protein expression in mouse brain
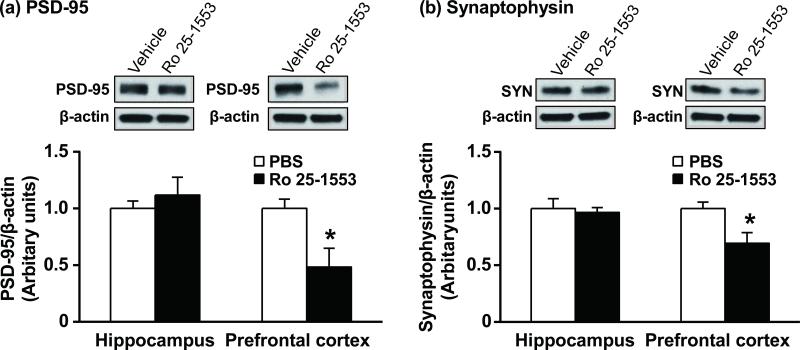
It was previously shown in neuronal culture models that PACAP, a high affinity ligand for the VPAC2 receptor, can regulate the interaction of synaptic proteins putatively involved in schizophrenia (Hattori et al. 2007; Yamada et al. 2012). To determine if activation of the VPAC2 receptor during early postnatal development could alter the levels of synaptic proteins in the mouse brain, we examined the effect of Ro 25-1553, a highly selective VPAC2 receptor agonist, on expression of PSD-95 and synaptophysin one week after the final dose of agonist (Fig. 1). Repeated administration of Ro 25-1553 (0.07 nmol/g, s.c., once daily) during P1-14 reduced significantly PSD-95 and synaptophysin protein levels in the prefrontal cortex, but not in the hippocampus, of mice at 3 weeks of age.
Fig. 1.
Effects of early postnatal treatment with the VPAC2 receptor agonist Ro 25-1553 on PSD-95 (a) and synaptophysin (b) levels one week later in the hippocampus and prefrontal cortex. Mice were injected s.c. once daily with phosphate-buffered saline (PBS) or Ro 25-1553 (0.07 nmol/g) during P1-14. One week after the last injection, the hippocampus and prefrontal cortex were dissected, and then PSD-95 and synaptophysin (SYN) expression was analyzed by Western Blot. Expression levels were normalized to β-actin and are presented as fold change relative to control PBS results. The results are expressed as the mean ± SEM of 3 mice/group. *P < 0.05 compared with the PBS-treated group.
Effects of the VPAC2 receptor activation during early postnatal development on behaviors of mice in adulthood
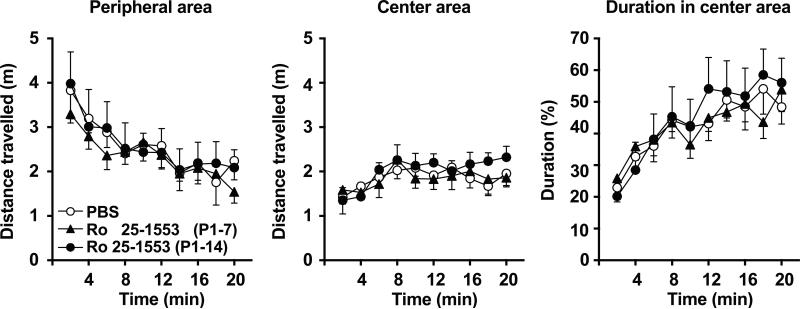
To investigate the effects of the VPAC2 receptor activation during early postnatal development on behaviors of mice in adulthood, mice were injected s.c. once daily with either Ro 25-1553 (0.07 nmol/g) or PBS from P1 to P7 or P1 to P14, and then behavioral analyses were carried out at 3–4 months of age. Fig. 2 shows the effects of Ro 25-1553 on the behaviors in the open-field test. The test provides a unique opportunity to systematically assess novel environment exploration, general locomotor activity, and provide an initial screen for anxiety-related behavior in rodents (Prut and Belzung 2003). Ro 25-1553 (P1-7 and P1-14)-treated mice and PBS-treated control mice had similar levels of locomotion and exhibited a typical pattern of habituation. There was no difference in the distance traveled in the peripheral area or center area between Ro 25-1553 (P1-7 and P1-14)-treated mice and PBS-treated mice. In addition, there was no difference in the time spent in the inner area of the open field between groups.
