Abstract
The Notch signaling pathway regulates intestinal epithelial cell homeostasis, including stem cell maintenance, progenitor cell proliferation and differentiation. Notch1 and Notch2 receptors are expressed in the epithelium, but individual contributions to these functions are unclear. We used genetic deletion to define receptor roles on stem cell function, cell proliferation/differentiation, and repair after injury. Loss of Notch1 induced a transient secretory cell hyperplasia that spontaneously resolved over time. In contrast, deletion of Notch2 had no secretory cell effect. Compound deletions of Notch1 and Notch2 resulted in a more severe secretory cell hyperplasia than deletion of Notch1 alone. Furthermore, only double deletion of Notch1 and Notch2 decreased cell proliferation, suggesting a low threshold for maintenance of proliferation compared to differentiation. Stem cells were affected by deletion of Notch1, with reduced expression of Olfm4 and fewer LGR5+ stem cells. Deletion of Notch2 had no apparent affect on stem cell homeostasis. However, we observed impaired crypt regeneration after radiation in both Notch1- and Notch2-deleted intestine, suggesting that higher Notch activity is required post-injury. These findings suggest that Notch1 is the primary receptor regulating intestinal stem cell function and that Notch1 and Notch2 together regulate epithelial cell proliferation, cell fate determination, and post-injury regeneration.
Keywords: Notch signaling; Notch1, Notch2, crypt base columnar stem cell; Olfm4, Lgr5, goblet cell hyperplasia; cell fate determination; irradiation injury
Introduction
The continuous renewal of the intestinal epithelium is fueled by active stem cells located at the crypt base. Although intestinal stem cells have been a topic of investigation for several decades, the discovery of LGR5 as a specific marker of the active stem cell population sparked a recent expansion of this field of study (Barker et al. 2007). LGR5+ intestinal stem cells divide to produce daughters that can become stem or transit-amplifying (TA) cells depending on competition for open stem cell niche spots (Snippert et al. 2010). The TA cells are short-lived, highly proliferative progenitors that expand epithelial cell numbers and differentiate into the various epithelial cell types, including absorptive enterocytes, mucous-secreting goblet cells, antimicrobial peptide-secreting Paneth cells and hormone-secreting enteroendocrine cells. The molecular mechanisms that regulate intestinal stem cell number and function, and TA cell proliferation and differentiation to maintain overall tissue homeostasis are not well understood.
The Notch signaling pathway regulates several aspects of intestinal epithelial cell homeostasis. Notch plays a key role in regulating epithelial cell fate, with pathway activation leading to enterocyte differentiation (Fre et al. 2005; Jensen et al. 2000; Stanger et al. 2005), while pathway inhibition promotes secretory cell differentiation, including goblet, Paneth and endocrine cells (Pellegrinet et al. 2011; Riccio et al. 2008; van Es et al. 2005; Vandussen et al. 2012). Notch regulation of absorptive vs. secretory cell fate occurs by transcriptional repression of the secretory lineage transcription factor Atoh1. The expression of Atoh1 has been shown to be both required (Shroyer et al. 2007; Yang et al. 2001) and sufficient (VanDussen and Samuelson 2010) for concerted differentiation of all secretory cell types. Conversely, enterocyte differentiation results when Atoh1 expression is repressed (Shroyer et al. 2007; Yang et al. 2001). Interestingly, the sole function of Notch to regulate cell fate appears to be through regulation of Atoh1 and pathway activation is not required for enterocyte differentiation if Atoh1 is genetically deleted (Kim and Shivdasani 2011).
In addition to regulation of intestinal epithelial cell fate, Notch signaling regulates epithelial cell proliferation, with pathway activation resulting in increased proliferation (Fre et al. 2005; Stanger et al. 2005), while pathway inhibition leads to a marked reduction in overall cell proliferation in the intestine (Pellegrinet et al. 2011; Riccio et al. 2008; van Es et al. 2005; VanDussen et al. 2012). Moreover, we and others have shown that Notch signaling is necessary for stem cell proliferation and cell survival (Pellegrinet et al. 2011; VanDussen et al. 2012). Pathway inhibition resulted in reduced expression of the stem cell marker Olfm4, decreased numbers of LGR5+ stem cells and reduced stem cell proliferation (VanDussen et al. 2012).
Expression of the 4 known Notch receptors (N1-4) and 5 ligands (Dll1, 3, 4 and Jag1 and 2) is temporally and spatially controlled for proper development and homeostasis of many tissues. N1 and N2 are both expressed in the adult intestinal epithelium (Fre et al. 2011; Sander and Powell 2004; Schroder and Gossler 2002), and lineage tracing studies suggest that both receptors are expressed in stem cells (Fre et al. 2011), but individual roles for each receptor is not well understood.
Previous studies investigating N1 and N2 function utilizing humanized inhibitory antibodies suggested a role for N1 in regulating intestinal homeostasis, as anti-N1 treatment showed a mild secretory cell hyperplasia (Wu et al. 2010) and decreased intestinal proliferation, and toxicity when paired with irradiation damage (Tran et al. 2013). In contrast to these findings, genetic deletion studies reported that individual loss of N1 or N2 in the intestinal epithelium had no phenotype, and thus N1 and N2 were thought to be fully functionally redundant in the gut (Riccio et al. 2008). Due to the important therapeutic implications of intestinal Notch regulation, it is important to reconcile these disparate findings.
In this study we used a genetic deletion model to definitively show that N1 is the predominantly active Notch receptor in the intestinal epithelium, as N1 receptor deletion led to secretory cell hyperplasia and reduced stem cell number. Furthermore, we investigate the dynamic regulation of lost N1 signal and expand on the understanding of how N1 and N2 function together to regulate proliferation and differentiation in the intestine. Finally, we uncover an unexpected sensitivity to loss of either N1 or N2 in irradiated intestine with implications on therapeutic use of Notch receptor blockade.
Materials and Methods
Mice
Floxed-Notch1 (N1F/F) (Yang et al. 2004) (Jackson Lab, no. 007181), floxed-Notch2 (N2F/F) (McCright et al. 2006) (Jackson Lab, no. 010525), floxed-Rbpjk (RbpjF/F) (Tanigaki et al. 2002) (gift from T. Honjo), Villin-CreERT2 (el Marjou et al. 2004) (gift from S. Robine) and Lgr5-GFP-IRES-CreERT2 (Lgr5-GFP) (Barker et al. 2007) (Jackson Lab, no. 008875) alleles were verified by PCR genotyping with the primers listed in Supplementary Table 1. All mouse strains were on a C57BL/6 strain background. Adult mice of both sexes aged 2-4 months were analyzed for all experiments except where indicated that juvenile mice aged 10 days were used. Mice were housed in ventilated and automated watering cages with a 12-hour light cycle under specific pathogen-free conditions. Protocols for mouse usage were approved by the University of Michigan Committee on Use and Care of Animals.
