Abstract
Objective
To examine the use and safety of rifampin in hospitalized infants.
Study Design
Observational study of clinical and laboratory adverse events among infants exposed to rifampin from 348 neonatal intensive care units managed by the Pediatrix Medical Group between 1997 and 2012.
Result
2500 infants received 4279 courses of rifampin; mean gestational age was 27 weeks (5th, 95th %tile; 23, 36) and mean birth weight was 1125 g (515, 2830). Thrombocytopenia (121/1000 infant days) and conjugated hyperbilirubinemia (25/1000 infant days) were the most common laboratory adverse events. The most common clinical adverse events were medical necrotizing enterocolitis (64/2500 infants, 3%) and seizure (60/2500 infants, 2%).
Conclusion
The overall incidence of adverse events among infants receiving rifampin appears low; however, additional studies to further evaluate safety and dosing of rifampin in this population are needed.
Keywords: rifampin, broad-spectrum antibiotic, infectious disease, neonatal intensive care unit
Introduction
Rifampin is a broad-spectrum antibiotic used for a variety of infections in children.1 Possessing excellent anti-staphylococcal activity, rifampin, when added to an anti-staphylococcal penicillin or vancomycin, is effective in the treatment of refractory cases of bacteremia due to coagulase-negative staphylococci (CoNS) in infants.2–5 Rifampin is also used as adjunctive therapy for infections of ventriculoperitoneal (VP) shunts with staphylococcal organisms in infants.6–9 CoNS and Staphylococcus aureus are the most common causes of sepsis in the neonatal intensive care unit (NICU).10 The use of rifampin as adjunctive therapy is a logical consideration for these infections10; therefore, understanding its safety profile in this population is important.
Adverse events (AEs) reported with rifampin use include hepatotoxicity, renal failure, rash, and hematological abnormalities including thrombocytopenia.1,11 However, few data exist on its safety in infants. Rifampin is not labeled by the Food and Drug Administration (FDA) for infants <3 months of age.12 The largest previous report of rifampin use in the neonatal population was a retrospective analysis of 137 premature infants that examined clearance of bacteremia and C-reactive protein levels, but this study did not report safety outcomes.4 Here, we describe the use of rifampin among a large cohort of hospitalized infants and the incidence of clinical and laboratory AEs associated with its use.
Methods
Data source and definitions
We identified all infants discharged from 348 NICUs managed by the Pediatrix Medical Group between 1997 and 2012 exposed to rifampin in the first 120 days of life. We excluded infants with major congenital anomalies. Data were obtained from a database that captures electronic medical record information from clinicians on all infants cared for by the Pediatrix Medical Group. Data collected include: maternal history, demographics, medications, laboratory results, microbiology results, and diagnoses. Dosing and dosing intervals were not available in the data.
We defined a rifampin course as uninterrupted days of exposure to rifampin. We classified rifampin courses as associated with a positive culture if a culture (blood, urine collected by in-and-out catheterization or suprapubic tap, or cerebrospinal fluid [CSF]) was positive on days of exposure to rifampin or up to 5 days prior to the first day of rifampin exposure. Multiple positive cultures for the same organism within a 21-day period were considered a single infectious episode. We defined CoNS sepsis as 2 positive cultures for CoNS within a 4-day period, 3 positive cultures for CoNS within a 7-day period, or 4 positive cultures for CoNS within a 10-day period. We excluded positive cultures from organisms considered contaminants, including non-speciated streptococci, Bacillus spp., gram-positive rods (not including Listeria spp.), Lactobacillus spp., Micrococcus spp., Stomatococcus spp., and Bacteroides spp.
An AE was attributed to rifampin if it occurred on a day of exposure to rifampin. AEs included laboratory and clinical AEs (surgical necrotizing enterocolitis [NEC], medical NEC, focal intestinal perforation, grade III–IV intraventricular hemorrhage [IVH], seizures, rash, and pulmonary hemorrhage). Each new clinical diagnosis occurring while an infant was exposed to rifampin was counted as a separate AE. Laboratory AEs were categorized as an AE or a severe adverse event (SAE) based on pre-specified cut-off values. Each laboratory abnormality was counted as a separate AE or SAE, and was counted each day that it occurred while an infant was exposed to rifampin. We defined concomitant antibiotic use as any antibiotic administered on a day of exposure to rifampin.