Fig. 2.
Effects of early postnatal treatment with the VPAC2 receptor agonist Ro 25-1553 on open-field behavior determined at three to four months of age. Each mouse was placed individually into a clear Plexiglas chamber (27.5 cm × 27.5 cm × 20.5 cm) and the behavior was recorded for 20 min. The distance traveled in the peripheral and center area and the time spent in the center area are calculated. The results are expressed as the mean ± SEM of 8 mice/group.
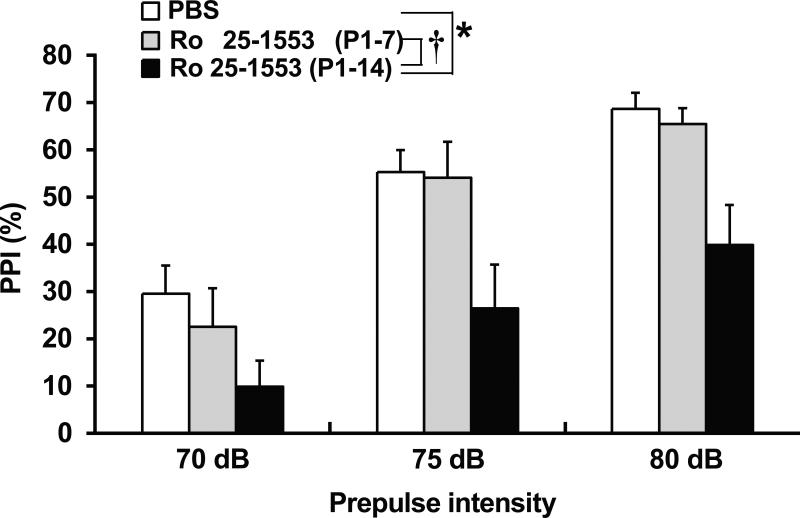
Fig. 3 shows the effects of Ro 25-1553 on PPI of the acoustic startle response in mice. PPI of the acoustic startle response was significantly less in mice treated with Ro 25-1553 during P1 to P14 than in PBS-treated mice and Ro 25-1553 (P1-7)-treated mice. Repeated measures two-way ANOVA revealed the significant main effects of the prepulse intensity [F2, 42 = 107.6, P < 0.0001] and treatment [F2, 21 = 4.6, P < 0.05], but there was no significant interaction between prepulse intensity and treatment. There was no difference in the baseline startle response between groups.
Fig. 3.
Effects of early postnatal treatment with the VPAC2 receptor agonist Ro 25-1553 on PPI of the acoustic startle response in mice. The percentage of PPI at each prepulse intensity is shown. The results are expressed as the mean ± SEM of 8 mice/group. *P < 0.05 compared with the PBS-treated group. †P < 0.05 compared with the Ro 25-1553 (P1-7)-treated group.
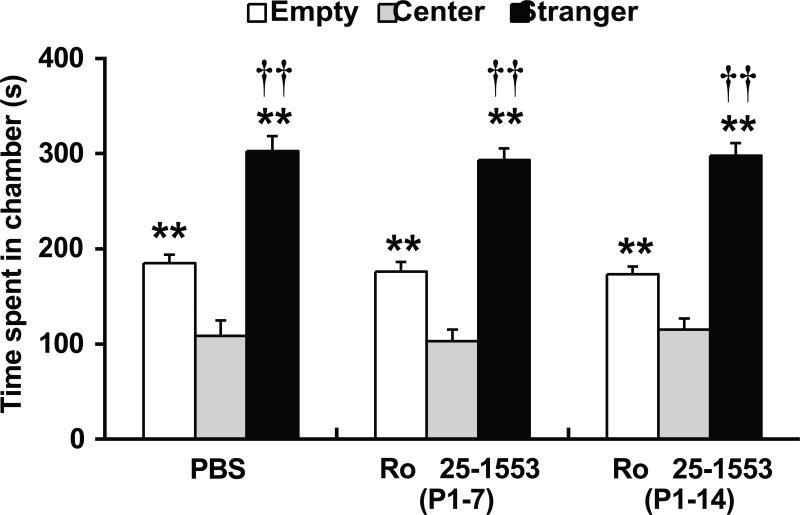
Fig. 4 shows the effects of Ro 25-1553 on performance in the three-chamber social interaction test. Test subjects spent more time in the chamber containing the unfamiliar mouse than in the empty side. Subjects generally spent more time in either side of the apparatus than in the middle chamber. Repeated measures ANOVA revealed the significant effect of side on duration [F2, 42 = 127.4, P < 0.0001]. However, there was no difference in the side preferences between Ro 25-1553 (P1-7 and P1-14)- and PBS-treated mice.