Animal treatment protocols and tissue collection
To activate CreERT2-mediated recombination, mice were injected intraperitoneally with 100mg/kg tamoxifen (10mg/ml in 5% ethanol and 95% corn oil, Sigma) or corn oil once per day for 5 days and tissue was collected on day 6, unless otherwise noted. Corn oil-treated control animals were found to have no background goblet cell hyperplasia (data not shown). Juvenile mice were injected at 10 days of age via the same protocol except with 20mg/ml tamoxifen to achieve 100mg/kg. To induce intestinal injury, animals were exposed to one dose of 12Gy whole body irradiation from a 137Cs source. Animals were fasted overnight and injected intraperitoneally with 25 mg/kg 5-ethynyl-2′-deoxyuridine (EdU) (Life Technologies) 2 hours prior to tissue collection. Intestinal tissue was harvested and fixed in 4% paraformaldehyde in PBS (PFA) overnight before paraffin processing as previously described (VanDussen et al. 2012). Tissue prepared for frozen sections was fixed in PFA for 1 hour and placed in 30% sucrose overnight before embedding in OCT (Tissue-Tek).
Immunohistochemistry
Paraffin sections (5μm) were stained with Periodic acid Schiff and Alcian Blue (PAS/AB) (Newcomer Supply) to visualize mucin-containing goblet cells. EdU-Click-it (Life Technologies) was used to evaluate proliferating cell number. Immunostaining with rat α-MMP7 (1:400, Vanderbilt Antibody and Protein Resource), rabbit α-MUC2 (1:200, Santa Cruz), rabbit α-CHGA (1:200, Abcam), rat α-PROM1 (1:100, eBioscience) and rabbit α-Ki67 (1:200, Thermo) was performed as described (Lopez-Diaz et al. 2006). For AB/CHGA co-staining, tissues were stained with α-CHGA (1:100, Abcam) and visualized with the DAB substrate kit (VectorLabs) per manufacturer’s instructions. Slides were then stained in AB for 30 minutes and counterstained in neutral red for 1 minute. Transgenic LGR5-GFP was directly imaged on frozen sections without antibody staining. Images were captured on a Nikon E800 microscope with Olympus DP controller software.
In situ hybridization
Olfm4 cDNA plasmid (IMAGE clone 9055739) was linearized and sense and antisense probes were made with DIG labeling kit according to the manufacturer’s instructions (Roche). Probes were purified using the Min-Elute Gel Extraction kit (Qiagen). Slides were deparaffinized, hydrated, and treated with 0.2N HCl for 15 minutes at room temperature. Tissues were treated with Proteinase K (30μg/mL, Roche) for 30 minutes at 37°C, post-fixed in 4% PFA for 10 minutes, acetylated for 10 minutes in 13.4% triethanolamine, 0.2M HCl and 2.5% acetic anhydride, incubated with hybridization buffer (50% deinonized formamide (Invitrogen), 10% dextran sulfate (Millipore), 2% Denhardts (Sigma), 1 mg/mL yeast tRNA, 0.2M NaCl, 0.1M Tris-HCl, pH 7.5, 0.1M phosphate buffer, pH 6.8, 5mM EDTA, pH 8 in DEPC-treated H2O) for 1 hour, followed by incubation with Olfm4 probe diluted in hybridization buffer at 68°C overnight. Tissue sections were then washed, incubated in blocking solution (20% heat-inactivated serum, 0.02g/mL blocking reagent (Roche) in buffer (0.1M Tris-HCl, pH7.5, 0.15M NaCl, 0.1% Tween 20 in sterile H2O) for 1 hour, and anti-DIG antibody (1:2500, Roche) overnight at 4°C. Slides were washed and developed with NBT/BCIP solution (1:100, Roche) in 0.1M Tris-HCl, pH9.5, 0.1M NaCl, 0.05M MgCl2, 0.5mg/mL levamisole (Sigma) in sterile H2O. Minimal signal was detected with Olfm4 sense probe control.
Quantitative morphometric analysis
All observers were blinded to slide identity for cell counting. Goblet cell hyperplasia was measured as the number of crypts that displayed increased goblet cells over total crypts per section. EdU morphometrics was achieved by counting the total number of epithelial EdU+ cells per well-oriented crypt and averaged per animal. CHGA+ cells were quantified as number of stained cells per crypt.
Crypt isolation and flow cytometry
Crypt isolation was performed on proximal jejunum (centimeters 9-15 as measured from the pylorus). Tissue was incubated in 15mM EDTA (Sigma) in DPBS (Gibco) at 4°C for 35 minutes, vortexed for 2 minutes, and filtered through a 70μm cell strainer (BD Bioscience). To obtain a single cell suspension for flow cytometry, purified crypts were resuspended in TrypLE Express (Gibco), shaken at 37°C for 10-12 minutes, and 0.1mg/ml DNase I (Roche) and 10% fetal bovine serum (FBS) were added. Cells were passed through a 40μm cell strainer (BD Bioscience), pelleted at 400×G, resuspended in 2% FBS, 0.05% sodium azide (Sigma), 2mM EDTA in DPBS and stained unfixed as follows. All cells were blocked with rat α-mouse CD16/CD32 (1:100, BD Bioscience), lymphocytes were excluded with CD45.2-PerCP-Cy5.5 (1:80, LifeTechnologies), epithelial cells were visualized with EpCAM-APC (1:80, eBioscience), and dead cells were excluded by DAPI (3.6mM) incorporation. Cells were analyzed on a BD FACSCanto II and analyzed with FlowJo software (Treestar). GFP+ cells were sequentially gated for size, singlets, DAPI-, CD45.2-, and EpCAM+. For EdU flow analysis cells were stained with CD45.2-PerCP-Cy5.5 and EpCAM-APC and then the EdU-Click-it Alexa-488 kit as per manufacturer’s instructions. EdU+ cells were gated for size, singlets, CD45.2-, and EpCAM+.
Gene expression analysis
RNA from full-thickness ileum or isolated jejunal crypts was isolated by Trizol (Invitrogen) extraction followed by the RNeasy Mini kit (Qiagen) with DNase I treatment as per manufacturer instructions. cDNA was reverse transcribed with the iScript cDNA synthesis kit (BioRad) using 1μg of total RNA. Quantitative RT-PCR was performed as described (Lopez-Diaz et al. 2006) with primer sequences as described (VanDussen et al. 2012). Assays for each sample were run in triplicate and normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh) as an internal control.
Gene deletion quantification
For quantification of N1 deletion, DNA was purified from isolated crypts using the Easy-DNA kit (Invitrogen, cat# K1800-01). Quantitative PCR was performed with 40ng DNA using the following primers: GCGCACGCTTTGGGTAGATA (forward) and ACACTTCCAGCGTCTTTGGG (reverse) within the deleted sequence, and CTGGGACAACAGCAGCCTAA (forward) and GCCTTGCAGGCCTTAAGAGA (reverse) in a reference sequence downstream of the N1 deletion site.
Statistical analyses
Quantitative data are presented as mean ± SEM, with all experimental groups normalized to time-matched tamoxifen-treated controls. Comparisons between two groups were conducted with unpaired two-tailed student t tests. Comparisons between 3 or more groups were analyzed by one-way ANOVA with Tukey’s or Dunett’s post-tests as noted. Prism software (Graphpad) was used for statistical analyses. Significance is reported as * (P<0.05), **(P<0.01), ***(P<0.001), and ****(P<0.0001).
Results
Weight loss and secretory cell hyperplasia in N1-deleted intestine
To conditionally delete N1 in the intestinal epithelium, we crossed the N1F/F mice (Yang et al. 2004) with transgenic mice carrying a tamoxifen-regulated Villin-CreERT2 allele (el Marjou et al. 2004). After tamoxifen treatment Villin-CreERT2; N1F/F (N1Δ/Δ) mice transiently lost weight with a nadir (92% of initial weight) occurring at day 8 (Fig. 1A). Although N1Δ/Δ animals began to gain weight after day 8, they remained significantly lighter than controls until after day 35.
