Abstract
Background and Purpose
Preterm birth is associated with alteration in cortico-thalamic development, which underlies poor neurodevelopmental outcomes. Our hypothesis was that preterm neonates with CHD would demonstrate abnormal thalamic microstructure when compared to critically ill neonates without CHD. A secondary aim was to identify any association between thalamic microstructural abnormalities and peri-operative clinical variables.
Material and Methods
We compared thalamic DTI measurements in 21 preterm neonates with CHD to two cohorts of neonates without CHD: 28 term and 27 preterm neonates, identified from the same neonatal intensive care unit. Comparison was made with three other selected white matter regions using ROI manual based measurements. Correlation was made with post-conceptional age and peri-operative clinical variables.
Results
In preterm neonates with CHD, there were age-related differences in thalamic diffusivity (axial and radial) compared to the preterm and term non-CHD group, in contrast to no differences in anisotropy. Contrary to our hypothesis, abnormal thalamic and optic radiation microstructure was most strongly associated with an elevated first arterial blood gas pO2 and elevated pre-operative arterial blood gas pH (p<0.05).
Conclusion
Age-related thalamic microstructural abnormalities were observed in preterm neonates with CHD. Perinatal hyperoxemia and increased peri-operative serum pH was associated with abnormal thalamic microstructure in preterm neonates with CHD. This study emphasizes the vulnerability of thalamo-cortical development in the preterm neonate with CHD.
Introduction
Approximately one in every 100 babies born in the United States is affected with congenital heart disease (CHD), and more than 15% of those are born prematurely. Interestingly, CHD is twice as common in preterm infants compared to term infants [1]. These facts have a vast impact on the newly born infant, his/her family, the healthcare team, and society as it has been consistently demonstrated that such an individual is at great risk for morbidity, mortality, and prolonged neurodevelopmental challenges [1 – 17].
Preterm birth in neonates without CHD has been shown to be associated with abnormal corticothalamic development, which is thought to underlie multi-domain neurocognitive deficits [18, 19]. Additionally, and perhaps not coincidentally, the peak peroid of perinatal brain injury in preterms concides with the development of the subplate, a structure that is critical to the development of thalamo-cortical connections [20 – 22]. We have previously reported on abnormal white matter findings in preterm CHD neonates using Diffusion Tensor Imaging and Tract Based Spatial Statistics [23]. Despite the large number of studies demonstrating how the thalamus and its connections are abnormal in the preterm brain, the microstructure of the thalamus in the preterm CHD patients has not been investigated.
In the present study, we used diffusion tensor imaging (DTI) to investigate the microstructural integrity of the thalamic parenchyma in preterm neonates with CHD. DTI is a quantitative MRI technique that can assess the microstructural integrity of the brain tissue based on the Brownian motion of water molecules. Our hypothesis was that preterm neonates with CHD would demonstrate thalamic microstructural abnormalities at near term-equivalent age when compared to other critically ill neonates without CHD. We included two separate comparison groups: term neonates without CHD and preterm neonates without CHD, both of which were identified from the same high risk NICU. The term neonates without CHD provided a comparison for what the images of the preterm non-CHD brains should approximate at term-equivalent age. The preterm neonates without CHD allowed us to determine whether the presence of a congenital heart defect resulted in further thalamic microstructural abnormality than would be accounted for by prematurity alone. Our secondary aim was to identify any association between thalamic microstructural abnormalities and both post-conceptional age and peri-operative clinical variables. We specifically tested the hypothesis that both age and hypoxic perinatal and peri-operative factors would be associated with abnormal thalamic microstructure in preterm CHD neonates.
Materials and Methods
Subjects
Cohort of preterm neonates with CHD
Neonates undergoing clinically-indicated brain MRIs at near term-equivalent age during the period of 2005 to 2010 were recruited as part of on-going longitudinal studies of neurodevelopment in neonates with prematurity and CHD at Children’s Hospital Los Angeles. In the preterm CHD group, we included neonates with any heart anomaly treated surgically in infancy, including: the combination of atrial septal defect (ASD) and ventricular septal defect (VSD) (requiring surgery after term-equivalency), hypoplastic left heart syndrome (HLHS), Ebstein's anomaly, coarctation of the aorta, truncus arteriosus, transposition of the great arteries (TGA), and double outlet right ventricle. CHD patients were excluded if: (1) the heart anomaly did not require surgery; (2) if they had an identified chromosomal abnormality; (3) if the brain MRI did not include DTI data which were analyzable (i.e., due to motion artifact or technical factors), or (4) if there was a congenital brain malformation or a significant brain abnormality/injury/infection, which could distort subsequent DTI measurements. Approximately 10 preterm CHD neonates were excluded.
Comparison cohorts of critically ill term and preterm neonates without CHD
For comparison, we included data from two cohorts of neonates without CHD identified from the same high-risk NICU--the first obtained as part of an IRB-approved retrospective review of neonatal MRIs conducted at the same institution as above between 2005–2010 and the second obtained as part of an ongoing longitudinal research program focused on prematurity. All near term-7 equivalent MRIs were completed under clinical-indications. The clinical indication for these studies included suspected abnormal brain morphology (not confirmed), suspected brain injury or infection (not confirmed by imaging or relevant laboratory studies); assessment of possible seizure activity, and assessment of a non-intracranial abnormality, including a facial or orbital lesion. For all neonates, medical records were reviewed, including pertinent outpatient follow-up, by a neonatologist (LP) and a pediatric neuropsychologist (JW). By choosing crtically ill term controls with normal conventional MRI, we hoped to learn more about the potential paths of aberrent brain development in high-risk neonates, term and preterms.