Fig. 4.
Effects of early postnatal treatment with the VPAC2 receptor agonist Ro 25-1553 development on performance in the three-chamber social interaction test. After habituation, a mouse was placed in the central chamber of a clear Plexiglas box (41 cm × 60 cm × 23.5 cm) divided into three interconnected chambers and was given the choice to interact with either an empty wire cup (Empty) or a similar wire cup with an unfamiliar mouse (Stranger). The amount of time spent in each of the three chambers was recorded for the test mouse in a 10-min. The results are expressed as the mean ± SEM of 8 mice/group. **P < 0.01 compared with the middle chamber. ††P < 0.01 compared with the empty side.
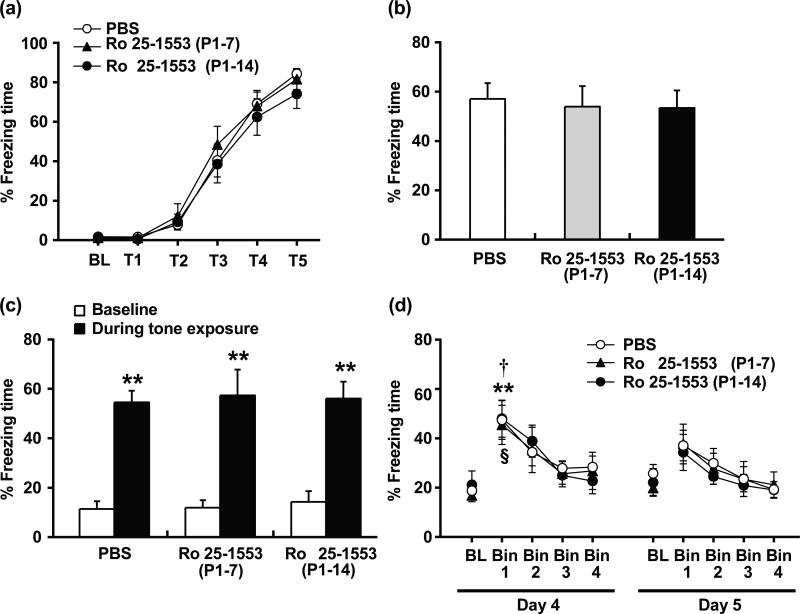
Fig. 5 shows the effects of Ro 25-1553 on memory formation, retention and extinction in the fear conditioning test. During the training phase of fear conditioning (Day 1), all groups had low levels of freezing before training began (Baseline) and froze about 80% of the time following the training session [F5, 105 = 122.6, P < 0.0001] (Fig. 5a). There was no significant difference in the acquisition between Ro 25-1553 (P1-7 and P1-14)-treated mice and PBS-treated mice. After the conditioning day, we performed the contextual fear test on Day 2 (Fig. 5b) and the tone-cued fear test on Day 3 (Fig. 5c). There were no significant differences in contextual freezing responses under context A, or in tone-cued freezing responses under context B between groups. For the 1st cued extinction on Day 4, freezing behaviors of mice were gradually decreased and then reached a similar level as the baseline (Fig. 5d). There was no significant difference in freezing responses between groups. On 2nd extinction test (Day 5), cued tone stimulation no longer caused a significant increase in freezing responses.
Fig. 5.
Effects of early postnatal treatment with the VPAC2 receptor agonist Ro 25-1553 on memory formation, retention and extinction in the fear conditioning test. (a) Acquisition. BL; baseline, T; tone. (b) Contextual fear test. (c) Tone-cued fear test. **P < 0.01 compared with the baseline. (d) Cued fear extinction. BL; baseline. **P < 0.01 compared with the baseline in the PBS-treated group. †P < 0.05 compared with the baseline in the Ro 25-1553 (P1-7)-treated group. §P < 0.05 compared with the baseline in the Ro 25-1553 (P1-14)-treated group. The results are expressed as the mean ± SEM of 8 mice/group.