Fig. 1. Intestinal epithelial N1 deletion leads to transient weight loss and goblet cell hyperplasia.
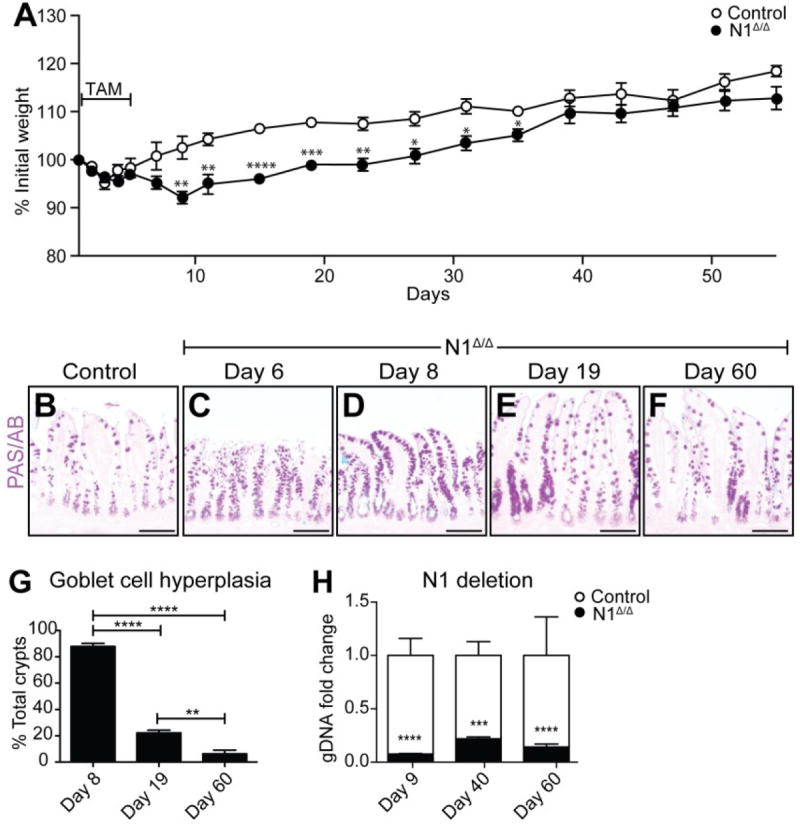
(A) Weight curve of Villin+/+; N1F/F (Control) and Villin-CreERT2; N1F/F (N1Δ/Δ) animals treated for 5 days with 100mg/kg tamoxifen (TAM). Bar represents duration of TAM treatment. Weights at each time point are compared with Student’s t tests. (B-F) PAS/AB stained goblet cells in control (B) or N1Δ/Δ ileum (C-F) at the time points indicated. (G) Quantification of ileal goblet cell hyperplasia presented as percent total crypts. Data were analyzed by ordinary one-way ANOVA with Tukey’s multiple comparisons test. N = 3-6 animals/group. (H) Quantification of N1 genomic DNA in control and N1Δ/Δ animals. Values were normalized to a reference sequence within N1, downstream of the deletion area, and shown as fold-change compared to control by Student’s t-test. N = 3-6 animals/group. * (P<0.05), **(P<0.01), ***(P<0.001), and ****(P<0.0001) Scale bar = 100μm.
We first assessed secretory cell populations in N1Δ/Δ intestines to determine if single receptor loss was sufficient to induce aberrant secretory cell differentiation. PAS/AB staining for mucin-containing cells revealed a striking increase in goblet cell abundance in the N1Δ/Δ terminal ileum (Fig. 1) and all other regions of the intestine (Supplementary Fig. 1). Interestingly, while increased goblet cells were observed in over 80% of crypts on day 8, the number of aberrant crypts significantly declined over time. By day 60, only 6% of crypts still maintained evidence of goblet cell hyperplasia (Fig. 1G), correlating with the normalization of N1Δ/Δ body weight (Fig. 1A). Of note, epithelial N1 deletion was maintained at similar levels throughout the time course (Fig. 1H) suggesting that the patchy restoration of normal goblet cell differentiation is through a N1-independent compensatory mechanism.
Interestingly, we observed a similar recovery pattern with complete genetic blockade of Notch signaling by crossing Villin-CreERT2 with RbpjF/F mice, and treating with tamoxifen (RbpjΔ/Δ). Administration of two doses of tamoxifen in this model results in complete goblet cell hyperplasia by day 7 followed by patchy normalization over time (Supplementary Fig. 2), indicating that this recovery response is not unique to N1 loss.
To investigate if other secretory cell types were increased in N1Δ/Δ mice, we stained for the Paneth cell marker MMP7 and endocrine cell marker CHGA, and observed increased numbers of both populations (Supplementary Fig.1I-M). Further analysis revealed co-localization of goblet and Paneth cell markers in N1Δ/Δ crypts (Supplementary Fig. 3). This finding is consistent with previous observations of global Notch inhibition in DBZ-treated mice (VanDussen et al. 2012). Although these co-stained cells do not follow typical mature goblet or Paneth cell differentiation programs, they do not co-express endocrine markers nor appear to be actively cycling (Supplementary Fig. 4).
Analysis of mRNA for secretory cell transcription factors and differentiated cell markers paralleled the observed secretory cell hyperplasia in the N1Δ/Δ intestine as well as the time-dependent phenotype regression (Fig. 2). Markers of all secretory cell lineages were significantly increased by day 8. Expression of the transcription factor Atoh1, which drives the differentiation of all secretory cell types (Shroyer et al. 2007; Yang et al. 2001), the goblet cell marker Muc2, and endocrine markers Neurog3 and ChgA normalized by day 60. In contrast, Spdef, which is important for the terminal differentiation of goblet and Paneth cells (Gregorieff et al. 2009; Noah et al. 2010) as well as Mmp7 remained amplified 2.4- and 1.7-fold respectively on day 60. Indeed, the MUC2/MMP7 co-stained cells were still observed in a patchy distribution in both the small intestine and colon 60 days after N1 deletion (Supplementary Fig. 3), consistent with the continued elevation of these markers.
Fig. 2. Secretory cell markers and Notch ligands are transiently upregulated in N1Δ/Δ intestine.
