Abstract
Low income, poor diet, obesity and a lack of exercise are inter-related lifestyle factors that can profoundly alter our biological make-up to increase cancer risk, growth and development. We recently reported a potential mechanistic link between carbohydrate derived metabolites and cancer which may provide a biological consequence of lifestyle that can directly impact tumor biology. Advanced glycation end-products (AGEs) are reactive metabolites produced as a by-product of sugar metabolism. Failure to remove these highly reactive metabolites can lead to protein damage, aberrant cell signaling, increased stress responses, and decreased genetic fidelity. Critically, AGE accumulation is also directly affected by our lifestyle choices and shows a race specific, tumor dependent pattern of accumulation in cancer patients. This review will discuss the contribution of AGEs to the cancer phenotype with a particular emphasis on their biological links with the socioeconomic and environmental risk factors that drive cancer disparity. Given the potential benefits of lifestyle changes and the potential biological role of AGEs in promoting cancer, opportunities exist for collaborations impacting basic, translational, epidemiological and cancer prevention initiatives.
Introduction
Despite great progress in the treatment of many cancers, specific populations across the world still suffer disproportionately high levels of cancer incidence and mortality. Cancer disparity is most evident in our African American populations who bear the highest cancer burden for many tumor types. Poor diet, low income, obesity and a lack of exercise are established lifestyle factors that are known to increase cancer burden and are often more prevalent in African American communities (1–3). As our understanding of tumor biology advances it is becoming increasingly clear that these inter-related lifestyle factors have distinct molecular consequences on the biological make-up of tumors, altering cell signaling events and gene expression profiles to contribute to cancer disparity outcomes such as its earlier development or its progression to more aggressive disease. Sparse information exists about the genetic and biological factors that contribute to differential cancer survival and mortality rates observed in minority populations. A greater understanding of the interplay between risk factors and the molecular mechanisms associated with cancer disparity will significantly impact minority health.
We recently reported a potential mechanistic link between sugar derived metabolites and cancer which may provide a molecular consequence of our lifestyle choices that can directly impact tumor biology and contribute to cancer disparity (4). Advanced glycation end products (AGEs) are reactive metabolites produced during the breakdown of sugar. AGEs accumulate in our tissues and organs over time and contribute to the development and complications associated with diseases of advancing age including diabetes, cardiovascular disease, renal failure, arthritis and neurodegenerative disorders (5). The rate of AGE accumulation in our bodies results from a balance between 1) their endogenous accumulation during the breakdown of sugar via the non-enzymatic, spontaneous glycosylation of proteins, lipids and DNA; 2) their exogenous intake through the foods we consume and other lifestyle factors such as drinking alcohol, smoking and a sedentary lifestyle; and 3) their inefficient removal via renal and/or enzymatic clearance, around 10–30% of exogenous AGEs are absorbed intestinally but only a third of those are excreted in urine and feces (6). Changes in this dynamic equilibrium, as seen as we grow older or as a consequence of poor lifestyle, causes increased levels of AGE accumulation which promote disease complications and progression.
While the mechanistic links between AGEs and lifestyle have been identified in diseases such as diabetes and cardiovascular disease (6), a potential contribution to the development and progression of cancer is relatively understudied. AGE presence in human tumors was first demonstrated in larynx, breast and colon tumors by immune-histochemical staining. Exogenous AGE treatment of breast (7) and prostate (8) immortalized cancer cell lines promotes cell growth, migration and invasion. In prostate cancer, AGE modified basement membrane promotes the invasive properties of prostate epithelial cells and correlates with decreased survival (8). A recent paper found that the dietary derived AGE carboxymethyl-lysine was associated with modestly increased risk of pancreatic cancer and may partially explain the positive association between red meat and pancreatic cancer (9).