Discussion
We have previously shown that VIPR2 gene expression in the developing mouse brain displays a pronounced peak at postnatal day (P) 12 (Waschek et al. 1996), a time of active synaptogenesis, pruning and myelination (O'Kusky et al. 2000; Paolicelli et al. 2011; Ye et al. 2002). The present study first demonstrated that repeated administration of a VPAC2 receptor agonist Ro 25-1553 during P1-14 decreased expression of synaptic marker proteins PSD-95 and synaptophysin in the prefrontal cortex, but not hippocampus, of mice at 3-weeks of age, and a selective effect on PPI measured at three to four months of age.
The selective reduction in PPI observed here with Ro 25-1553 would appear to contrast with a previous study in which a similar treatment regimen with PACAP, acts on three different receptors, VPAC1, VPAC2, and PAC1, resulted in multiple neurodevelopmental alterations (Reglödi et al. 2003). However, endpoints in that study were determined from birth to three weeks of age only, so it uncertain what behavioral alterations would have been observed in adults. Several lines of evidence indicate that Ro 25-1553 is highly selective for the VPAC2 receptor (Gourlet et al. 1997; Harmar et al. 2012). For example, in addition to results from classical ligand binding studies on cultured cells expressing the receptor, an autoradiographic study showed that specific binding of [125I]-Ro 25-1553 was detectable in tissues known to express the receptor, including the suprachiasmatic nuclei (SCN), in wild-type mice, but not in Vipr2 null mice (Harmar et al. 2002). Furthermore, application of Ro 25-1553, like VIP, triggers a slow inward current in a subpopulation of SCN neurons from wild-type, but not Vipr2 null, mice (Pakhotin et al. 2006). Our findings suggest that overactivation of the VPAC2 receptor during early postnatal development causes synaptic alterations in the prefrontal cortex of infant mice. Interestingly, Zupan et al. (2000) showed that treatment of late gestation mice with a VIP/neurotensin hybrid peptide (a putative non-selective VIP receptor antagonist) resulted in increased densities of synaptophysin and N-methyl-d-aspartate receptors and expression of microtubule-associated protein-5 and neurofilament 160 kD in the neocortex of offspring at P12 and 45, suggesting abnormal synaptogenesis. Previous studies have also shown the physiological role of PACAP signaling in synaptic development and plasticity in the central nervous system (Allais et al. 2007; Girard et al. 2004; Matsuyama et al. 2003; Otto et al. 2001). Of note, present findings provide the first evidence that modulation of the VPAC2 receptor signaling affects synaptic maturation. Synaptic abnormalities have been proposed as a cause of various sensory processing impairments associated with schizophrenia (Faludi and Mirnics 2011; McGlashan and Hoffman 2000). Reduced PSD-95 and synaptophysin protein levels in cortical regions of schizophrenic patients are observed (Glantz and Lewis 1997; Kristiansen et al. 2006) and in situ hybridization study also shows that PSD-95 mRNA expression was decreased in Brodmann area 9 of the prefrontal cortex, but not in the hippocampus (Ohnuma et al. 2000). These findings prompt us to examine the effect of activation of the VPAC2 receptor during early postnatal development on behaviors of mice in adulthood.
Among behaviors tested here, we observed that Ro 25-1553 treatment during P1-14 selectively caused a PPI deficit without any effects on the baseline startle response. On the other hand, Ro 25-1553 treatment during P1-7 was insufficient to affect prepulse inhibition. We previously demonstrated that expression of the VPAC2 receptor was very low in mouse brain at P0 and P6, but was then rapidly induced, with a pronounced peak at P12 (Waschek et al. 1996). PPI of startle is an operational measure of the preattentive filtering process known as sensorimotor gating, and abnormalities in preattentive information processing may be predictive of or lead to complex cognitive deficits in schizophrenia (Braff et al. 1999; Swerdlow et al. 2006). Animal models of PPI deficits have been investigated to gain a greater understanding of the underlying pathological mechanisms involved in schizophrenia. Prenatal lipopolysaccharide exposure was reported to induce a transient reduction of synaptophysin expression in the frontal cortex (measured at three weeks of age) and a deficit in PPI that persisted throughout adult life in mice (Romero et al. 2010). While we did not measure synaptic proteins in adult mice, our finding are in agreement that PPI deficits can be associated with alterations in synaptic proteins at a time when neuronal microcircuits are forming. Decreased PSD-95 protein expression has also been found in the prefrontal cortex of isolation-reared female rats, an animal model of neuropsychiatric disorders that include features reminiscent of anxiety- and schizophrenia-like disorders including PPI deficits (Hermes et al. 2011). Interestingly, early postnatal social isolation was shown to impair dendritic arborization in the prefrontal cortex, and was accompanied by increase in the number of VIP-immunoreactive neurons (Pascual et al. 2006). Our findings suggest that activation of the VPAC2 receptor during early postnatal development in mice leads to a long-term and selective disruption of PPI associated with changes in prefrontal synaptic protein expression.