Statistical analysis
We used standard summary statistics including counts and percentages and means, medians, and percentiles to describe categorical and continuous study variables, respectively. We reported laboratory AEs occurring while on rifampin as both number of days with an AE per 1000 infant days of exposure to rifampin and the proportion of courses of rifampin during which an AE was reported on at least 1 day. We reported clinical AEs as proportions occurring at both the course and infant level. All analyses were performed using Stata 12 (College Station, TX). The study was approved by the Duke University Institutional Review Board without the need for written informed consent as the data were collected without identifiers.
Results
Infant characteristics and outcomes
We identified 2500 infants who received 4279 courses of rifampin for a total of 23,701 infant days. The mean gestational age (GA) was 27 weeks (5th, 95th%tile; 23, 36), and the mean birth weight was 1125 g (515, 2830) (Table 1). Mean weight at the time of first rifampin exposure was 1518 g (650, 3380).
Table 1.
Demographics
| Infants exposed to rifampin (N=2500) | ||
|---|---|---|
| Gestational age, weeks | ||
| <26 | 888 (36%) | |
| 26–28 | 822 (33%) | |
| 29–32 | 450 (18%) | |
| 33–36 | 203 (8%) | |
| ≥37 | 137 (5%) | |
|
| ||
| Birth weight, g | ||
| <1000 | 1531 (61%) | |
| 1000–1499 | 524 (21%) | |
| 1500–2499 | 252 (10%) | |
| 2500–3499 | 140 (6%) | |
| ≥3500 | 49 (2%) | |
|
| ||
| Age at first rifampin exposure, days | ||
| <3 | 5 (0.2%) | |
| 3–6 | 8 (0.3%) | |
| 7–29 | 1634 (65%) | |
| 30–59 | 628 (25%) | |
| 60–120 | 225 (9%) | |
|
| ||
| Race/ethnicity | ||
| White | 1019 (42%) | |
| African American | 624 (26%) | |
| Hispanic | 683 (28%) | |
| Other | 95 (4%) | |
|
| ||
| Male | 1334 (53%) | |
|
| ||
| Caesarean delivery | 1771 (72%) | |
|
| ||
| Mean (5th, 95th percentile) length of NICU stay, days | 85 (20, 160) | |
|
| ||
| Died | 242 (11%) | |
Abbreviation: NICU, neonatal intensive care unit.
Microbiology
There were 1455 courses (34%) administered to 1249 infants for a total of 8874 infant days in the setting of positive cultures and 2824 courses (66%) of rifampin administered to 1251 infants for a total of 14,827 infant days in the setting of negative cultures. The majority of positive cultures were obtained from blood—1332/1380 (97%). The most commonly cultured organisms were gram-positive (1273/1380 [92%]), and CoNS was the most common pathogen (670/1380 [49%]) (Table 2). Among the infants with negative cultures, 715/1251 (57%) had either a single culture positive for CoNS or a surface culture positive for methicillin-resistant Staphylococcus aureus (MRSA).
Table 2.
Culture results for rifampin courses associated with a positive culture
| Blood N=1332 | Urine N=8 | CSF N=40 | Total N=1380 | |
|---|---|---|---|---|
| Gram-positive | ||||
| CoNS | 649 (49%) | 0 | 21 (53%) | 670 (49%) |
| S. aureus | 477 (36%) | 0 | 13 (33%) | 490 (36%) |
| Other* | 105 (8%) | 3 (38%) | 5 (13%) | 113 (8%) |
| Gram-negative | ||||
| E. coli | 3 (0.2%) | 1 (13%) | 0 | 4 (0.3%) |
| Other† | 40 (3%) | 0 | 0 | 40 (3%) |
| Fungus | 41 (3%) | 4 (50%) | 0 | 45 (3%) |
| Other‡ | 17 (1%) | 0 | 1 (3%) | 18 (1%) |
Abbreviations: CSF, cerebrospinal fluid; CoNS, coagulase-negative staphylococci; S. aureus, Staphylococcus aureus; E. coli, Escherichia coli.