Our group examined circulating and tumor AGE levels in clinical specimens of prostate cancer and identified a race specific, tumor dependent pattern of accumulation (4). AGE levels were significantly elevated in both serum and tumor with highest accumulation occurring in more aggressive tumors. When examined in a matched cohort of patients, high AGE levels in the serum correlated with high AGE accumulation in cancer tissue (4). Significantly, when the data was stratified by race, AGE metabolite levels were significantly higher in serum from African American cancer patients compared to Caucasian. These initial data indicate that AGEs may represent a potential mechanistic link between cell metabolism and cancer which may also provide a biological consequence of the lifestyle risk factors that drive cancer health disparity.
This review aims to highlight the social and mechanistic associations that exist between AGE metabolites, cellular stress response and the lifestyle factors known to increase cancer incidence and mortality. Given the potential benefits of lifestyle changes and the role of AGEs in promoting disease phenotypes, opportunities exist for impacting cancer disparity via basic and translational studies in defined population groups as well as health and nutritional education and community outreach initiatives.
AGE accumulation is inherently linked to lifestyle
Systematic reviews and meta-analysis studies support the view that eating unhealthily, being overweight or obese and/or sustaining a sedentary lifestyle can increase risk of cancer, risk of cancer recurrence and decrease overall survival rates (10,11). A recent statement from Cancer Research UK estimated that lifestyle accounts for around 40% of cancer cases, 2nd only to smoking. This is racially significant as the highest rates of being overweight and obese (defined as a BMI >25) and the lowest adherence to CDC recommended physical activity guidelines (defined as a minimum of 150mins per week) occur among the African American populations at highest risk of developing and dying of cancer (12). A family tradition of high calorie “soul foods” with a heavy use of fat and sugar exists for many African American families. Additionally, low income promotes the use of cheap, unhealthy and highly processed foods which can lead to weight gain, obesity and increased cancer risk. Poverty rates within African Americans communities are amongst the highest in the country and they are also more likely to live in designated “food deserts” where people have limited access to healthy affordable food (13). All of these lifestyle factors not only contribute to health disparity and increase cancer risk but significantly contribute to the exogenous AGE accumulation pool in our bodies:
The typical Western diet comprising of red meat, refined grains and high fat/high sugar foods are associated with systemic disease and are particularly AGE-laden, contributing as much as 30% of the AGEs accumulated within our bodies (6). The consumption of AGE-rich diets by mice increases circulating and tissue AGE content to promote conditions such as atherosclerosis, diabetes and kidney disease all of which are inhibited by dietary AGE restriction (6). Although human studies are limited, associations between elevated AGE and serum biomarkers of oxidative stress, endothelial dysfunction, inflammation, hyperlipidemia and hyperglycemia have been identified in patients with impaired renal function and diabetes (6). Evidence supports dietary AGE restriction for the reduction of 8-isoprostanes and tumor necrosis factor-α (TNFα) in healthy adults and reduced glucose and insulin resistance and AGE-modified low-density lipoprotein in type-2 diabetes patients (14).
AGE content in foods is not only dependent on nutritional content but also on how the food is prepared. Cooking methods involving dry heat such as grilling, broiling, and searing, used to improve food flavor, aroma and appearance, accelerate the glycation reaction between sugars and proteins to significantly increase overall AGE content (6). Frying meats for example can increase AGE content by as much as ten-fold. Thermal processing and/or irradiation by food manufacturers, used to improve food safety, preservation, and taste also rapidly accelerates the AGE forming reaction (6). Due to beneficial effects on flavor, synthetic AGEs are now directly added into the manufacturing process for several food items. Processed foods are now one of the most common food items in groceries baskets across the country and due to their relatively low cost are often most heavily used by low income families.
Recent data from the European Prospective Investigation into Cancer and Nutrition Study concludes that a sedentary lifestyle poses twice the risk of premature death as being overweight or obese (15). Studies of the effects of physical activity on AGE levels are limited and are mainly carried out using animal models but indicate that regular physical activity can help maintain or even reduce AGE levels in our bodies. In obese rats, regular moderate exercise reduced advanced glycation early diabetic nephropathy, lowered plasma AGE-associated fluorescence as well as overall renal AGE content (16). Similarly, increased physical activity in middle aged senescent rats reduced both cardiac fibrosis and circulating AGE levels (17). In non-diabetic middle-aged women, a 12 week lifestyle modification consisting of an initial educational session followed by encouragement showed that the number of daily walking steps significantly correlated with AGE levels. Decreases in AGEs correlated with reduced body weight and body fat content (18).