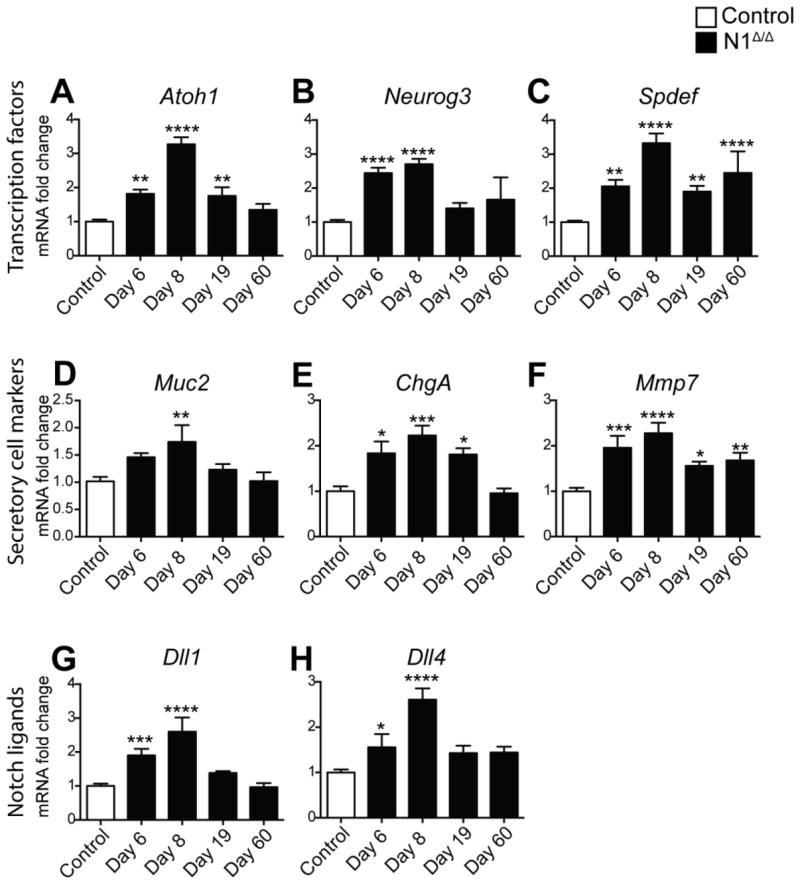
Quantitative RT-PCR analysis of secretory cell transcription factors (A-C), differentiated secretory cell markers (D-F) and Notch ligands (G,H) in tamoxifen-treated Villin+/+; N1F/F (Control) and N1Δ/Δ animals at the time points indicated. RNA was isolated from full-thickness ileum. All values were normalized to Gapdh expression level and reported as fold change compared to control. Data were compared with ordinary one-way ANOVA with Dunnett’s post-test. N = 3-6 animals/group.
Notch ligand expression in secretory cells
DLL1 and DLL4 have been shown to be the primary ligands regulating the intestinal stem and progenitor compartment (Pellegrinet et al. 2011). Therefore, we analyzed transcript levels of Dll1 and Dll4 in N1Δ/Δ intestine. Expression of both ligands was elevated 2.6-fold compared to control on day 8 (Fig. 2G-H). Importantly, the overexpression of Dll1 and Dll4 in N1Δ/Δ animals subsided over time, similar to the secretory cell markers discussed above, suggesting increased ligand presentation is limited to the secretory cell expansion period.
N2-deleted intestine shows no change in differentiation
We next analyzed N2 deletion to determine if genetic deletion of this receptor led to any epithelial cell changes. To achieve specific intestinal epithelial deletion Villin-CreERT2 was crossed to the N2F/F alleles (McCright et al. 2006). In contrast to N1Δ/Δ, tamoxifen-treated Villin-CreERT2; N2F/F (N2Δ/Δ) animals did not lose weight post-treatment (data not shown), and no goblet cell changes were evident in N2Δ/Δ intestine (Fig. 3). Furthermore, no transcriptional changes were observed in genes encoding secretory cell transcription factors, differentiated cell markers, or Notch ligands (Fig. 3).
Fig. 3. N2 deletion in the intestinal epithelium does not result in secretory cell changes.
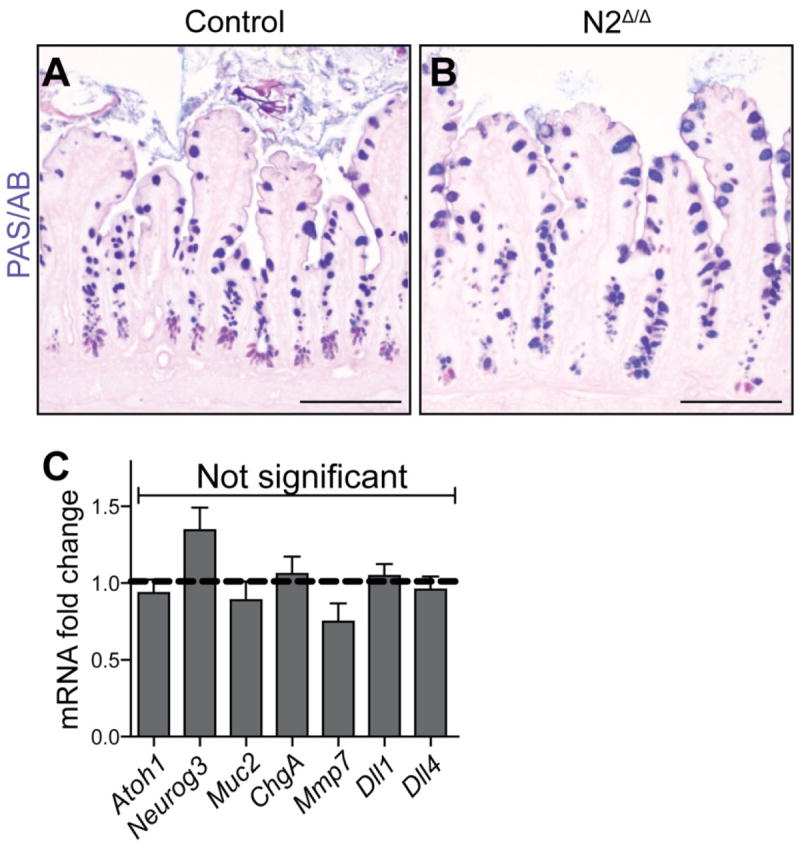
Villin+/+; N2F/F (Control) or Villin-CreERT2; N2F/F (N2Δ/Δ) animals were treated with 100mg/kg TAM daily for 5 days and harvested on day 6. (A,B) PAS/AB staining for goblet cells in control (A) and N2Δ/Δ (B) ileum. No marked increases in goblet cells were observed in the N2Δ/Δ animals. (C) qRT-PCR analysis of secretory transcription factors, differentiated secretory cell markers, and Notch ligands. Data are presented as mRNA fold-change and comparisons were made with Student’s t tests compared to control, which was normalized to 1 (dashed line). No significant changes were observed for any marker gene. (Neurog3, P=0.14; Mmp7, P=0.18). N = 3-6 animals/group. Scale bar =100μm.
N1 and N2 function additively for secretory cell differentiation
While N1Δ/Δ animals displayed a distinct secretory cell hyperplasia, the severity of the phenotype was less profound than published reports of double Notch receptor gene deletion (N1Δ/Δ; N2Δ/Δ) (Riccio et al. 2008) as well as pharmacologic Notch inhibition (van Es et al. 2005; VanDussen et al. 2012). Thus, we analyzed compound deletions of N1 and N2 to assess dosage effects of Notch receptors on secretory cell fate. Goblet cells were visualized by PAS/AB staining in N1 heterozygotes (N1Δ/+), compound partial deletions (N1Δ/+; N2Δ/Δ and N1Δ/Δ; N2Δ/+) and double knockout (N1Δ/Δ; N2Δ/Δ) animals. Heterozygote N1Δ/+ animals displayed no increase in goblet cells (Fig. 4), indicating that a loss of both N1 alleles is required for the secretory cell effect. Compound partial deletions N1Δ/+; N2Δ/Δ and N1Δ/Δ; N2Δ/+ each displayed a more severe goblet cell hyperplasia compared to N1Δ/Δ, albeit less severe than the total knockout N1Δ/Δ; N2Δ/Δ. These findings were paralleled by analysis of mRNA transcripts for secretory cell markers (Fig. 4G), suggesting that N1 and N2 function additively to regulate epithelial cell differentiation.