Social withdrawal is a classical disabling feature of schizophrenia and ASD which has been modeled in mice (Hanks et al. 2013; Silverman et al. 2010). Furthermore, mice with a homozygous PSD-95 gene deletion (Dlg4–/–) show a complex phenotype reminiscent of autism, which includes increased repetitive behaviors, abnormal communication and social behaviors, increased stress- and anxiety-related responses (Feyder et al. 2010). However, Ro 25-1553 treatment during early postnatal development did not affect the behaviors in the open-field test or sociability in the three-chamber social interaction test. It has been proposed that impaired emotional function plays an important role in the negative symptoms of schizophrenia, and that this impairment arises from a disruption of the basic neural circuitry that underlies encoding, modulating, and retrieving emotional memories. The present study examined the effect of activation of the VPAC2 receptor during early postnatal development on fear learning (conditioning, retrieval, and extinction), alterations of which represent an endophenotype that has been documented in schizophrenic patients and recapitulated in multiple mouse models of schizophrenia (Amann et al. 2010; Holt et al. 2009, 2012). However, early postnatal treatment with Ro 25-1553 did not affect the fear memory functions examined here.
Rare copy number variants (CNVs) have a prominent role in the etiology of schizophrenia, ASD and other neuropsychiatric disorders (Sebat et al. 2009). Recent studies have indicated that duplication of the VIPR2 gene confers the susceptibility to schizophrenia (Levinson et al. 2011; Vacic et al. 2011; Yuan et al. 2014). Lymphocytes from patients with these microduplications exhibited higher VIPR2 gene expression and VIP responsiveness, indicating the overactivity of the VPAC2 receptor signaling. The present study showed that activation of the VPAC2 receptor by Ro 25-1553 during early postnatal development causes alterations in the expression of synaptic proteins in the prefrontal cortex and leads to a disruption in PPI. These findings implicate a potential pathological role of VPAC2 receptor overactivation during early development, providing pharmacological support for the hypothesis that VIPR2 duplications increase the risk of schizophrenia due to overactivity of VPAC2 signaling in the brain at a critical stage of brain development.
Acknowledgments
We thank Dr. Felix E. Schweizer (UCLA) and Dr. Thomas J. O'Dell (UCLA) for their kind gifts of anti-synaptophysin and anti-PSD-95 antibodies, respectively. This study was supported in part by grants from Simons Foundation, Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers (Grant No. S2603, Japan) and National Institutes of Health (MH098506 and HD04612).
Footnotes
Conflicts of interest
The authors state no conflicts of interest.