MR Imaging Protocol
All imaging was obtained in a 1.5T General Electric System (GE-Medical Systems, Milwaukee, WI) with a neonatal head coil and neonatal incubator (if clinically necessary). The following imaging sequences were acquired: T2-weighted FSE in axial and coronal plane (TE/TR = 85/5000 msec, FOV = 20 cm, matrix = 320×160 or 256×128; slice thickness 3 mm, spacing=0); Coronal T1 3D SPGR (TE/TR=6/25 msec, FOV=18 cm, matrix= 256 × 160; slice thickness=1mm, spacing=0). The DTI protocol included an Echo-planar imaging (EPI) sequence with the following parameters: TE/TR = 80/10000 msec, FOV = 22 cm, matrix = 128×128, slice thickness = 4.5 mm, spacing = 0, with an in-plane resolution of 1.7 mm applied along 25 non-colinear directions with a b-value of 700 s/mm2.
ROI analysis
DTI analysis was performed off-line using DTI Studio (Johns Hopkins University, Baltimore, MD). Diffusivity and anisotropy metrics were measured in a total of 4 standardized ROI’s [24]. Specifically, the manually-delineated regions included the thalamus (drawn on a single axial slice at the level of the thalamus and genu/splenium of the callosum); and three other white matter structures: optic radiations, parietal periventricular cross-roads (a region known to be commonly affected by pWML or PVL involving intersections between longitudinal cortico-cortical tracts in anterior-posterior and left-right orientation and thalamocortical tracts radiating centrally toward the peripheral cortex), and the splenium of the corpus callosum These specific white matter regions were chosen as these involved projections from the thalamus and abnormalities of these areas have been demonstrated previously in CHD populations [9,23]. Standardized axial slices were used for all regions of interest. In cases of head tilt, ROIs were delineated at multiple slice levels and then averaged. For structures such as the thalamus, ROIs were drawn so as to outline the structure. For other ROIs, a standardized circular ROI of a given diameter was used. The senior neuroradiologist (AP) ensured consistency of positioning for all regions. In all regions, standard measurements were obtained: fractional anisotropy (FA), eigenvalue 1 (λ1), eigenvalue 2 (λ2), eigenvalue 3 (λ3) and mean diffusivity (MD) were calculated. Eigenvalue 1 was also considered as axial diffusivity (λ11)(AD). Radial diffusivity (λ⊥)(RD) was calculated as the average of eigenvalues 2 & 3. Definition of these diffusion tensor metrics have been previously described [23].
Clinical Variables
The first arterial blood gas was the first documented blood gas for the neonate. The admission blood gas was the first blood gas at our facility upon arrival. The pre-operative blood gas done on the day of surgery immediately prior to surgery and the post-operative blood gas was the first blood gas obtained after surgery. Necrotizing enterocolitis was defined by the clinical team caring for the infant.
Statistical Analyses
Continous clinical variables were compared using a three-way ANOVA. Pairwise comparisons were then made using Tukey’s analysis. Categortical clinical variables were compared using Kruskal-Wallis or chi-square tests, as appropriate. Differences in peri-operative variables were determined by non-parametric tests. R Developmental Core Team and SPSS (Version 19, IBM Corporation) were used for all statistical analyses.
Results
Characterization of the Clinical Population and Clinical Variable Data
There were 76 cases that met the inclusion criteria for this study: 21 preterm neonates with CHD, 28 neonates born at term (≥ 37 weeks; term non-CHD comparison group) and 27 neonates born between 23 and 36 weeks gestational age (preterm non-CHD comparison group). There were no significant differences in post-conceptional age (PCA; defined as gestational age at birth plus post-natal age) at time of MRI among the three groups, including the subset of preterm CHD cases identified with pWMLs (p=0.58 ANOVA) (Table 1. Diagnoses, clinical course, MRI and surgical variables for preterm CHD cases are summarized in Table 1–2. Of note, 8/21 (38%) of the preterm CHD MRIs were obtained (clinically) prior to undergoing cardiac surgery.
Table 1.
Comparison of Clinical Variables Among Preterm CHD cases and High Risk Term Non-CHD and Preterm Non-CHD Groups
| Preterm CHD group (n=21) |
Term Comparison Group (n=28) |
Preterm Comparison Group (n=27) |
p value (all groups) |
||
|---|---|---|---|---|---|
| GA (weeks) | mean (SD) | 32.2(3) | 39.8 (0.9) | 30.7 (4.6) | 0.000 |
| PNA at MRI (weeks) | mean (SD) | 11.5 (9) | 4.7 (3.9) | 11.1 (8.4) | 0.009 |
| PCA at MRI (weeks) | mean (SD) | 43.7 (8) | 44.4 (4.2) | 42.6 (6.5) | 0.585 |
| Apgar 1 min | median (n) | 7 (19) | 8 (9) | 6 (21) | 0.037 |
| Apgar 5 mins | median (n) | 8 (19) | 9 (9) | 8 (21) | 0.086 |
| Apgar 10 mins | median (n) | 7 (3) | − (0) | 7 (3) | 0.406 |
| Size for GA | Small: % | 35 | 14 | 8 | 0.0001 |
| Appropriate: % | 59 | 81 | 84 | ||
| Large:% | 6 | 5 | 8 | ||
| ISAM | % (n) | 0 (10) | 0 (18) | 8.3 (24) | 0.08 |
| Postnatal Sepsis | % (n) | 67 (42) | 14.8 (27) | 62.5 (24) | 0.001 |
| Hydrocortisone | % (n) | 28 (18) | 10.7 (28) | 18.5 (27) | 0.188 |
| Days on Hydrocortisone | mean (SD) | 1.33 (4.68) | 0.6 (1.9) | 1.7 (6.1) | 0.770 |
| Inotropes | % (n) | 72 (18) | 17.9 (28) | 46.2 (26) | 0.000 |
| Days on Dopamine | mean (SD) | 3.78 (4.4) | 1.3 (3.2) | 3.8 (6) | 0.073 |
| NEC | % (n) | 28 (18) | 3.6 (28) | 19.2 (26) | 0.061 |
CHD= Congenital heart disease; GA= gestational age at birth; PNA=Postnatal age; PCA=post-conceptional age (calculated as GA plus PNA); ISAM=Infant of Substance-Abusing Mother NEC=Necrotizing Enterocolitis
Exposure to hydrocortisone and/or inotropes includes immediately pre- and post-operatively
Sepsis and NEC includes the entire hospitalization from birth until first discharge
Table 2.