Reported organisms included Enterococcus and group B Streptococcus; also includes those reported as gram-positive cocci for which speciation was not available.
Reported organisms include Acinetobacter, Citrobacter, Enterobacter, Klebsiella, Serratia, and Pseudomonas; also includes those reported as gram-negative rods for which speciation was not available.
Organism not clearly specified or not clearly categorized (e.g., Mycoplasma).
Nineteen infants (0.8%) with VP shunts received 40 courses (0.9%) of rifampin for a total of 4270 infant days. There were 15 positive cultures obtained in these infants; 6/15 (40%) of these were from blood, and 9/15 (60%) from the CSF. All 9 positive CSF cultures grew gram-positive organisms, while the 6 positive blood cultures included 2 gram-positive, 2 gram-negative, and 2 fungal organisms.
The mean duration of a rifampin treatment course was 7 days (1, 17) in those infants with positive blood cultures and 9 days (1, 28) in those with positive CSF cultures. Vancomycin was the most common concomitantly administered antibiotic, followed by penicillins and cephalosporins (Table 3).
Table 3.
Concomitant antibiotics administered
| Days of concomitant rifampin exposure (N=23,701) | |
|---|---|
| Vancomycin | 18,270 (77%) |
| Penicillins | 4175 (18%) |
| Cephalosporins | 2425 (10%) |
| Linezolid | 734 (3%) |
| Clindamycin | 702 (3%) |
| Carbapenems | 628 (3%) |
| Trimethoprim/sulfamethoxazole | 134 (1%) |
| Daptomycin | 29 (0.1%) |
Safety
Laboratory AEs and SAEs were observed on 249 per 1000 infant days and 53 per 1000 infant days, respectively (Table 4). The majority of rifampin courses had at least 1 laboratory AE (3493/4279 [82%]). Laboratory SAEs occurred in a lower proportion of courses (1782/4279 [42%]). The most common electrolyte abnormality was hyperkalemia (19/1000 infant days). Conjugated hyperbilirubinemia was the most common laboratory AE associated with liver dysfunction (25/1000 infant days). Renal laboratory AEs, including elevated blood urea nitrogen (BUN) or creatinine, occurred on 13/1000 infant days, with SAEs occurring at 5 per 1000 infant days. On the 22,548/27,979 (81%) days that vancomycin and rifampin were given concomitantly, the incidence of elevated creatinine as an AE was similar to the days where rifampin was given without concomitant vancomycin at 8.4/1000 infant days and 7.2/1000 infant days, respectively. Leukocytosis (61/1000 infant days) and thrombocytopenia (121/1000 infant days) were the most common complete blood count abnormalities (Table 4). Clinical AEs occurred in 197/4279 (8%) of infants (Table 5). The most commonly observed clinical AEs were seizure, medical NEC, and IVH (Table 5).
Table 4.