References
- Allais A, Burel D, Isaac ER, Gray SL, Basille M, Ravni A, Sherwood NM, Vaudry H, Gonzalez BJ. Altered cerebellar development in mice lacking pituitary adenylate cyclase-activating polypeptide. Eur J Neurosci. 2007;25:2604–2618. doi: 10.1111/j.1460-9568.2007.05535.x. [DOI] [PubMed] [Google Scholar]
- Amann LC, Gandal MJ, Halene TB, Ehrlichman RS, White SL, McCarren HS, Siegel SJ. Mouse behavioral endophenotypes for schizophrenia. Brain Res Bull. 2010;83:147–161. doi: 10.1016/j.brainresbull.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am J Psychiatry. 1999;156:596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- Charitidi K, Meltser I, Canlon B. Estradiol treatment and hormonal fluctuations during the estrous cycle modulate the expression of estrogen receptors in the auditory system and the prepulse inhibition of acoustic startle response. Endocrinology. 2012;153:4412–4421. doi: 10.1210/en.2012-1416. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Cairns NJ, Harrison PJ. Synaptophysin gene expression in schizophrenia. Investigation of synaptic pathology in the cerebral cortex. Br J Psychiatry. 2000;176:236–242. doi: 10.1192/bjp.176.3.236. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A, Chafai M, Vaudry D, Basille M, Cazillis M, Aubert N, Louiset E, de Jouffrey S, Le Bigot JF, Fournier A, Gressens P, Rostène W, Vaudry H, Gonzalez BJ. The neuropeptide pituitary adenylate cyclase-activating polypeptide exerts anti-apoptotic and differentiating effects during neurogenesis: focus on cerebellar granule neurones and embryonic stem cells. J Neuroendocrinol. 2007;19:321–327. doi: 10.1111/j.1365-2826.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- Faludi G, Mirnics K. Synaptic changes in the brain of subjects with schizophrenia. Int J Dev Neurosci. 2011;29:305–309. doi: 10.1016/j.ijdevneu.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyder M, Karlsson RM, Mathur P, Lyman M, Bock R, Momenan R, Munasinghe J, Scattoni ML, Ihne J, Camp M, Graybeal C, Strathdee D, Begg A, Alvarez VA, Kirsch P, Rietschel M, Cichon S, Walter H, Meyer-Lindenberg A, Grant SG, Holmes A. Association of mouse Dlg4 (PSD-95) gene deletion and human DLG4 gene variation with phenotypes relevant to autism spectrum disorders and Williams' syndrome. Am J Psychiatry. 2010;167:1508–1517. doi: 10.1176/appi.ajp.2010.10040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GD, Yazdi RD, Khan AH, Lusis AJ, Davis RC, Smith DJ. A genome-wide panel of congenic mice reveals widespread epistasis of behavior quantitative trait loci. Mol Psychiatry. 2009;14:631–645. doi: 10.1038/mp.2008.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani C, Gallo V. Inhibition of cyclin E-cyclin-dependent kinase 2 complex formation and activity is associated with cell cycle arrest and withdrawal in oligodendrocyte progenitor cells. J Neurosci. 2001;21:1274–1282. doi: 10.1523/JNEUROSCI.21-04-01274.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Keller ET, Schutz KC, May V, Braas KM. Pituitary adenylate cyclase activating polypeptide and PAC1 receptor signaling increase Homer 1a expression in central and peripheral neurons. Regul Pept. 2004;123:107–116. doi: 10.1016/j.regpep.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54:943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vertongen P, Vandermeers A, Vandermeers-Piret MC, Rathe J, De Neef P, Waelbroeck M, Robberecht P. The long-acting vasoactive intestinal polypeptide agonist RO 25-1553 is highly selective of the VIP2 receptor subclass. Peptides. 1997;18:403–408. doi: 10.1016/s0196-9781(96)00322-1. [DOI] [PubMed] [Google Scholar]
- Hanks AN, Dlugolenski K, Hughes ZA, Seymour PA, Majchrzak MJ. Pharmacological disruption of mouse social approach behavior: relevance to negative symptoms of schizophrenia. Behav Brain Res. 2013;252:405–414. doi: 10.1016/j.bbr.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Harmar AJ. An essential role for peptidergic signalling in the control of circadian rhythms in the suprachiasmatic nuclei. J Neuroendocrinol. 2003;15:335–338. doi: 10.1046/j.1365-2826.2003.01005.x. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP, Wank SA, Waschek JA. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Fahrenkrug J, Gozes I, Laburthe M, May V, Pisegna JR, Vaudry D, Vaudry H, Waschek JA, Said SI. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br J Pharmacol. 2012;166:4–17. doi: 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC2 receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Hattori T, Baba K, Matsuzaki S, Honda A, Miyoshi K, Inoue K, Taniguchi M, Hashimoto H, Shintani N, Baba A, Shimizu S, Yukioka F, Kumamoto N, Yamaguchi A, Tohyama M, Katayama T. A novel DISC1-interacting partner DISC1-Binding Zinc-finger protein: implication in the modulation of DISC1-dependent neurite outgrowth. Mol Psychiatry. 2007;12:398–407. doi: 10.1038/sj.mp.4001945. [DOI] [PubMed] [Google Scholar]