Preterm CHD Cases: Frequency of Diagnoses
| Heart Defects | n=21 |
|---|---|
| Hypoplastic Left Heart Syndrome | 5 |
| Ebstein’s Anomaly | 2 |
| Coarctation of the aorta | 3 |
| Transposition of the Great Arteries | 2 |
| Atrial Septal Defect and Ventricular Septal Defect, and/or Patent Ductus Arteriosus requiring surgery | 8 |
| Double Outlet Right Ventricle | 1 |
Assessment of Thalamic Parenchyma
Using ROI technique, there was no significant difference between the preterm CHD group and the two non-CHD groups (term and preterm) in the thalamic anisotropy measurements (Table 3). In contrast, there was increased axial diffusivity (AD) in the thalamus of the preterm CHD group compared to the term non-CHD group (p value = 0.042). There was also a trend towards increased radial diffusivity (RD) and ADC of both preterm groups compared to the term non-CHD group (Table 3).
TABLE 3.
DTI-Manual ROI-based results comparing Preterm CHDs with High Risk Term Non-CHD and Preterm Non-CHD Comparison Groups
| Preterm | Term | Preterm CHD |
|||
|---|---|---|---|---|---|
| n=27 mean (SD) |
n=28 mean (SD) |
n=21 mean (SD) |
p * | ||
| Peripheral White Matter | |||||
| Parietal** | FA | .17 (.06) | .18 (.05) | .17 (.06) | .948 |
| RA | .14 (.05) | .15 (.04) | .14 (.06) | .953 | |
| Axial | 1.95 (.33) | 1.80 (.35) | 1.91 (.40) | .378 | |
| Radial | 1.52 (.33) | 1.39 (.31) | 1.49 (.38) | .492 | |
| ADC | 1.67 (.33) | 1.53 (.32) | 1.63 (.38) | .475 | |
| Optic | FA | .32 (.05) | .34 (.06) | .29 (.08) | # .045 |
| Radiation** | RA | .27 (.04) | .29 (.05) | .25 (.07) | # .032 |
| Axial | 2.07 (.32) | 1.95 (.34) | 2.05 (.32) | .387 | |
| Radial | 1.26 (.24) | 1.15 (.24) | 1.30 (.26) | .162 | |
| ADC | 1.53 (.26) | 1.41 (.26) | 1.54 (.26) | .243 | |
| Deep White Matter | |||||
| Splenium | FA | .54 (.09) | .59 (.07) | .46 (.12) | ##.00001 |
| RA | .50 (.10) | .57 (.08) | .42 (.13) | ##.00003 | |
| Axial | 2.32 (.34) | 2.20 (.34) | 2.23 (.35) | .615 | |
| Radial | .91 (.20) | .77 (.17) | 1.02 (.22) | ##.00008 | |
| ADC | 1.38 (.22) | 1.24 (.21) | 1.42 (.21) | #.012 | |
| Gray Matter | |||||
| Thalamus** | FA | .17 (.02) | .17 (.02) | .16 (.03) | .716 |
| RA | .14 (.02) | .14 (.02) | .14 (.02) | .624 | |
| Axial | 1.36 (.20) | 1.23 (.19) | 1.34 (.21) | #.042 | |
| Radial | 1.07 (.16) | .98 (.16) | 1.07 (.18) | .066 | |
| ADC | 1.17 (.17) | 1.07 (.18) | 1.16 (.19) | .063 | |
ANOVA
Measurements are average of L and R readings
p-value ≤ 0.05 significant
Tukey’s Pairwise Comparisons:
Term controls significantly different from CHD’s. 2 other pairwise comparisons (term controls vs. preterm controls, preterm controls vs. CHD’s) non-significant
CHD’s significantly different from both preterm and term controls. Pairwise comparison of preterm and term controls non-significant
Comparative Assessment of Adjacent Central Cerebral White Matter
To compare the thalamic findings with adjacent central white matter regions, we also performed similar ROI measurements in three central white matter structures: parietal white matter, optic radiations, and splenium of the corpus callosum. The parietal white matter showed no differences across the three study groups. In contrast, there were significant differences seen in the optic radiations including reduced FA (p value =0.045). There was also reduced FA seen in the splenium (p-value < 0.0001) with increased radial diffusivity (p value < 0.0001) between the preterm CHD group compared to the term and preterm comparison groups (Table 3).