Laboratory adverse events and serious adverse events
| Adverse events | Serious adverse events | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Courses, n (%) N=4279 | Days (/1000 infant days) | Courses, n (%) N=4279 | Days (/1000 infant days) | |||
| Serum electrolytes | ||||||
| Hyperglycemia | > 250 mg/dL | 145 (3%) | 1 | > 400 mg/dL | 7 (0.2%) | 0 |
| Hypoglycemia | < 40 mg/dL | 345 (8%) | 5 | < 20 mg/dL | 74 (2%) | 0.8 |
| Hypernatremia | > 150 mmol/L | 215 (5%) | 3 | > 160 mmol/L | 10 (0.2%) | 0.1 |
| Hyponatremia | < 125 mmol/L | 321 (8%) | 4 | < 115 mmol/L | 10 (0.2%) | 0.1 |
| Hyperkalemia | > 6 mmol/L | 1069 (25%) | 19 | > 7.5 mmol/L | 135 (3%) | 2 |
| Hypokalemia | < 3 mmol/L | 504 (12%) | 10 | < 2.5 mmol/L | 123 (3%) | 2 |
| Hypercalcemia | > 12.5 mg/dL | 64 (2%) | 1 | > 13.5 mg/dL | 25 (0.6%) | 0.3 |
|
| ||||||
| Renal dysfunction | ||||||
| Elevated BUN | > 70 mg/dL | 204 (5%) | 8 | > 100 mg/dL | 58 (1%) | 2 |
| Elevated creatinine | > 1.7 mg/dL | 232 (5%) | 8 | > 3.0 mg/dL | 60 (1%) | 3 |
|
| ||||||
| Liver dysfunction | ||||||
| Elevated AST | >500 U/L | 7 (0.1%) | 0 | > 1 000 U/L | 2 (0.05%) | 0 |
| Elevated ALT | > 500 U/L | 15 (0.4%) | 0.4 | > 1 000 U/L | 2 (0.05%) | 0 |
| Elevated GGT | > 100 U/L | 178 (4%) | 4 | > 200 U/L | 81 (2%) | 2 |
| Conjugated bilirubin | > 5 mg/dL | 743 (17%) | 25 | > 10 mg/dL | 199 (5%) | 6 |
|
| ||||||
| Complete blood count | ||||||
| Leukocytosis | > 25,000/mm3 | 1567 (37%) | 61 | > 40,000/mm3 | 546 (13%) | 14 |
| Leukopenia | < 5000/mm3 | 493 (12%) | 8 | < 2 000/mm3 | 65 (2%) | 1 |
| Thrombocytopenia | < 100 000/mm3 | 2045 (48%) | 121 | < 30,000/mm3 | 606 (14%) | 11 |
| Thrombocytosis | > 600 000/mm3 | 145 (3%) | 4 | > 1,000,000/mm3 | 4 (0.09%) | 0.1 |
Abbreviations: BUN, blood urea nitrogen; AST, aspartate aminotransferase; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase.
Table 5.
Clinical adverse events associated with rifampin use
| Courses, n (%) N=4279 | Patient level, n (%) N=2500 | |
|---|---|---|
| Gastrointestinal | ||
| Necrotizing enterocolitis – medical | 133 (3%) | 64 (3%) |
| Necrotizing enterocolitis – surgical | 41 (1%) | 19 (1%) |
| Focal intestinal perforation | 3 (0.07%) | 1 (0.04%) |
| Neurologic | ||
| Intraventricular hemorrhage – grade III or IV | 91 (2%) | 36 (1%) |
| Seizure | 132 (3%) | 60 (2%) |
| Dermatologic | ||
| Rash | 33 (1%) | 15 (0.6%) |
| Pulmonary | ||
| Pulmonary hemorrhage | 50 (1%) | 22 (1%) |
| Any clinical adverse event | 426 (10%) | 197 (8%) |
Use of rifampin over time
There was an increase in rifampin use from 1997 (1/1000 infants) through 2007 (4/1000 infants) (Figure 1). The use of rifampin decreased to 1/1000 infants in 2012.
Figure 1.
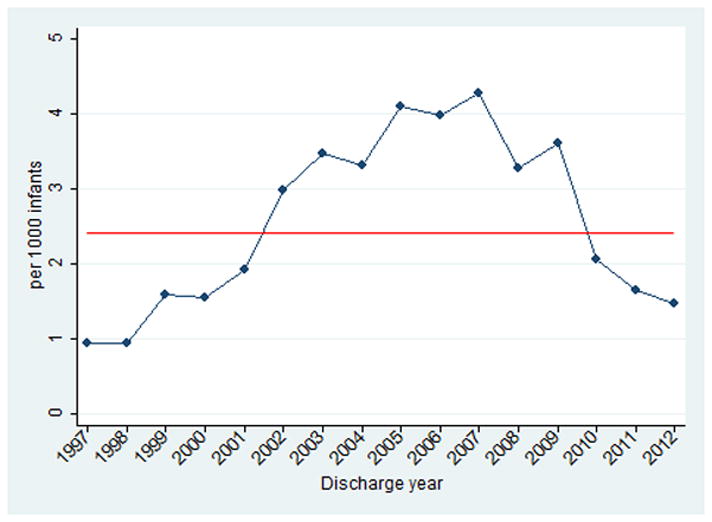
Rifampin use over time. The red line indicates the mean number of infants exposed to rifampin during the study period.