Fig. 4. N1 and N2 function additively to regulate epithelial differentiation.
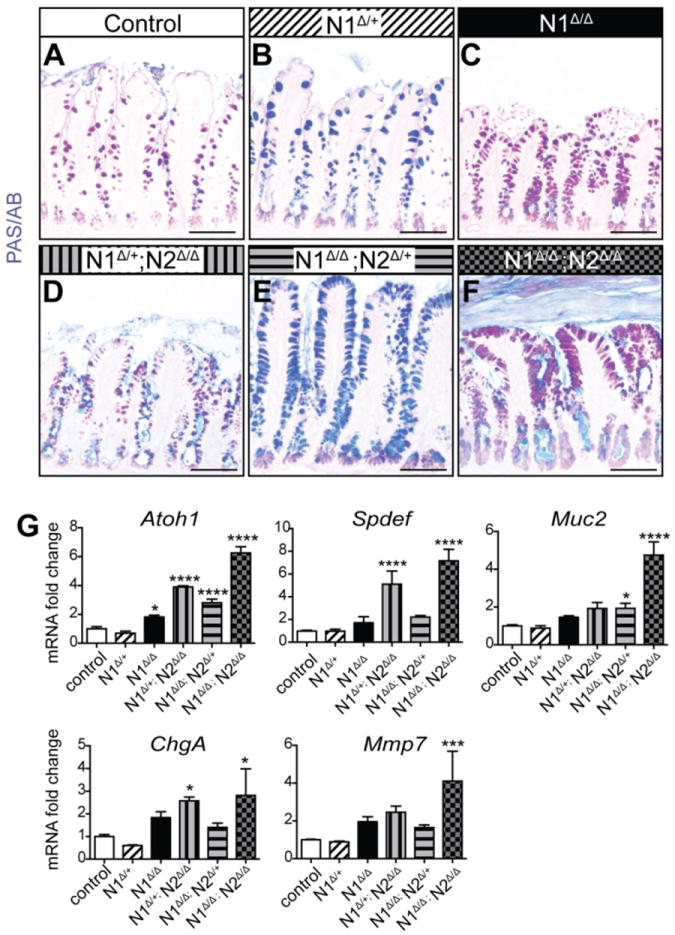
Control animals were pooled Villin+/+; N1F/+; N2F/F and Villin+/+; N1F/+. All control and experimental groups were injected with 100mg/kg TAM daily for 5 days and harvested on day 6. (A-F) PAS/AB staining for goblet cells in control (A), N1Δ/+ (B) N1Δ/Δ (C), N1Δ/+; N2Δ/Δ (D) N1Δ/Δ; N2Δ/+ (E) N1Δ/Δ; N2Δ/Δ (F) ileum. (G) Quantitative RT-PCR analysis for secretory cell markers in all groups. Data are compared to control with ordinary one-way ANOVA and Dunnett’s post-test. N = 3- 5 animals/group. Scale bar =100μm.
N1 and N2 function redundantly for proliferation
Another hallmark of intestinal Notch inhibition is decreased epithelial proliferation (Pellegrinet et al. 2011; Riccio et al. 2008; van Es et al. 2005; VanDussen et al. 2012). Despite the robust secretory cell phenotype, no loss of proliferation was observed in N1Δ/Δ, as measured by flow cytometry for crypt epithelial EdU+ cells (Fig. 5A). To determine the contribution of each receptor to regulation of epithelial proliferation, EdU+ cells were assessed in all Notch receptor deletion combinations (Fig. 5B-H). Interestingly, only double Notch receptor deletion resulted in a significant reduction in proliferation, suggesting that N1 and N2 function fully redundantly for this process, and that markedly reduced levels of Notch signaling can sustain the bulk of intestinal epithelial proliferation.
Fig. 5. N1 and N2 are fully redundant for intestinal epithelial proliferation.
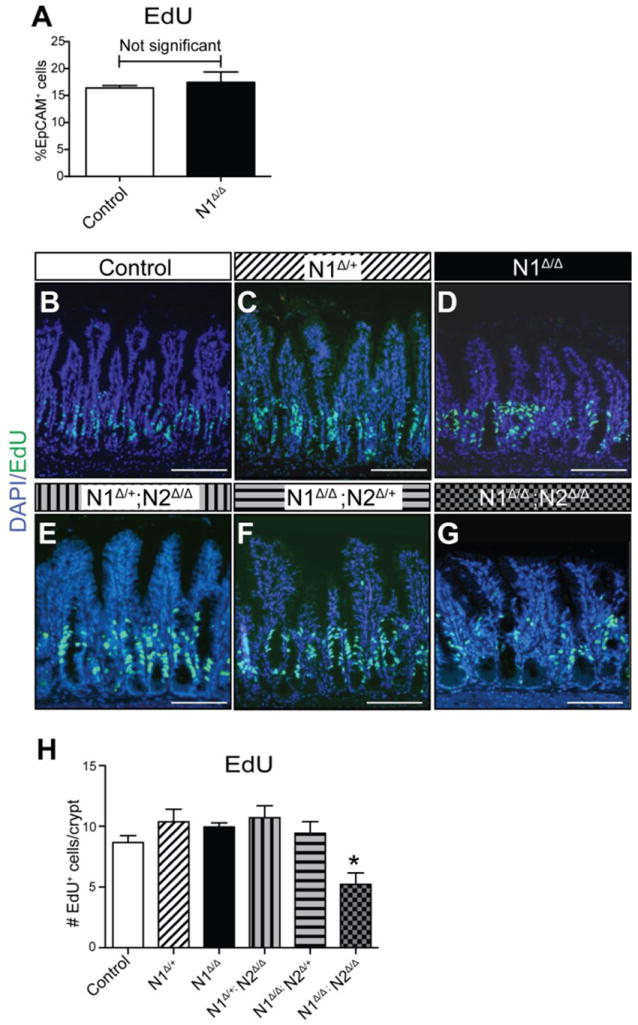
Animals were treated with EdU+ 2 hours before tissue collection. (A) EdU+ cells in dissociated epithelial cells from tamoxifen-treated Villin+/+; N1F/F (Control) and N1Δ/Δ jejunum were analyzed by flow cytometry. Data are presented as percent of EpCAM+ cells gated for size, singlets and CD45-. No change was observed in N1Δ/Δ (day 6) compared to control. (B-G) Representative images of ileal proliferation as visualized by EdU uptake in all genotypes as indicated. (H) Morphometric quantification of proliferative cells. Data are presented as average EdU+ cells/crypt. Control group is pooled day 6 and day 8 tamoxifen-treated Villin+/+; N1F/F controls, which were shown to be the same. Quantitative data are compared with ordinary one-way ANOVA and Dunnett’s post-test. N = 3-5 animals/group. Scale bar =100μm.