Developmental Trajectories of Thalamic Microstructure Across Groups
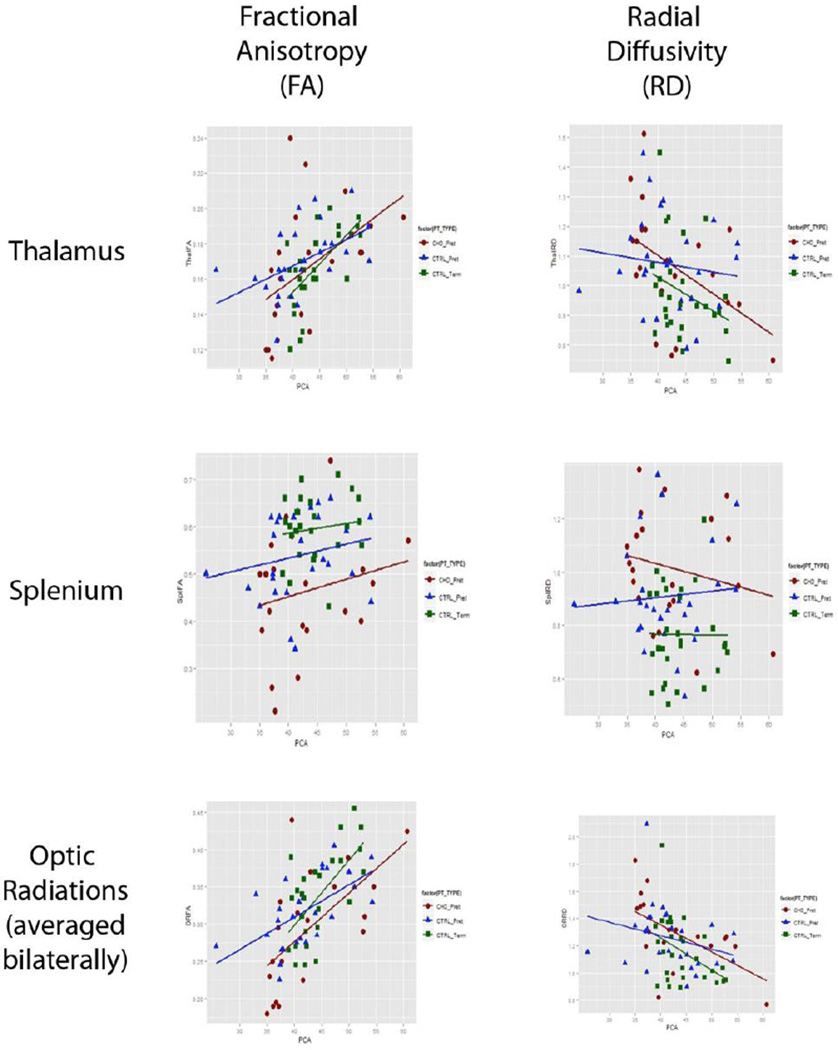
In order to investigate how development of microstructure in the thalamus might be differentially impacted across groups, we computed the correlation between age and each metric for the neonates in our preterm CHD and term/preterm non-CHD comparison groups. In the thalamus and optic radiations, there were increases in FA with post conceptional age in all three groups, without a significant age interaction. Significant age-related changes in radial diffusivity were also observed in the thalamus and optic radiations, and although these appeared to be associated with different correlations in preterms with CHD compared to other critically ill neonates, the interaction terms were not significant (Table 5). In the thalamus, radial diffusivity decreased with age in the preterm CHD group (r=−0.486, p=0.026), but not in the preterm neonates without CHD (r=−0.129, p=0.520) or the term neonates without CHD (r=−0.307, p=0.111). In the optic radiation, radial diffusivity decreased with age in the preterm CHD group (r=−0.587, p=0.007) and the term neonates without CHD (r=−0.423, p=0.025) but not in the preterm neonates without CHD (r=−0.269, p=0.175) (see Figure 2).
Table 5.
Study Group Comparisons of DTI Metric Correlations with Gestational Age
| Fractional Anisotropy | Radial Diffusivity | ||||||
|---|---|---|---|---|---|---|---|
| Correlation | p−value | Interaction by group |
Correlation | p−value | Interaction by group |
||
| Thal | Preterm CHD | 0.482 | 0.027 | p< 0.68 | −0.486 | 0.026 | p<0.49 |
| Preterm | 0.488 | 0.0097 | −0.129 | 0.520 | |||
| Term | 0.644 | .0002 | −0.307 | 0.111 | |||
| Splen | Preterm CHD | 0.220 | 0.338 | p<0.62 | −0.213 | 0.352 | p<0.80 |
| Preterm | 0.212 | 0.288 | 0.082 | 0.686 | |||
| Term | 0.133 | 0.500 | 0.010 | 0.959 | |||
| Optic Rad | Preterm CHD | 0.628 | 0.003 | p<0.63 | −0.587 | 0.007 | p<0.32 |
| Preterm | 0.587 | 0.001 | −0.269 | 0.175 | |||
| Term | 0.643 | 0.0002 | −0.423 | 0.025 | |||
Correlations significantly different from 0 are in BOLD. Thal=thalamus; Splen=Splenium; Optic Rad=Optic Radiations
Figure 2.
Age-interaction graphs: Fractional Anisotropy and Radial Diffusivity measurements in Thalamus, Splenium, and Optic Radiations correlating to Gestational Age (weeks) at time of MRI on x-axis. Preterm CHD—Red, Preterm Non-CHD—Blue, Term Non-CHD—Green.
Correlations among Preterm CHD DTI metrics and clinical-perioperative variables
Clinical variables (NEC, 1st ABG [arterial blood gas] pH, 1st ABG pO2, admission ABG pH, admission ABG pO2, pre-op ABG pH, pre-op ABG pO2, post-op ABG pH, post-op ABG pO2) and DTI measurements (FA, AD, RD, MD) of the thalamus and associated cerebral white matter structures (optic radiation parietal white matter and splenium) were correlated within the preterm CHD group. Our results indicated that the DTI metrics obtained in the thalamus were most strongly associated (0.60 or better) with an elevated first arterial blood gas pO2 (pO2 range 23–232) and elevated pre-operative arterial blood gas pH (pH range 7.13–7.59) (Table 4). Additionally, there were a number of strong correlations between DTI metrics in the vicinity of the optic radiations and the first arterial blood gas pO2 and the post-operative arterial blood gas pH (Table 4).