Discussion
We present the largest study of rifampin use in infants to date, with 2500 infants receiving the drug over a 15-year period. Thirty-four percent of rifampin courses were given in the setting of positive cultures. Laboratory AEs were commonly observed during rifampin exposure, with conjugated hyperbilirubinemia and thrombocytopenia having the highest incidence. In this cohort of infants receiving rifampin, clinical AEs occurred infrequently.
Rifampin is often used as adjunctive treatment for refractory gram-positive bacteremia, particularly bacteremia caused by staphylococci.2–5,13 The majority of patients in our cohort with culture-proven infection had staphylococcal bacteremia (predominantly CoNS). While our study suggests that the use of rifampin in the setting of persistently positive cultures is consistent with the limited existing evidence, there was a significant amount of rifampin use in situations where the cause of infection was less well-defined.
The use of rifampin increased from 1997 to a peak in 2007 before declining. Although the reasons for this trend are unclear, the increasing use of rifampin coincides with the emergence of community-acquired MRSA in infants, children, and adults.14,15 Similarly, the subsequent decline in rifampin use may have been influenced by an increasing emphasis on improving central line care practices, which several studies indicate has resulted in an overall decline in central line-associated blood stream infections, which are most commonly caused by gram-positive organisms.16–18 Alternatively, the decrease in rifampin use could be due to increasing attention on antibiotic stewardship programs. In our data, there was no significant change in the proportion of rifampin use in the setting of negative cultures from 2007–2012.
Laboratory AEs were common in our cohort, occurring in 249 per 1000 infant days of administration. The FDA label for rifampin recommends that liver function tests be monitored due to the potential for a transient rise in transaminases and bilirubin.12 In our cohort, the incidence of conjugated hyperbilirubinemia was high—743/4279 (17%) of courses.19 Hematologic laboratory AEs were also common in this cohort of infants. Thrombocytopenia is a known potential effect of rifampin therapy.20,21 The FDA label states that thrombocytopenia occurs primarily with high-dose, intermittent therapy and “rarely occurs with well supervised daily therapy.”12 In our cohort, thrombocytopenia occurred frequently (48% of infants). Although leukocytosis was also common, leukocytosis and thrombocytopenia are often seen in the setting of infection and may be an indicator of coexisting disease pathology rather than direct drug effect. The FDA label includes elevated BUN as a potential adverse reaction. The incidence of renal dysfunction in our cohort was relatively low, with elevated BUN and creatinine occurring in 5% of infants. As a comparison, other cohorts of infants with sepsis have reported incidences of acute kidney injury as high as 26%.22–24 The overall incidence of clinical AEs was only 10% at the course level and 8% at the patient level. Rash, included on the FDA label and commonly associated with rifampin use, was uncommon (<1%) in this cohort of hospitalized infants.
This study has important limitations. Because this is an observational study, we are limited to identifying associations rather than assessing causal relationships. Identification of clinical AEs was limited to what was documented by the clinicians, and laboratory AEs may be affected by the frequency of laboratory draws, which occurred at the discretion of the treating clinician. In addition, attribution of AEs to rifampin is difficult because it is almost universally administered in combination with other drugs in the setting of an acutely ill infant. Finally, information about drug dose and interval was not available, thus limiting our ability to evaluate any dose-dependent effects.
Using prospectively collected data from a large, diverse, multicenter cohort of infants, we examined AEs associated with rifampin use. Our study suggests that rifampin is generally safe for use in infants, although bilirubin levels and platelets should be monitored while on therapy. Further studies are needed to determine the optimal dosing, timing, and duration of rifampin use in infants.