In summary, AGEs are inherently linked with poor lifestyle and play a pathogenic role in multiple diseases associated with growing older. Approaches to define the molecular consequences of AGE accumulation may define novel therapeutic targets and potential biomarkers with which to reduce cancer incidence and mortality particularly in minority populations.
AGE accumulation, RAGE and stress response
Persistent, unchecked inflammation and a related increase in oxidative stress are major biological consequences of poor lifestyle and an underlying factor behind most, if not all, systemic diseases. A healthy diet and regular exercise has been shown to reduce chronic inflammation associated with diabetes and cardiovascular disease in the absence of weight loss, and studies indicate that increased physical activity is associated with lower inflammatory marker levels (19,20). It is now generally accepted that chronic inflammation, oxidative stress and cancer are intrinsically linked, and an inflammatory microenvironment is an essential element for both the onset and growth of tumors. In pre-cancerous lesions, a constant state of inflammatory response and increased reactive oxygen species (ROS) production can cause both genetic and epigenetic alterations to alter gene expression and increase cancer risk (21). In established tumors, inflammation is thought to mediate crosstalk between cancers cells and the stroma resulting in the active recruitment of immune cells to the tumor microenvironment and increased oxidative stress (21). The inflammatory milieu created contributes to tumor onset and progression by promoting genetic instability, cell survival, growth and metastatic potential.
A major pathogenic consequence of AGE accumulation is the perpetual activation of immune mediated chronic inflammation and the generation of ROS which results in a perpetual inflammatory microenvironment susceptible to disease development. C-reactive protein (CRP) is a marker of inflammation that is linked with increased risk of heart disease, diabetes and some cancers. In diabetes, serum AGE levels are an independent determinant of CRP levels due to a chronic inflammatory response (22). Increases in the exogenous AGE pool mediated by poor diet and a sedentary lifestyle may contribute to tumor development and growth through the perpetual activation of immune response (Fig 1). This would be particularly significant in population groups with the highest prevalence of poor lifestyle and cancer risk, such as our African American communities, which evidence suggests may have higher AGE accumulation levels (4). African Americans have an increased burden of chronic inflammation which is independent of body mass index (BMI) and other potential confounding factors (23). African Americans have higher CRP levels than Caucasian American which correlates with obesity and other metabolic and disease risk factors (24). Clinical and epidemiological evidence also identifies African American race as an independent risk factor for elevated oxidative stress (25) and the increased expression and/or activity of critical oxidative stress markers (26).
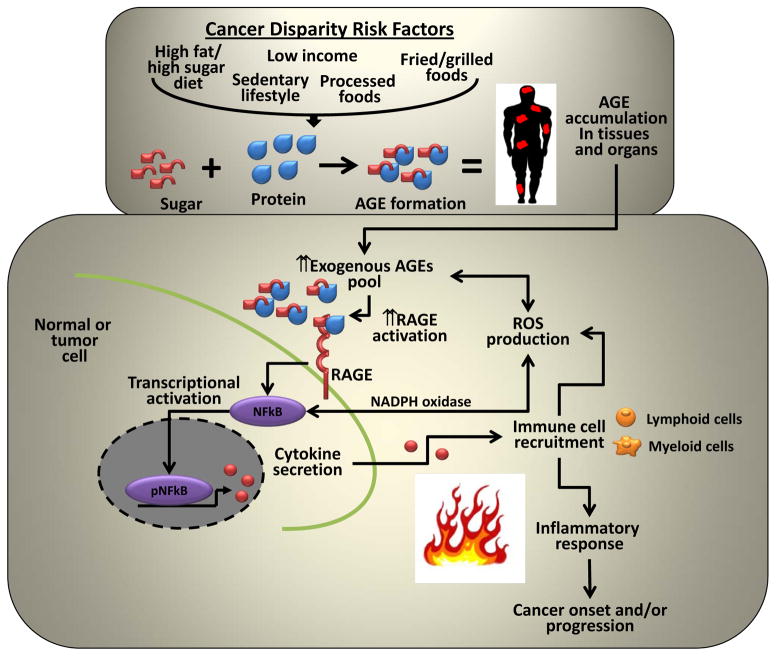
Figure 1. Hypothetical model for AGE mediated activation of inflammatory response in tumors.