Table 4.
Correlations between DTI metrics and Preterm CHD Clinical Variables
| FA | AD | RD | ADC | ||
|---|---|---|---|---|---|
| NEC | Splenium | 0.08 | −0.142 | −0.157 | −0.226 |
| Parietal WM | −0.106 | −0.269 | −0.189 | −0.223 | |
| Thalamus | 0.172 | −0.329 | −0.347 | −0.349 | |
| Optic Rad | 0.149 | −0.089 | −0.152 | −0.128 | |
| Ist ABG pH | Splenium | 0.285 | −0.020 | −0.428 | −0.333 |
| Parietal WM | −0.002 | 0.015 | 0.013 | 0.011 | |
| Thalamus | 0.025 | 0.001 | −0.007 | −0.007 | |
| Optic Rad | 0.203 | 0.181 | 0.105 | 0.169 | |
| 1st ABG pO2 | Splenium | 0.296 | 0.883 | 0.374 | 0.740 |
| Parietal WM | −0.22 | 0.810 | 0.831 | 0.824 | |
| Thalamus | −0.035 | 0.846 | 0.746 | 0.783 | |
| Optic Rad | 0.453 | 0.936 | 0.935 | 0.944 | |
| Adm ABG pH | Splenium | −0.017 | 0.075 | −0.009 | −0.054 |
| Parietal WM | 0.398 | 0.047 | −0.129 | −0.068 | |
| Thalamus | −0.055 | 0.298 | 0.279 | 0.275 | |
| Optic Rad | 0.194 | 0.209 | 0.028 | 0.102 | |
| Adm ABG pO2 | Splenium | −0.16 | −0.141 | −0.006 | −0.107 |
| Parietal WM | 0.416 | −0.173 | −0.323 | −0.275 | |
| Thalamus | 0.143 | −0.104 | −0.125 | −0.113 | |
| Optic Rad | 0.187 | 0.172 | 0.022 | 0.072 | |
| Pre-op ABG pH | Splenium | −0.114 | 0.084 | 0.211 | 0.225 |
| Parietal WM | 0.034 | 0.336 | 0.258 | 0.287 | |
| Thalamus | −0.600 | 0.249 | 0.315 | 0.328 | |
| Optic Rad | −0.429 | −0.112 | 0.149 | 0.041 | |
| Pre-OP ABG pO2 | Splenium | 0.121 | 0.331 | 0.241 | 0.248 |
| Parietal WM | 0.436 | 0.314 | 0.14 | 0.208 | |
| Thalamus | 0.012 | 0.348 | 0.315 | 0.328 | |
| Optic Rad | 0.166 | 0.418 | 0.323 | 0.385 | |
| Post-OP ABG pH | Splenium | 0.107 | −0.060 | −0.262 | −0.241 |
| Parietal WM | −0.103 | 0.104 | 0.079 | 0.085 | |
| Thalamus | −0.140 | 0.153 | 0.166 | 0.164 | |
| Optic Rad | 0.138 | 0.225 | 0.600 | 0.134 | |
| Post-OP ABG pO2 | Splenium | 0.013 | 0.225 | 0.090 | 0.216 |
| Parietal WM | 0.208 | 0.161 | 0.063 | 0.098 | |
| Thalamus | −0.021 | 0.324 | 0.300 | 0.300 | |
| Optic Rad | 0.156 | 0.302 | 0.107 | 0.183 |
Discussion
This is the first study to describe selective microstructural imaging abnormalities in the thalamus and central white matter matter regions in premature babies born with CHD. When correlated with clinical variables, hyperoxemia at birth was predictive of abnormal diffusivity in the thalamus, splenium, parietal white matter, and optic radiations at term-equivalent age in the preterm CHD group. Additionally, a greater pre-operative pH was associated with a lower FA at term-equivalent in the preterm CHD group. Our data of the non-CHD diffusivity measurements is corroborated by measurements previously reported in the literature [24, 25]. Additionally, the white matter findings are consistent with Tract Based Spatial Statistics results recently reported [23].
In regards to the thalamus, the diffusivity of the preterm groups (CHD and non-CHD) were similarly greater than the term group, suggesting a difference in degree of myelination. Interestingly, the lag in myelination is demonstrating a trend toward being more pronounced in the CHD group (as illustrated by the greater slope of the line on Figure 2); and neither preterm group approximates the diffusivity of the term neonates even by 60 weeks post-conceptional age. It is difficult to know the exact reason for such delay as the mechanism and pathophysiology may be different between these two prematurely born populations. Delayed maturation has been demonstrated in the premature brain [19], as well as the term infant with CHD [26 – 28]. However, arrested development versus disarrayed development may appear grossly equivalent but in fact have a profound impact on future potential.