- Hermes G, Li N, Duman C, Duman R. Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav. 2011;104:354–359. doi: 10.1016/j.physbeh.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of neural responses to safety cues in schizophrenia. Arch Gen Psychiatry. 2012;69:893–903. doi: 10.1001/archgenpsychiatry.2011.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC. Extinction memory is impaired in schizophrenia. Biol Psychiatry. 2009;65:455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Itri J, Colwell CS. Regulation of inhibitory synaptic transmission by vasoactive intestinal peptide (VIP) in the mouse suprachiasmatic nucleus. J Neurophysiol. 2003;90:1589–1597. doi: 10.1152/jn.00332.2003. [DOI] [PubMed] [Google Scholar]
- Jacobs NS, Cushman JD, Fanselow MS. The accurate measurement of fear memory in Pavlovian conditioning: Resolving the baseline issue. J Neurosci Methods. 2010;190:235–239. doi: 10.1016/j.jneumeth.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karson CN, Mrak RE, Schluterman KO, Sturner WQ, Sheng JG, Griffin WS. Alterations in synaptic proteins and their encoding mRNAs in prefrontal cortex in schizophrenia: a possible neurochemical basis for ‘hypofrontality’. Mol Psychiatry. 1999;4:39–45. doi: 10.1038/sj.mp.4000459. [DOI] [PubMed] [Google Scholar]
- Kristiansen LV, Beneyto M, Haroutunian V, Meador-Woodruff JH. Changes in NMDA receptor subunits and interacting PSD proteins in dorsolateral prefrontal and anterior cingulate cortex indicate abnormal regional expression in schizophrenia. Mol Psychiatry. 2006;11:737–747. doi: 10.1038/sj.mp.4001844. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, Zhang N, Mowry BJ, Olincy A, Amin F, Cloninger CR, Silverman JM, Buccola NG, Byerley WF, Black DW, Kendler KS, Freedman R, Dudbridge F, Pe'er I, Hakonarson H, Bergen SE, Fanous AH, Holmans PA, Gejman PV. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Matsumoto A, Hashimoto H, Shintani N, Baba A. Impaired long-term potentiation in vivo in the dentate gyrus of pituitary adenylate cyclase-activating polypeptide (PACAP) or PACAP type 1 receptor-mutant mice. Neuroreport. 2003;14:2095–2098. doi: 10.1097/00001756-200311140-00017. [DOI] [PubMed] [Google Scholar]
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Nakamachi T, Farkas J, Watanabe J, Ohtaki H, Dohi K, Arata S, Shioda S. Role of PACAP in neural stem/progenitor cell and astrocyte: from neural development to neural repair. Curr Pharm Des. 2011;17:973–984. doi: 10.2174/138161211795589346. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC.: 1996. [Google Scholar]
- Nelson KB, Grether JK, Croen LA, Dambrosia JM, Dickens BF, Jelliffe LL, Hansen RL, Phillips TM. Neuropeptides and neurotrophins in neonatal blood of children with autism or mental retardation. Ann Neurol. 2001;49:597–606. [PubMed] [Google Scholar]
- Niewiadomski P, Zhujiang A, Youssef M, Waschek JA. Interaction of PACAP with Sonic hedgehog reveals complex regulation of the hedgehog pathway by PKA. Cell Signal. 2013;25:2222–2230. doi: 10.1016/j.cellsig.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma T, Kato H, Arai H, Faull RL, McKenna PJ, Emson PC. Gene expression of PSD95 in prefrontal cortex and hippocampus in schizophrenia. Neuroreport. 2000;11:3133–3137. doi: 10.1097/00001756-200009280-00019. [DOI] [PubMed] [Google Scholar]
- O'Kusky JR, Ye P, D'Ercole AJ. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J Neurosci. 2000;20:8435–8442. doi: 10.1523/JNEUROSCI.20-22-08435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, Gröne HJ, Kellendonk C, Tronche F, Maldonado R, Lipp HP, Konnerth A, Schütz G. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci. 2001;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakhotin P, Harmar AJ, Verkhratsky A, Piggins H. VIP receptors control excitability of suprachiasmatic nuclei neurones. Pflugers Arch. 2006;452:7–15. doi: 10.1007/s00424-005-0003-z. [DOI] [PubMed] [Google Scholar]