N1 is the primary receptor regulating stem cells
We have previously shown that Notch signaling is crucial for maintenance of the LGR5+ crypt base columnar (CBC) stem cell population (VanDussen et al. 2012), and thus questioned whether the secretory cell changes in the N1Δ/Δ intestine were coupled with altered stem cell homeostasis. Olfm4 is a CBC marker (van der Flier et al. 2009) as well as a direct Notch target gene (VanDussen et al. 2012), which can be used as a faithful read-out of Notch suppression in active stem cells. In situ hybridization for Olfm4 showed profound loss of expression in N1Δ/Δ intestine 6 days after tamoxifen induction (Fig. 6A). Expression of classic Notch target genes Hes1 and Myc were also significantly decreased in N1Δ/Δ intestine (Supplementary Fig. 5). Interestingly, while Hes1 and Myc expression fully recovered to baseline by Day 60, Olfm4 expression only partially recovered, with maintenance of patchy crypts negative for Olfm4 expression at 60 days (Supplementary Fig. 5).
Fig. 6. LGR5+ stem cells are depleted with N1 deletion.
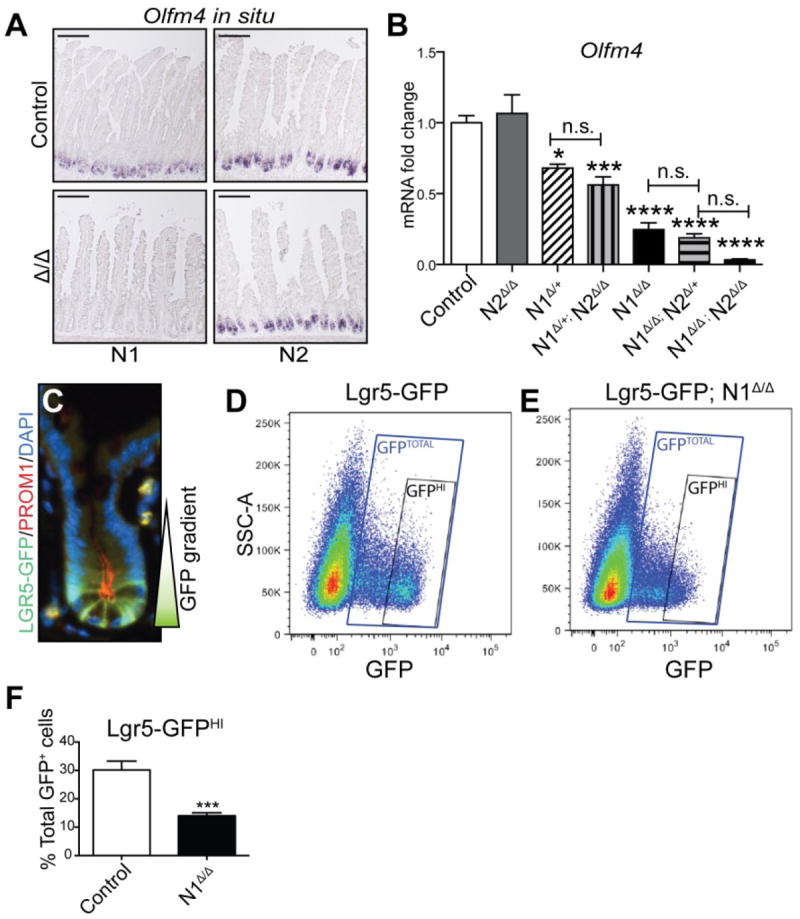
(A) In situ hybridization for Olfm4 in N1Δ/Δ and N2Δ/Δ jejunum. Controls are tamoxifen-treated Villin+/+; N1F/F and Villin+/+; N2F/F respectively. (B) Quantitative qRT-PCR for Olfm4 in all Villin-CreERT2 Notch receptor deletion groups. Control group is pooled tamoxifen-treated Villin+/+; N1F/+; N2F/F and Villin+/+; N1F/+ animals. Comparisons are made by ordinary one-way ANOVA and Tukey post-test. (C) High powered view of an Lgr5-GFP (green) mouse crypt co-stained for Prominin-1 (PROM1, red) and DAPI (blue). GFP shows a gradient of expression, with highest intensity in CBCs at the crypt base. (D-F) Flow cytometric analysis of Lgr5-GFP cells. Control (Lgr5-GFP; N1+/+) and Villin-CreERT2; Lgr5-GFP; N1F/F animals were injected with 100mg/kg TAM daily for 5 days and harvested on day 6. (D,E) Scatter plots of GFP expression in single, live, CD45.2- EpCAM+ crypt epithelial cells in control (D) and Lgr5-GFP; N1Δ/Δ (E) animals. Gates indicate GFPHI populations and GFPTOTAL populations. (F) Quantification of GFPHI cells. Data are presented as percentage of GFPTOTAL cells. N = 3-4 animals/group.
Consistent with the absence of other epithelial changes, N2Δ/Δ animals displayed no change in Olfm4 expression by in situ hybridization or mRNA expression analysis (Fig. 6 A, B). All other combinations of receptor gene deletion, however, showed significantly reduced Olfm4 transcript levels (Fig. 6B). Interestingly, the N1Δ/+ animals showed reduced Olfm4 expression that was not significantly different from N1Δ/+; N2Δ/Δ intestine, suggesting that the additional loss of both N2 alleles has very little effect on Olfm4. Likewise, while N1Δ/Δ animals expressed significantly less Olfm4 than N1Δ/+ heterozygotes, expression was not further reduced in N1Δ/Δ; N2Δ/+ animals. These results suggest that N1 is the primary receptor required for Notch target gene activation in the active intestinal stem cell population.
To more specifically determine the N1-dependence of CBC stem cells, we crossed the Villin-CreERT2; N1F/F mice to Lgr5-GFP mice (Barker et al. 2007) to allow visualization of LGR5+ stem cells by GFP expression. We employed flow cytometry on single cells isolated from N1Δ/Δ and control crypts to quantify LGR5-GFP stem cell expression on day 6 after the start of tamoxifen treatment. As there is a gradient of GFP expression in LGR5-GFP crypts (Sato et al. 2009) (Fig. 6C), we focused on GFPHI cells to explicitly measure changes in LGR5+ CBCs (Fig. 6D-F). In controls, 30% of total GFP+ cells were GFPHI, while N1Δ/Δ mice retained only 14% GFPHI cells, a 53% reduction from baseline. Consistent with the Olfm4 results, Lgr5 mRNA abundance only partially recovered after 60 days (Supplementary Fig. 5).
Both N1 and N2 are required for post-irradiation recovery
Finally, we investigated the role of Notch receptors on intestinal repair. To assess this, we treated N1Δ/Δ and N2Δ/Δ animals and their respective controls with tamoxifen and then administered 12Gy of whole body irradiation. Both groups quickly lost weight compared to controls, and mice were euthanized 3 days post-irradiation when the N1Δ/Δ group was moribund (Fig. 7A,B). In contrast, although the control groups also lost weight, a parallel experiment demonstrated that they survived at least 8 days post-irradiation and regained stem cell marker expression before succumbing to bone marrow insufficiency (Supplementary Fig. 6). PAS/AB staining showed increased goblet cells in the N1Δ/Δ group (Fig. 7E), consistent with the phenotype observed in non-irradiated animals at this time point (Fig. 1). Irradiated N2Δ/Δ tissues did not have increased goblet cells, but displayed abnormal secretory cell distribution reflective of altered epithelial architecture (Fig. 7G). Normal intestine experiences a proliferative surge post-irradiation as part of the recovery response (Paulus et al. 1992; Van Landeghem et al. 2012; Yan et al. 2012). To determine if N1 and N2 were essential for this process, we analyzed EdU incorporation in all groups (Fig. 7H-N). While irradiated control animals displayed a visible increase in the number of EdU+ cells compared to unirradiated controls, the N1Δ/Δ and N2Δ/Δ tissues were almost completely devoid of proliferative cells, suggesting that while neither receptor is critical for proliferation during normal homeostasis, they are both required for post-injury proliferation.