There was a positive correlation between the first measured arterial pO2 and the thalamic diffusivity, as well as the diffusivity of the associated central cerebral white matter regions. This is potentially very interesting as it was consistently a strong association and suggestive of seeing the end-result imaging measurements at term-equivalent of the effects of perinatal hyperoxemia in the preterm baby. As only approximately 30% of the premature CHD group was prenatally diagnosed as having complex congenital heart disease, it is possible that the clinical course involved the premature infant being given progressively more supplemental oxygen to achieve acceptable oxygenation. Once on significant amounts of oxygen and the preterm neonate was not responding as expected, an echocardiogram was obtained that revealed the heart lesion; then, oxygen goals were revised and the supplemental oxygen likely weaned. But, exposure to hyperoxic conditions had already occurred and possibly affected the developing brain. It is known that the developing brain is vulnerable to hyperoxemia effects which causes apoptosis, increased inflammation, and cell death [29]. Specifically in the preterm neonate, the cells necessary for myelin production are forming and at risk. Oligodendroglial precursors and premyelinating oligodendroglia are susceptible to injury by free radicals, reactive oxygen and nitrogen species, and inflammatory cytokines [29 – 31]. The microglia defense network of the brain has also been demonstrated to become activated by an increase in cerebral gene expression of inflammatory mediators (IL-1beta, IL-12p40, TLR-2, and TRL-4) [29]. And, neurotrophic factor production is suppressed by hyperoxemia [32]. Such oxidative stress effects have been demonstrated to have a predilection for the deep and central white matter [29, 30]. The association of abnormal diffusivity in the associated central cerebral white matter regions in our study is supported by such previous findings. It has been demonstrated in the rat that exposure to hyperoxia (80% oxygen) over a two-hour duration lead to significant cell death in the brain 24 hours later [32]. Such timing is consistent with the timing of hyperoxemia exposure being seen in this study’s patients. As well, a critical time period of vulnerability seems to exist for such oxidative damage to the developing brain. Again, in the rat hyperoxemic population, the largest numbers of cell death within cortical white matter tracts occurred within the first seven days. In humans, this time period is thought to begin with the third trimester of gestation [32]. The suggestion of increased risk of cell death in the developing preterm brain is important as it has been demonstrated that decreased volume of grey matter structures correlates with cognitive deficits [32]. As well, following such ideas it is tempting to speculate that hyperoxemia-therapy in undiagnosed complex congenital heart disease neonates may exacerbate the delayed regional development and/or volumetric brain growth [28].
Additionally, there was an inverse correlation between the pre-operative arterial pH and the thalamic FA and a trend of the same in the optic radiations, which was unexpected. This may be a function of a sicker premature neonate who is being over-ventilated, perhaps acutely due to vigorous resuscitation. Or, it may be due to more chronic management attempting to maintain stability while trying to delay cardiac repair in a small baby such as aggressive diuretic therapy or over-ventilating in making an effort to achieve a normal pH in the face of evolving metabolic acidosis due to hypoperfusion resulting from the cardiac lesion. Due to cerebral vasoregulation, low carbon dioxide levels lead to vasoconstriction in the brain. In the premature brain, there is not much vasculature extending to the white matter, which may explain why the greatest effect of elevated pH was seen in the thalamus. Or, perhaps it is because the thalamus is a very active region of development during this time period as thalamocortical projections are extending through this region and are affected.
Perhaps the critical structure that deserves more attention in the preterm CHD individual’s brain development is the subplate. The subplate is the origin of many cortical synapses and is critical for the development of cortical thalamic development [30]. By the late preterm period, most corticothalamic connections have been made and corticocortical pathways are still being established [30]. Subplate neurons have been demonstrated to result in cortical visual impairment and they are known to be vulnerable to hypoxia/ischemia [20]. It is generally accepted that these neurons are helpful for working around areas of damage and they typically involute by 34 weeks of gestation [30]. It is plausible that in preterm CHD neonates who have likely delayed brain maturation by an average four weeks [27], a significant insult to their developing brain that affects the subplate could disrupt/delay crucial thalamocortical connections from being established. Such deficits could explain, in part, the neurodevelopmental challenges faced by children with CHD [33].
Limitations
It could be suggested that we should have focused on the complex cyanotic heart lesions and excluded infants with other lesions such as ASD and VSD due to marked differences in the hemodynamics in these neonates both in utero and postnatally [34]. However, the incidence of complex CHD among preterm infants is relatively rare and we did not have sufficient sample size to study individual lesions. A second limitation in this study is the inclusion of cases with MRI scans performed preoperatively and postoperatively, with the majority of the scans being performed postoperatively. Because the post-operative status cannot tease out the innate effects of brain microstructural maldevelopment versus those therapeutically influenced (bypass, timing of surgery, etc), the demonstrates microstructural changes may be the beginning or the evolution of aberrant development. Third, there is the potential for sampling bias as we relied on clinical scans to identify our neonates prior to study inclusion. Additionally, it would have been interesting to look at the correlation of initial blood gas findings in the non-CHD premature group in relation to their microstructural findings. However, these data were not available as these babies were not inborn. Lastly, our comparison groups were also critically ill neonates and not truly normal controls, who were found to have structurally normal brains on screening studies.
Conclusion
Our study provides evidence that preterm neonates with CHD demonstrate DTI-metric age-related microstructural abnormalities in the thalamus when compared to other critically ill neonates without CHD. In addition, perinatal hyperoxemia and elevated preoperative pH appear to be predictive of abnormal thalamic microstructure in preterm neonates with CHD. This study emphasizes the vulnerability of thalamo-cortical development in the preterm neonate with CHD.