Cancer disparity risk factors contribute to the increased exogenous AGE pool in our tissues and organs leading to the increased expression and activation of its cognate receptor RAGE. RAGE activation in turn triggers the activation of inflammatory associated transcription factors such as NFkB, STAT3 and HIF1α which increases cytokine secretion leading to the increased recruitment of lymphoid and myeloid immune cells into the tumor microenvironment, elevated ROS production and an inflammatory response. To perpetuate the cycle, reactive intermediates generated during AGE formation can directly increase ROS production to further promote the immune response. Oxidizing conditions and ROS presence can in turn further promote the formation of AGEs to create a cyclic and persistent oxidative response.
AGEs contribute to immune mediated chronic inflammation by functioning as ligand for the transmembrane receptor for AGE known as RAGE (or AGER) (Fig 1). RAGE is overexpressed in a number of tumors and evidence suggests a direct link between RAGE activation with the proliferation, survival, migration, and invasion of tumor cells. Loss of RAGE in inflammatory mouse models confers resistance to skin carcinogenesis and suppresses tumor growth (27). In prostate cancer, RAGE preferentially interacts with AGE over other potential RAGE ligands and AGE treatment of prostate cancer cells induces both cell growth and invasion (28). Mechanistically, AGE mediated activation of RAGE results in the increased activation of pro-inflammatory transcriptional regulators including nuclear factor-kappa B (NFkB), signal transducer activator of transcription 3 (STAT3) and hypoxia inducible factor 1 (HIF1). In diabetes, RAGE activation perpetuates NF-kB activation in a feedback loop involving de novo synthesis of NFkB-p65 which functions to maintain a persistent pool of this key pro-inflammatory regulator. Increased activation of these critical transcription factors increases the secretion of cytokines/chemokines such as interleukin-1β (IL1β), interleukin-6 (IL6) and TNFα leading to the increased recruitment of lymphoid and myeloid immune cells into the tumor microenvironment, elevated ROS production and an inflammatory response (27). To perpetuate the cycle and add further fuel to the fire, reactive intermediates generated during AGE formation (i.e. Schiff’s Bases and Amadori products) can directly increase ROS production and increased ROS presence can in turn further promote the formation of AGE precursors such as methylglyoxal to create a cyclic and persistent inflammatory response (29). Significantly, anti-oxidants can inhibit AGE induced changes in glucose consumption and lower ROS levels. AGE activation of RAGE increases heme oxygenase-1, nuclear translocation of NF-kB, and increased endothelial expression of vascular cell adhesion molecule-1 (VCAM-1) all of which function to increase oxidative stress and elevated levels of ROS (30). RAGE loss of function inhibits all of these AGE mediated effects.
Significance to cancer research
Based on associations between active metabolism, lifestyle, and immune response, increases in exogenous AGE accumulation may represent a biological mechanism contributing to cancer disparity and may represent a novel paradigm to explaining the increased cancer incidence and mortality figures observed within minority populations (Fig 1). A series of recent articles has highlighted the tumor associated immune response as a critical pathway contributing to cancer disparity in African Americans. An examination of expression differences based upon tumor composition shows that cytokine signaling associated with an increased immune response was found to be a predominant pathway increased in African American prostate cancer patients (2). Upon closer analysis, the majority of race specific differential gene expression was found in the stromal compartment of the tumor (2). A similar race specific increase in immune response gene copy number and gene expression was seen in matched radical prostatectomy tissues (31) and in Gleason 6 prostate tumors (1).