Acknowledgments
Sources of support: This work was funded under contract HHSN2752010000031 from the National Institute of Child Health and Human Development (NICHD) for the Pediatric Trials Network; award number 5T32-HD060558 from the NICHD; and award number 1R25-HD076475-01 from the NICHD. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award number UL1TR001117.
The Pediatric Trials Network Administrative Core Committee
Katherine Y. Berezny, Duke Clinical Research Institute, Durham, NC; Edmund Capparelli, University of California–San Diego, San Diego, CA; Michael Cohen-Wolkowiez, Duke Clinical Research Institute, Durham, NC; Gregory L. Kearns, Children’s Mercy Hospital, Kansas City, MO; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Andre Muelenaer, Virginia Tech Carilion School of Medicine, Roanoke, VA; T. Michael O’Shea, Wake Forest Baptist Medical Center, Winston Salem, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; John van den Anker, George Washington University School of Medicine and Health, Washington, DC; Kelly Wade, Children’s Hospital of Philadelphia, Philadelphia, PA; Thomas J. Walsh, Weill Cornell Medical College of Cornell University, New York, NY.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development: David Siegel, Perdita Taylor-Zapata, Anne Zajicek, Alice Pagan
The EMMES Corporation (Data Coordinating Center): Ravinder Anand, Gina Simone
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of Interest
Dr. Hornik receives salary support for research from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) (UL1TR001117). Dr. Benjamin receives support from the United States government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-05, 1K24HD058735-05, UL1TR001117, and NICHD contract HHSN275201000003I) and the nonprofit organization Thrasher Research Fund for his work in neonatal candidiasis (www.thrasherresearch.org); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr. Smith receives salary support for research from the NIH and the National Center for Advancing Translational Sciences of the NIH (HHSN267200700051C, HHSN275201000003I, and UL1TR001117); he also receives research support from industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). Dr. Ericson receives support from the National Institute of Child Health and Human Development of the NIH (5T32HD060558). The remaining authors have no potential conflicts of interest to disclose.
References
- 1.Alsayyed B. Rifampin. Pediatr Rev. 2004;25:216–217. doi: 10.1542/pir.25-6-216. [DOI] [PubMed] [Google Scholar]
- 2.Shama A, Patole SK, Whitehall JS. Intravenous rifampicin in neonates with persistent staphylococcal bacteraemia. Acta Paediatr. 2002;91:670–673. doi: 10.1080/080352502760069098. [DOI] [PubMed] [Google Scholar]
- 3.Soraisham AS, Al-Hindi MY. Intravenous rifampicin for persistent staphylococcal bacteremia in premature infants. Pediatr Int. 2008;50:124–126. doi: 10.1111/j.1442-200X.2007.02519.x. [DOI] [PubMed] [Google Scholar]
- 4.van der Lugt NM, Steggerda SJ, Walther FJ. Use of rifampin in persistent coagulase-negative staphylococcal bacteremia in neonates. BMC Pediatr. 2010;10:84. doi: 10.1186/1471-2431-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan TQ, Mason EO, Jr, Ou CN, Kaplan SL. Use of intravenous rifampin in neonates with persistent staphylococcal bacteremia. Antimicrob Agents Chemother. 1993;37:2401–2406. doi: 10.1128/aac.37.11.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bayston R. Hydrocephalus shunt infections. J Antimicrob Chemother. 1994;34 (Suppl A):75–84. doi: 10.1093/jac/34.suppl_a.75. [DOI] [PubMed] [Google Scholar]
- 7.Forward KR, Fewer HD, Stiver HG. Cerebrospinal fluid shunt infections. A review of 35 infections in 32 patients. J Neurosurg. 1983;59:389–394. doi: 10.3171/jns.1983.59.3.0389. [DOI] [PubMed] [Google Scholar]