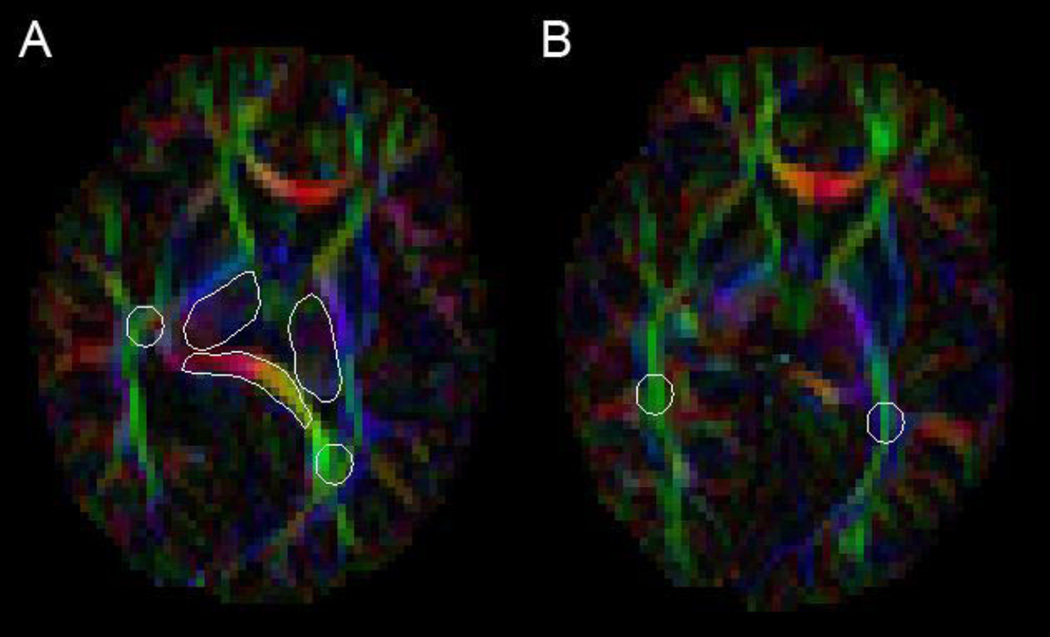
Figure 1.
ROI placement: Image A: Thalamus (darker outilined region), Splenium (yellow and red outlined region), Parietal WM (green outlined region); Image B: Optic Radiations
ACKNOWLEGMENTS
The authors thank Julie Castro, Lisa Padilla, and Hesham Mahmoud for their assistance with patient recruitment and data management. We thank Melanie Gieraltowski for manuscript preparation. Support for this study is provided by the National Institutes of Health: K23NS063371 to AP, P50NA019632 to JLW, 1UL1RR031986 to AP, JLW, LP; and Rudi Schulte Foundation to SB, CHLA Clinical Investigation Center, The Children’s Heart Foundation, SUPPORTED IN PART BY THE NIH NCRR GCRC GRANT 5 MO1 RR00043-44 AT CHILDRENS HOSPITAL LOS ANGELES.
Abbreviation List
- CHD
congenital heart disease
- NICU
neonatal intensive care unit
- ASD
atrial septal defect
- VSD
ventricular septal defect
- HLHS
hypoplastic left heart syndrome
- TGA
transposition of the great arteries
- DORV
double outlet right ventricle
- FA
fractional anisotropy
- RD
radial diffusivity
- AD
axial diffusivity
References
- 1.Tanner K, Sabrine N, Wren C. Cardiovascular malformations among preterm infants. Pediatrics. 2005;116:e833–e838. doi: 10.1542/peds.2005-0397. [DOI] [PubMed] [Google Scholar]
- 2.Dees E, Lin H, Cotton RB, Graham TP, Dodd DA. Outcome of preterm infants with congenital heart disease. J Pediatr. 2000;137:653–659. doi: 10.1067/mpd.2000.108568. [DOI] [PubMed] [Google Scholar]
- 3.Cheng HH, Almodovar MC, Laussen PC, et al. Outcomes and risk factors for mortality in premature neonates with critical congenital heart disease. Pediatr Cardiol. 2011;32:1139–1146. doi: 10.1007/s00246-011-0036-3. [DOI] [PubMed] [Google Scholar]
- 4.Natarajan G, Anne SR, Aggarwal S. Outcomes of congenital heart disease in late preterm infants: double jeopardy? Acta Paediatr. 2011;100:1104–1107. doi: 10.1111/j.1651-2227.2011.02245.x. [DOI] [PubMed] [Google Scholar]
- 5.Gelehrter S, Fifer CG, Armstrong A, Hirsch J, Gajarski R. Outcomes of hypoplastic left heart syndrome in low-birth-weight patients. Pediatr Cardiol. 2011;32:1175–1181. doi: 10.1007/s00246-011-0053-2. [DOI] [PubMed] [Google Scholar]
- 6.Costello JM, Polito A, Brown DW, et al. Birth before 39 weeks' gestation is associated with worse outcomes in neonates with heart disease. Pediatrics. 2010;126:277–284. doi: 10.1542/peds.2009-3640. [DOI] [PubMed] [Google Scholar]
- 7.Goff DA, Luan X, Gerdes M, et al. Younger gestational age is associated with worse neurodevelopmental outcomes after cardiac surgery. J Thorac Cardiov Sur. 2012;143:535–542. doi: 10.1016/j.jtcvs.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kostovic I, Judas M. Patterns of cortical lamination during prenatal life: Do they have implications for treatment? Neuroscience and Biobehavioral Reviews. 2007;31:1157–1168. doi: 10.1016/j.neubiorev.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Miller SP, McQuillen PS, Hamrick S, et al. Abnormal brain development in newborns with congenital heart disease. New Engl J Med. 2007;357:1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe K, Matsui M, Matsuzawa J, et al. Impaired neuroanatomic development in infants with congenital heart disease. J Thorac Cardiov Sur. 2009;137:146–153. doi: 10.1016/j.jtcvs.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 11.Miller SP, Ferriero DM, Leonard C, et al. Early brain Injury in premature newborns detected with magnetic resonance imaging is associated with adverse neurodevelopmental outcomes. J Pediatr. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Newburger JW, Sleeper LA, Bellinger DC, et al. Early developmental outcome in children with hypoplastic left heart syndrome and related anomalies: The single ventricle reconstruction trial. Circulation. 2012;125:2081–2091. doi: 10.1161/CIRCULATIONAHA.111.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballweg JA, Wernovsky G, Gaynor JW. Neurodevelopmental outcomes following congenital heart surgery. Pediatr Cardiol. 2007;28:126–133. doi: 10.1007/s00246-006-1450-9. [DOI] [PubMed] [Google Scholar]
- 14.Bellinger DC, Wypij D, Rivkin MJ, et al. Adolescents with d-transposition of the great arteries corrected with the arterial switch procedure: neuropsychological assessment and structural brain imaging. Circulation. 2011;124:1361–1369. doi: 10.1161/CIRCULATIONAHA.111.026963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics. 2000;105:1082–1089. doi: 10.1542/peds.105.5.1082. [DOI] [PubMed] [Google Scholar]