An analysis of over 500 genes previously associated with prostate cancer shows that African American prostate tumors have significant up-regulation of NFkB and inflammatory cytokine factors (IL6, IL8, IL1B, C-X-C chemokine receptor type 4, and fatty acid synthase) compared to European Americans (32). In breast cancer, race is an independent predictor of elevated IL6 levels (33). Clinical and epidemiological evidence also identifies African American race as an independent risk factor for elevated oxidative stress and ROS levels. For example, nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) catalyzes the reduction of superoxide (O2·) radicals to ROS. Significantly, HUVEC cells from African Americans show higher levels of nitric oxide, lower superoxide dismutase activity and increased expression of the NADPH oxidase subunit p47phox protein than their Caucasian counterparts (26). These combined data further indicate that the immune mediated inflammatory response may be elevated in African American cancer patients and therefore may be more susceptible to the pathogenic effects of AGE accumulation. Such a heightened inflammatory response may be a major contributor to the development and progression of cancer and contribute to the dire cancer incidence and survival rates observed in this population.
The existence of AGE metabolites, their connections with diet and lifestyle and their contribution to systemic disease are relatively unfamiliar to the general public as well as the cancer research community. While emerging research has identified increased levels of AGEs in the circulation and tumor of cancer patients and has identified a significant role in carcinogenesis for their cognate receptor RAGE, it is not known if the same AGE-RAGE mediated biological pathways established in other systemic diseases are at play in the tumor microenvironment and to what extent AGEs derived from poor diet and a sedentary lifestyle contribute. Overall, supporting evidence for dietary restriction and/or physical activity interventions to reduce AGE levels in humans is hampered by the need for long term high-quality randomized control trials with larger cohorts and defined disease outcomes. Multidisciplinary, collaborative research teams are needed involving behavioral intervention experts, dieticians as well as basic, translational and population scientists in order to fully comprehend the link between lifestyle, cancer and AGE accumulation. Given the benefits of lifestyle changes on cancer incidence and progression and the role of dietary-AGE in promoting disease phenotypes, opportunities exist for impacting cancer prevention initiatives arising through health and nutritional education and community outreach: while the accumulation of AGEs in our tissues and organs cannot be prevented, we can make changes to our everyday lifestyle to keep their accumulation at a minimum (6):
Avoid/cut down on foods high in protein, sugar and fat as well as processed foods
Increase intake of natural grains, vegetables and fruits
Cook foods at lower temperatures for longer periods of time
Do not overcook meats and where possible skip the browning step
Substitute high-sugar, oil-based marinades with lemon juice, vinegar, and tomato juice and experiment with different spices and rubs for enhanced flavor
Exercise regularly, take steps to change your sedentary lifestyle towards a more active one
Due to the success of earlier detection and more effective treatments for many cancers, the number of cancer survivors is ever increasing with the U.S. cancer survivor population expected to reach almost 20 million by 2024. Poor lifestyle, including obesity and physical inactivity are major challenges facing all cancer survivors that may negatively affect recurrence and represent modifiable risk factors which may augment risk reduction through lifestyle interventions and education strategies. The accumulation of AGEs may represent a unique common cancer risk factor associated with early recognition of cancer onset and/or its potential recurrence in cancer survivors. This would allow for intensive risk reduction and improved identification of high-risk patients requiring defined dietary and physical activity intervention aimed at reducing the rate of AGE accumulation. It may also lead to innovative insights for pharmacologic and lifestyle adjustment and could identify protective factors that may underlie the observed differences in health outcomes observed between health disparity populations and the general population.
Acknowledgments
Grant Support
This work was supported in part by grants from the NIH (P20 CA157071 to M.E. Ford and J.D. Salley; R21 CA176135 to D.P. Turner).