Fig. 7. N1 and N2 are required for post-irradiation intestinal recovery.
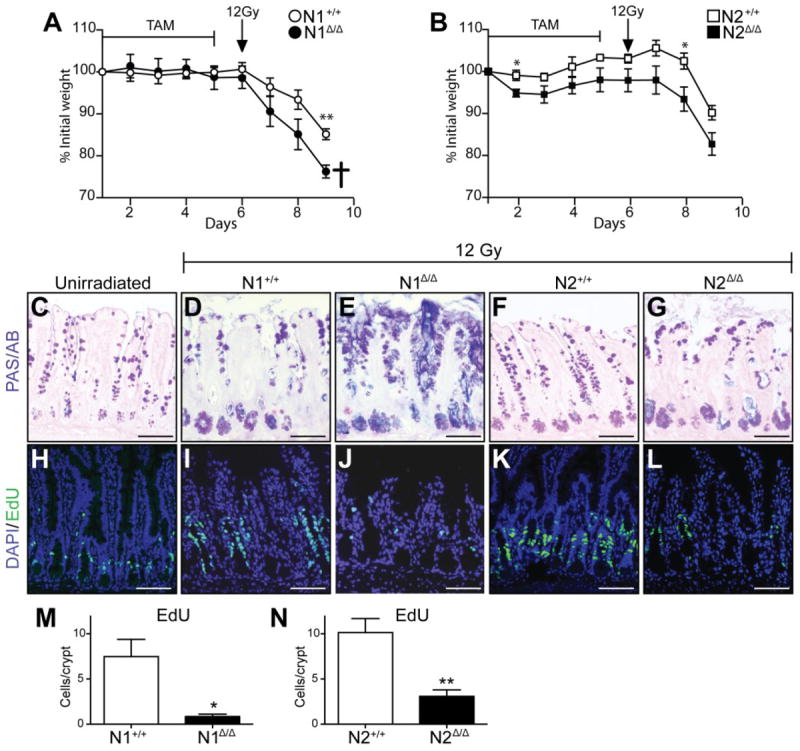
(A,B) N1Δ/Δ and N2 Δ/Δ animals and their respective controls Villin+/+; N1F/F (N1+/+) and Villin+/+; N2F/F (N2+/+) were treated with 100mg/kg TAM daily for 5 days, administered 12Gy of whole body irradiation on day 6 and harvested on day 9 when the N1Δ/Δ group had lost more than 20% body weight and was moribund (cross). (C-G) PAS/AB staining for goblet cells in ileum of the above groups. (H-L) Proliferation was assessed in ileum by EdU incorporation. (C,H) An unirradiated N1+/+ control group treated with tamoxifen for 5 days and harvested on day 9 is included for comparison. (M, N) Quantification of EdU uptake in irradiated animals. N = 3-4 animals/group. Scale bar =100μm.
Discussion
We present here an expanded understanding of Notch receptor function in the intestinal epithelium. During normal homeostasis N1 is the primary receptor responsible for differentiation and stem cell maintenance. Epithelial deletion of N1 results in a transient secretory cell hyperplasia, with overproduction of all secretory cell types. Additionally, our study used the Lgr5-GFP mouse model in combination with N1 deletion to show that loss of N1 acutely disrupts CBC stem cell homeostasis. We report new evidence that expression of either N1 or N2 is sufficient for normal proliferation during homeostasis, but that expression of both N1 and N2 is required for crypt regeneration in the post-irradiation setting.
Previous studies suggested that N1 is important for stem cells since its expression was found to be highest in CBCs compared to other crypt cells (Munoz et al. 2012; Sato et al. 2010). Additionally, N1-expressing cells (Fre et al. 2011) as well as cells undergoing active N1 cell-surface cleavage (Vooijs et al. 2007) have been shown to lineage trace into entire crypts, the gold-standard for defining stem cells in vivo. To our knowledge, only one other study has investigated the function of N1 in intestinal stem cells. Vooijs et al.(Vooijs et al. 2007) engineered blastocyst chimeras with wild-type and LacZ-expressing N1 deficient cells and analyzed the resulting adult intestines. Rare LacZ expression was observed in adult chimeras, suggesting that stem cells lacking N1 were capable of persisting over time. They concluded that while N1 was active in stem cells, it was not required for stem cell maintenance. Our results are in agreement with this study as we observed that N1-deleted stem cells can persist for several weeks. However, our observation that stem cell marker Olfm4 and Lgr5 expression remain decreased after two months of N1 deletion while other Notch target genes fully recover suggests that stem cell number is sensitive to N1 expression.
Additionally, while our study bolsters the secretory cell findings reported with N1-inhibitory antibody treatment (Wu et al. 2010) and chimeric N1-deleted intestine (Vooijs et al. 2007), another study using the same model system we have employed in our experiments found that Villin-CreERT2; N1Δ/Δ mice had no phenotype (Riccio et al. 2008) and is thus in direct conflict with our results. Importantly, the Riccio et al. study was conducted in juvenile rather than adult mice. To determine if there is an age-dependent window during which N1 plays a more minimal role in secretory cell differentiation we analyzed Villin-CreERT2; N1F/F mice injected with tamoxifen starting at 10 days of age. In concordance with our findings in adults, we observed increased goblet cell production in juvenile mice (Supplementary Fig. 7) suggesting that N1 becomes critical for proper epithelial differentiation early in the post-natal period. Thus, at this time, while we cannot explain why the Riccio et al. study did not observe a phenotype in N1Δ/Δ intestine, we presume that differences in tamoxifen treatment concentrations, chase times post-treatment, as well as variation in recombination frequencies may have contributed to the different results.
Our study is the first to show dynamic regulation of secretory cell fate in Notch deletion models. Interestingly, although overexpression of goblet and endocrine cell markers resolved by 2 months after N1 deletion, the presence of Paneth/goblet co-stained cells persisted in N1Δ/Δ animals. While it is agreed that Paneth cells are much longer lived than other differentiated intestinal epithelial cells, the exact length of the Paneth cell lifespan is disputed, with estimates ranging from 18 to 60 days (Cheng et al. 1969; Ireland et al. 2005). The continued presence of co-stained goblet/Paneth cells after 60 days suggests that these aberrant co-stained cells have a lifespan similar to mature Paneth cells and that once cells are specified as expressing both Paneth and goblet cell markers, they do so for the duration of their lifespan.
This study uniquely offers a detailed analysis of compound Notch receptor deletions in the intestine. By serially deleting 1 to 4 alleles of the N1 and N2 genes, we have discovered differences in the regulation of differentiation, proliferation, and stem cell maintenance. Although N1Δ/Δ results in secretory cell hyperplasia while N2Δ/Δ does not, combined deletions result in a more severe secretory cell phenotype. This finding suggests that these receptors function additively for secretory cell fate. In contrast, only double receptor deletion (N1Δ/Δ; N2Δ/Δ) showed a significant loss of epithelial proliferation, indicative of full redundancy of N1 and N2 function for proliferation and suggestive that N2 signaling in the absence of N1 is capable of maintaining proper proliferation but unable to maintain normal differentiation. Furthermore, this reveals that maintenance of proliferation requires less overall Notch signaling tone. The separation of these two phenotypes may allow pharmacological opportunities to regulate differentiation while sparing proliferation.