- 16.Karsdorp PA, Everaerd W, Kindt M, Mulder BJM. Physiological and cognitive functioning in children and adolescents with congenial heart disease. Pediatr Psychol. 2007;32:527–541. doi: 10.1093/jpepsy/jsl047. [DOI] [PubMed] [Google Scholar]
- 17.Shillingford AJ, Glanzman MM, Ittenbach RF, Clancy RR, Gaynor JW, Wernovsky G. Inattention, hyperactivity, and school performance in a population of school-age children with complex congenital heart disease. Pediatrics. 2008;121:e759–e767. doi: 10.1542/peds.2007-1066. [DOI] [PubMed] [Google Scholar]
- 18.Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, Gousias IS, Edwards AD, Counsell SJ. The effect of preterm birth on thalamic and cortical development. Cereb Cortex. 2012;22:1016–1024. doi: 10.1093/cercor/bhr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chau V, Synnes A, Grunau RE, Poskitt KJ, Brant R, Miller SP. Abnormal brain maturation in preterm neonates associated with adverse developmental outcomes. Neurology. 2013;81:2082–2089. doi: 10.1212/01.wnl.0000437298.43688.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McQuillen PS, Ferriero DM. Perinatal subplate neuron injury: implications for cortical development and plasticity. Brain Pathol. 2005;15:250–260. doi: 10.1111/j.1750-3639.2005.tb00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kostovic I, Judas M. The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatrica. 2010;99:1119–1127. doi: 10.1111/j.1651-2227.2010.01811.x. [DOI] [PubMed] [Google Scholar]
- 22.Kanold PO, Luhmann HJ. The subplate and early cortical circuits. Annu Rev Neurosci. 2010;33:23–48. doi: 10.1146/annurev-neuro-060909-153244. [DOI] [PubMed] [Google Scholar]
- 23.Paquette LB, Wisnowski JL, Pruetz JD, et al. Abnormal cerebral microstructure in premature neonates with congenital heart disease. AJNR. 2013;34:2026–2033. doi: 10.3174/ajnr.A3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartha AI, Yap KR, Miller SP, Jeremy RJ, Nishimoto M, Vigneron DB, Barkovich AJ, Ferriero DM. The normal neonatal brain: MR imaging, diffusion tensor imaging, and 3D MR spectroscopy in healthy term neonates. AJNR. 2007;28:1015–1021. doi: 10.3174/ajnr.A0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Counsell SJ, Dyet LE, Larkman DJ. Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. Neuroimage. 2007;34:896–904. doi: 10.1016/j.neuroimage.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 26.Andropoulos DB, Hunter JV, Nelson DP, Stayer SA, Stark AR, McKenzie ED, Heinle JS, Graves DE, Fraser CD., Jr Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac and Cardiovasc Surg. 2010;139(3):543–556. doi: 10.1016/j.jtcvs.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Licht DJ, Shera DM, Clancy RR. Brain maturation is delayed in infants with complex congenital heart defects. J Thorac Cardiovasc Surg. 2009;137:529–536. doi: 10.1016/j.jtcvs.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clouchoux C, du Plessis AJ, Bouyssi-Kobar M, et al. Delayed cortical development in fetuses with complex congenital heart disease. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs281. [DOI] [PubMed] [Google Scholar]
- 29.Markus T, Hansson S, Amer-Wahlin I, Hellstrom-Westas L, Saugstad OD, Ley D. Cerebral inflammatory response after fetal asphyxia and hyperoxic resuscitation in newborn sheep. Pediatric Research. 2007;62(1):71–77. doi: 10.1203/PDR.0b013e31811ead6e. [DOI] [PubMed] [Google Scholar]
- 30.Haynes RL, Folkerth RD, Keefe RJ, Sung I, Swzeda LI, Rosenberg PA, Volpe JJ, Kinney HC. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2013;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 31.Kaindl AM, Favrais G, Gressens P. Molecular mechanism involved in injury to the preterm brain. J Child Neurol. 2009;24(9):1112–1118. doi: 10.1177/0883073809337920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felderhoff-Mueser U, Bittigau P, Sifringer M, Jarosz B, Korobowicz E, Mahler L, Piening T, Moysich A, Grune T, Thor F, Heumann R, Buhrer C, Ikonomidou C. Oxygen causes cell death in the developing brain. Neurobiology of disease. 2004;13:273–282. doi: 10.1016/j.nbd.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 33.Bellinger DC, Bernstein JH, Kirkwood MW. Visual-spatial skills in children after open-heart surgery. J Dev Behav Pediatr. 2003;24:169–179. doi: 10.1097/00004703-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 34.Ball G, Counsell SJ, Anjari M, Merchant N, Arichi T, Doria V, Rutherford MA, Edwards AD, Rueckert D, Boardman JP. An optimised tract-based spatial statistics protocol for neonates: applications to prematurity and chronic lung disease. Neuroimage. 2010;53:94–102. doi: 10.1016/j.neuroimage.2010.05.055. [DOI] [PubMed] [Google Scholar]