Footnotes
Disclosure of Potential Conflicts of Interest
The authors disclose no potential conflicts of interest
Bibliography
- 1.Reams RR, Agrawal D, Davis MB, Yoder S, Odedina FT, Kumar N, et al. Microarray comparison of prostate tumor gene expression in African-American and Caucasian American males: a pilot project study. Infectious agents and cancer. 2009;4 (Suppl 1):S3. doi: 10.1186/1750-9378-4-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kinseth MA, Jia Z, Rahmatpanah F, Sawyers A, Sutton M, Wang-Rodriguez J, et al. Expression differences between African American and Caucasian prostate cancer tissue reveals that stroma is the site of aggressive changes. International journal of cancer Journal international du cancer. 2014;134(1):81–91. doi: 10.1002/ijc.28326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin DN, Starks AM, Ambs S. Biological determinants of health disparities in prostate cancer. Current opinion in oncology. 2013;25(3):235–41. doi: 10.1097/CCO.0b013e32835eb5d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster D, Spruill L, Walter KR, Nogueira LM, Fedarovich H, Turner RY, et al. AGE metabolites: a biomarker linked to cancer disparity? Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2014;23(10):2186–91. doi: 10.1158/1055-9965.EPI-14-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho SJ, Roman G, Yeboah F, Konishi Y. The road to advanced glycation end products: a mechanistic perspective. Current medicinal chemistry. 2007;14(15):1653–71. doi: 10.2174/092986707780830989. [DOI] [PubMed] [Google Scholar]
- 6.Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(6):911–16. e12. doi: 10.1016/j.jada.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharaf H, Matou-Nasri S, Wang Q, Rabhan Z, Al-Eidi H, Al Abdulrahman A, et al. Advanced glycation endproducts increase proliferation, migration and invasion of the breast cancer cell line MDA-MB-231. Biochimica et biophysica acta. 2015;1852(3):429–41. doi: 10.1016/j.bbadis.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Teja M, Gronau JH, Breit C, Zhang YZ, Minamidate A, Caley MP, et al. AGE-modified basement membrane cooperates with Endo180 to promote epithelial cell invasiveness and decrease prostate cancer survival. The Journal of pathology. 2014 doi: 10.1002/path.4485. [DOI] [PubMed] [Google Scholar]
- 9.Jiao L, Stolzenberg-Solomon R, Zimmerman TP, Duan Z, Chen L, Kahle L, et al. Dietary consumption of advanced glycation end products and pancreatic cancer in the prospective NIH-AARP Diet and Health Study. Am J Clin Nutr. 2015;101(1):126–34. doi: 10.3945/ajcn.114.098061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkin DM. 9. Cancers attributable to inadequate physical exercise in the UK in 2010. British journal of cancer. 2011;105 (Suppl 2):S38–41. doi: 10.1038/bjc.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Key TJ, Schatzkin A, Willett WC, Allen NE, Spencer EA, Travis RC. Diet, nutrition and the prevention of cancer. Public health nutrition. 2004;7(1A):187–200. doi: 10.1079/phn2003588. [DOI] [PubMed] [Google Scholar]
- 12.Sauaia A, Byers T. Obesity in US children and adults. Jama. 2012;307(20):2145. doi: 10.1001/jama.2012.4726. author reply 45–6. [DOI] [PubMed] [Google Scholar]
- 13.Zenk SN, Lachance LL, Schulz AJ, Mentz G, Kannan S, Ridella W. Neighborhood retail food environment and fruit and vegetable intake in a multiethnic urban population. American journal of health promotion : AJHP. 2009;23(4):255–64. doi: 10.4278/ajhp.071204127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kellow NJ, Savige GS. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: a systematic review. European journal of clinical nutrition. 2013;67(3):239–48. doi: 10.1038/ejcn.2012.220. [DOI] [PubMed] [Google Scholar]