Finally, we discovered that deletion of either N1 or N2 renders the intestine highly susceptible to irradiation injury, resulting in altered epithelial architecture and almost complete loss of proliferation. This is consistent with the irradiation susceptibility of N1-inhibitory antibody-treated animals (Tran et al. 2013), and extends this finding to an epithelial-specific effect of N1 deletion. Importantly, this is the first report of N2 sensitivity in the post-irradiation setting and the first intestinal phenotype to be described in N2Δ/Δ mice. As deletion of N2 has no apparent affect on the active stem cell population, these findings may suggest a higher level of required Notch activity in reserve stem cell populations. Indeed Qu et al.(Qu et al. 2014) found that Notch inhibition resulted in fewer Dclk1+ cells after irradiation, a putative reserve stem cell population. Our findings are significant for the potential use of Notch as a pharmacologic target for cancer treatment. Because most successful therapeutic schemes are combined with injury-inducing chemotherapies or radiation, concomitant treatment with N1 or N2 inhibition could be deleterious.
In conclusion, the N1 receptor plays a primary role in regulating intestinal epithelial cell fate, stem cell maintenance and repair. N2 has a minimal role during homeostasis but is required for crypt regeneration in the post-injury setting. Therapeutic inhibition of these receptors should be approached with caution due to the important role they play in homeostasis and repair.
Supplementary Material
Acknowledgments
We thank John Kao and Mohamad El-Zaatari for equipment and advice regarding flow protocols, Scott Magness, Jason Spence, and Noah Shroyer for expertise with crypt and single cell isolation protocols, and the University of Michigan Microscopy and Image Laboratory and Flow Cytometry cores for equipment and expertise. Jordan Onopa provided vital technical support and Jessica Crowley was indispensible for animal management. Support for A. Carulli was graciously provided by the MSTP training fellowship (T32-GM07863), the Center for Organogenesis Training Program (T32-HD007515), and a Ruth L. Kirschstein NRSA (F30-DK095517). I.M. was supported by the National Institutes of Health (RO1-AI091627) and the Leukemia and Lymphoma Society. The research was funded by the National Institutes of Health, including a grant to LCS (RO1-DK078927) and Core support from the Michigan Gastrointestinal Peptide Center (P30-DK34933) and a Program Project Grant (P01-DK062041).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijinsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Cheng H, Merzel J, Leblond CP. Renewal of Paneth cells in the small intestine of the mouse. The American journal of anatomy. 1969;126:507–525. doi: 10.1002/aja.1001260409. [DOI] [PubMed] [Google Scholar]
- el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–193. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- Fre S, Hannezo E, Sale S, Huyghe M, Lafkas D, Killel H, Louvi A, Greve J, Louvard D, Artavanis-Tsakonas S. Notch lineages and activity in intestinal stem cells determined by a new set of knock-in mice. PloS One. 2011;6:e25785. doi: 10.1371/journal.pone.0025785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Ireland H, Houghton C, Howard L, Winton DJ. Cellular inheritance of a Cre-activated reporter gene to determine Paneth cell longevity in the murine small intestine. Devel Dynamics. 2005;233:1332–1336. doi: 10.1002/dvdy.20446. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Kim TH, Shivdasani RA. Genetic evidence that intestinal Notch functions vary regionally and operate through a common mechanism of Math1 repression. J Biol Chem. 2011;286:11427–11433. doi: 10.1074/jbc.M110.188797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y, Brunkan CS, Keeley T, Eaton KA, Merchant JL, Chew CS, Samuelson LC. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol - Gastrointest Liver Physiol. 2006;290:G970–979. doi: 10.1152/ajpgi.00461.2005. [DOI] [PubMed] [Google Scholar]
- McCright B, Lozier J, Gridley T. Generation of new Notch2 mutant alleles. Genesis. 2006;44:29–33. doi: 10.1002/gene.20181. [DOI] [PubMed] [Google Scholar]
- Munoz J, Stange DE, Schepers AG, van de Wetering M, Koo BK, Itzkovitz S, Volckmann R, Kung KS, Koster J, Radulescu S, Myant K, Versteeg R, Sansom OJ, van Es JH, Barker N, van Oudenaarden A, Mohammed S, Heck HJ, Clevers H. The Lgr5 intestinal stem cell signature: robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 2012;31:3079–3091. doi: 10.1038/emboj.2012.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp Cell Res. 2010;316:452–465. doi: 10.1016/j.yexcr.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus U, Potten CS, Loeffler M. A model of the control of cellular regeneration in the intestinal crypt after perturbation based solely on local stem cell regulation. Cell Prolif. 1992;25:559–578. doi: 10.1111/j.1365-2184.1992.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Pellegrinet L, Rodilla V, Liu Z, Chen S, Koch U, Espinosa L, Kaestner KH, Kopan R, Lewis J, Radtke F. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology. 2011;140:1230–1240. doi: 10.1053/j.gastro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu D, May R, Sureban SM, Weygant N, Chandrakesan P, Ali N, Li L, Barrett T, Houchen CW. Inhibition of Notch signaling reduces the number of surviving Dclk1+ reserve crypt epithelial stem cells following radiation injury. Am J Physiol - Gastrointest Liver physiol. 2014;306:G404–411. doi: 10.1152/ajpgi.00088.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LH, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–383. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander GR, Powell BC. Expression of notch receptors and ligands in the adult gut. J Histochem Cytochem. 2004;52:509–516. doi: 10.1177/002215540405200409. [DOI] [PubMed] [Google Scholar]
- Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2010;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Schroder N, Gossler A. Expression of Notch pathway components in fetal and adult mouse small intestine. Gene Expr Patterns. 2002;2:247–250. doi: 10.1016/s1567-133x(02)00060-1. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132:2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, Clevers H. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci USA. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigaki K, Han H, Yamamoto N, Tashiro K, Ikegawa M, Kuroda K, Suzuki A, Nakano T, Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Imm. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- Tran IT, Sandy AR, Carulli AJ, Ebens C, Chung J, Shan GT, Radojcic V, Friedman A, Gridley T, Shelton A, Reddy P, Samuelson LC, Yan M, Siebel CW, Maillard I. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013;123:1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol - Gastrointest Liver Physiol. 2012;302:G1111–1132. doi: 10.1152/ajpgi.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud A, Grosse AS, Gumucio DL, Ernst SA, Tsai YH, Dempsey PJ, Samuelson LC. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488–497. doi: 10.1242/dev.070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDussen KL, Samuelson LC. Mouse atonal homolog 1 directs intestinal progenitors to secretory cell rather than absorptive cell fate. Dev Biol. 2010;346:215–223. doi: 10.1016/j.ydbio.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vooijs M, Ong CT, Hadland B, Huppert S, Liu Z, Korving J, van den Bon M, Stappebeck T, Wu Y, Clevers H, Kopan R. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development. 2007;134:535–544. doi: 10.1242/dev.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Yang X, Klein R, Tian X, Cheng HT, Kopan R, Shen J. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol. 2004;269:81–94. doi: 10.1016/j.ydbio.2004.01.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