- Pascual R, Zamora-León SP, Valero-Cabré A. Effects of postweaning social isolation and re-socialization on the expression of vasoactive intestinal peptide (VIP) and dendritic development in the medial prefrontal cortex of the rat. Acta Neurobiol Exp (Wars) 2006;66:7–14. doi: 10.55782/ane-2006-1582. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Reglıdi D, Kiss P, Tamás A, Lengvári I. The effects of PACAP and PACAP antagonist on the neurobehavioral development of newborn rats. Behav Brain Res. 2003;140:131–139. doi: 10.1016/s0166-4328(02)00289-9. [DOI] [PubMed] [Google Scholar]
- Romero E, Guaza C, Castellano B, Borrell J. Ontogeny of sensorimotor gating and immune impairment induced by prenatal immune challenge in rats: implications for the etiopathology of schizophrenia. Mol Psychiatry. 2010;15:372–383. doi: 10.1038/mp.2008.44. [DOI] [PubMed] [Google Scholar]
- Sebat J, Levy DL, McCarthy SE. Rare structural variants in schizophrenia: one disorder, multiple mutations; one mutation, multiple disorders. Trends Genet. 2009;25:528–535. doi: 10.1016/j.tig.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J, Lu N, Nicot A, Tatsuno I, DiCicco-Bloom E. PACAP is an anti-mitogenic signal in developing cerebral cortex. Nat Neurosci. 2001;4:123–124. doi: 10.1038/83936. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function. Arch Gen Psychiatry. 2006;63:1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, Makarov V, Yoon S, Bhandari A, Corominas R, Iakoucheva LM, Krastoshevsky O, Krause V, Larach-Walters V, Welsh DK, Craig D, Kelsoe JR, Gershon ES, Leal SM, Dell Aquila M, Morris DW, Gill M, Corvin A, Insel PA, McClellan J, King MC, Karayiorgou M, Levy DL, DeLisi LE, Sebat J. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;471:499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Koonce C, Manley K, Frye CA. Proestrous compared to diestrous wildtype, but not estrogen receptor beta knockout, mice have better performance in the spontaneous alternation and object recognition tasks and reduced anxiety-like behavior in the elevated plus and mirror maze. Behav Brain Res. 2009;196:254–260. doi: 10.1016/j.bbr.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschek JA, Ellison J, Bravo DT, Handley V. Embryonic expression of vasoactive intestinal peptide (VIP) and VIP receptor genes. J Neurochem. 1996;66:1762–1765. doi: 10.1046/j.1471-4159.1996.66041762.x. [DOI] [PubMed] [Google Scholar]
- Waschek JA. VIP and PACAP receptor-mediated actions on cell proliferation and survival. Ann N Y Acad Sci. 1996;805:290–300. doi: 10.1111/j.1749-6632.1996.tb17491.x. [DOI] [PubMed] [Google Scholar]
- Yamada K, Matsuzaki S, Hattori T, Kuwahara R, Taniguchi M, Hashimoto H, Shintani N, Baba A, Kumamoto N, Yamada K, Yoshikawa T, Katayama T, Tohyama M. Increased stathmin1 expression in the dentate gyrus of mice causes abnormal axonal arborizations. PLoS One. 2010;5:e8596. doi: 10.1371/journal.pone.0008596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Li L, Richards RG, DiAugustine RP, D'Ercole AJ. Myelination is altered in insulin-like growth factor-I null mutant mice. J Neurosci. 2002;22:6041–6051. doi: 10.1523/JNEUROSCI.22-14-06041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Jin C, Sha W, Zhou Z, Zhang F, Wang M, Wang J, Li J, Feng X, Yu S, Wang J. A competitive PCR assay confirms the association of a copy number variation in the VIPR2 gene with schizophrenia in Han Chinese. Schizophr Res. 2014;156:66–70. doi: 10.1016/j.schres.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Zelikowsky M, Hast TA, Bennett RZ, Merjanian M, Nocera NA, Ponnusamy R, Fanselow MS. Cholinergic blockade frees fear extinction from its contextual dependency. Biol Psychiatry. 2013;73:345–352. doi: 10.1016/j.biopsych.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zupan V, Nehlig A, Evrard P, Gressens P. Prenatal blockade of vasoactive intestinal peptide alters cell death and synaptic equipment in the murine neocortex. Pediatr Res. 2000;47:53–63. doi: 10.1203/00006450-200001000-00012. [DOI] [PubMed] [Google Scholar]