- 15.Ulf Ekelund HAW, Norat Teresa, Luan Jian’an, May Anne M, Weiderpass Elisabete, Sharp Stephen S, et al. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: the European Prospective Investigation into Cancer and Nutrition Study (EPIC) The American Journal of Cancer Research. 2015 doi: 10.3945/ajcn.114.100065. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boor P, Celec P, Behuliak M, Grancic P, Kebis A, Kukan M, et al. Regular moderate exercise reduces advanced glycation and ameliorates early diabetic nephropathy in obese Zucker rats. Metabolism. 2009;58(11):1669–77. doi: 10.1016/j.metabol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Wright KJ, Thomas MM, Betik AC, Belke D, Hepple RT. Exercise training initiated in late middle age attenuates cardiac fibrosis and advanced glycation end-product accumulation in senescent rats. Experimental gerontology. 2014;50:9–18. doi: 10.1016/j.exger.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa T, Miyazaki A, Fujimoto S. Decrease in serum levels of advanced glycation end-products by short-term lifestyle modification in non-diabetic middle-aged females. Med Sci Monit. 2009;15(6):PH65–73. [PubMed] [Google Scholar]
- 19.Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging and disease. 2012;3(1):130–40. [PMC free article] [PubMed] [Google Scholar]
- 20.Galland L. Diet and inflammation. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 2010;25(6):634–40. doi: 10.1177/0884533610385703. [DOI] [PubMed] [Google Scholar]
- 21.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 22.Tan KCCW, Tam S, Bucala R, Betteridge J. Association between acute-phase reactants and advanced glycation end products in type 2 diabetes. Diabetes care. 2004;(27):223–8. doi: 10.2337/diacare.27.1.223. [DOI] [PubMed] [Google Scholar]
- 23.Chandler PD, Scott JB, Drake BF, Ng K, Manson JE, Rifai N, et al. Impact of vitamin D supplementation on inflammatory markers in African Americans: results of a four-arm, randomized, placebo-controlled trial. Cancer prevention research. 2014;7(2):218–25. doi: 10.1158/1940-6207.CAPR-13-0338-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiner AP, Beleza S, Franceschini N, Auer PL, Robinson JG, Kooperberg C, et al. Genome-wide association and population genetic analysis of C-reactive protein in African American and Hispanic American women. American journal of human genetics. 2012;91(3):502–12. doi: 10.1016/j.ajhg.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris AA, Zhao L, Patel RS, Jones DP, Ahmed Y, Stoyanova N, et al. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) study. Metabolic syndrome and related disorders. 2012;10(4):252–9. doi: 10.1089/met.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feairheller DL, Park JY, Sturgeon KM, Williamson ST, Diaz KM, Veerabhadrappa P, et al. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clinical and translational science. 2011;4(1):32–7. doi: 10.1111/j.1752-8062.2011.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riehl A, Nemeth J, Angel P, Hess J. The receptor RAGE: Bridging inflammation and cancer. Cell communication and signaling : CCS. 2009;7:12. doi: 10.1186/1478-811X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. The Prostate. 2005;64(1):92–100. doi: 10.1002/pros.20219. [DOI] [PubMed] [Google Scholar]
- 29.Rojas A, Mercadal E, Figueroa H, Morales MA. Advanced Glycation and ROS: a link between diabetes and heart failure. Current vascular pharmacology. 2008;6(1):44–51. doi: 10.2174/157016108783331312. [DOI] [PubMed] [Google Scholar]
- 30.Guimaraes EL, Empsen C, Geerts A, van Grunsven LA. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J Hepatol. 2010;52(3):389–97. doi: 10.1016/j.jhep.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Rose AE, Satagopan JM, Oddoux C, Zhou Q, Xu R, Olshen AB, et al. Copy number and gene expression differences between African American and Caucasian American prostate cancer. Journal of translational medicine. 2010;8:70. doi: 10.1186/1479-5876-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell IJ, Dyson G, Land S, Ruterbusch J, Bock CH, Lenk S, et al. Genes associated with prostate cancer are differentially expressed in African American and European American men. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2013;22(5):891–7. doi: 10.1158/1055-9965.EPI-12-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park NJ, Kang DH. Inflammatory cytokine levels and breast cancer risk factors: racial differences of healthy Caucasian and African American women. Oncology nursing forum. 2013;40(5):490–500. doi: 10.1188/13.ONF.40-05AP. [DOI] [PubMed] [Google Scholar]