- 8.Gombert ME, Landesman SH, Corrado ML, Stein SC, Melvin ET, Cummings M. Vancomycin and rifampin therapy for Staphylococcus epidermidis meningitis associated with CSF shunts: report of three cases. J Neurosurg. 1981;55:633–636. doi: 10.3171/jns.1981.55.4.0633. [DOI] [PubMed] [Google Scholar]
- 9.McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ. Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis. 2003;36:858–862. doi: 10.1086/368191. [DOI] [PubMed] [Google Scholar]
- 10.Chapman RL, Faix RG. Persistent bacteremia and outcome in late onset infection among infants in a neonatal intensive care unit. Pediatr Infect Dis J. 2003;22:17–21. doi: 10.1097/00006454-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien RJ, Long MW, Cross FS, Lyle MA, Snider DE., Jr Hepatotoxicity from isoniazid and rifampin among children treated for tuberculosis. Pediatrics. 1983;72:491–499. [PubMed] [Google Scholar]
- 12.Rifadin IV (rifampin for injection) package insert. Bridgewater, NJ: Sanofi-Aventis US LLC; 2013. [Google Scholar]
- 13.Bliziotis IA, Ntziora F, Lawrence KR, Falagas ME. Rifampin as adjuvant treatment of gram-positive bacterial infections: a systematic review of comparative clinical trials. Eur J Clin Microbiol Infect Dis. 2007;26:849–856. doi: 10.1007/s10096-007-0378-1. [DOI] [PubMed] [Google Scholar]
- 14.Fortunov RM, Hulten KG, Hammerman WA, Mason EO, Jr, Kaplan SL. Community-acquired Staphylococcus aureus infections in term and near-term previously healthy neonates. Pediatrics. 2006;118:874–881. doi: 10.1542/peds.2006-0884. [DOI] [PubMed] [Google Scholar]
- 15.Gerber JS, Coffin SE, Smathers SA, Zaoutis TE. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin Infect Dis. 2009;49:65–71. doi: 10.1086/599348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borghesi A, Stronati M. Strategies for the prevention of hospital-acquired infections in the neonatal intensive care unit. J Hosp Infect. 2008;68:293–300. doi: 10.1016/j.jhin.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Bizzarro MJ. Prevention of central line associated bloodstream infections in critical care units. Curr Opin Pediatr. 2011;23:85–90. doi: 10.1097/MOP.0b013e328341d1da. [DOI] [PubMed] [Google Scholar]
- 18.Schulman J, Stricof R, Stevens TP, Horgan M, Gase K, Holzman IR, et al. Statewide NICU central-line-associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127:436–444. doi: 10.1542/peds.2010-2873. [DOI] [PubMed] [Google Scholar]
- 19.Moyer V, Freese DK, Whitington PF, Olson AD, Brewer F, Colletti RB, et al. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39:115–128. doi: 10.1097/00005176-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Grosset J, Leventis S. Adverse effects of rifampin. Rev Infect Dis. 1983;5 (Suppl 3):S440–S450. doi: 10.1093/clinids/5.supplement_3.s440. [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Lee CJ. Thrombocytopenia—a rare but potentially serious side effect of initial daily and interrupted use of rifampicin. Chest. 1989;96:202–203. doi: 10.1378/chest.96.1.202. [DOI] [PubMed] [Google Scholar]
- 22.Mathur NB, Agarwal HS, Maria A. Acute renal failure in neonatal sepsis. Indian J Pediatr. 2006;73:499–502. doi: 10.1007/BF02759894. [DOI] [PubMed] [Google Scholar]
- 23.Agras PI, Tarcan A, Baskin E, Cengiz N, Gurakan B, Saatci U. Acute renal failure in the neonatal period. Ren Fail. 2004;26:305–309. doi: 10.1081/jdi-200026749. [DOI] [PubMed] [Google Scholar]
- 24.Stapleton FB, Jones DP, Green RS. Acute renal failure in neonates: incidence, etiology and outcome. Pediatr Nephrol. 1987;1:314–320. doi: 10.1007/BF00849230. [DOI] [PubMed] [Google Scholar]


