Abstract
Rationale
A substantial number of clinical studies indicate associations between sleep abnormalities and drug abuse; however, the role played by the circadian system in the development of addiction is largely unknown.
Objective
The aim of this study was to examine the effects of experimentally induced chronic jet lag on methamphetamine consumption in a rat model of methamphetamine drinking.
Methods
Male Sprague-Dawley rats (n=32) were housed in running wheel cages in a 12:12 light:dark cycle. One group of rats (n=16) was given two weeks of forced methamphetamine consumption (0.01% in drinking water; meth pre-exposed) while a second group (n=16, not pre-exposed) received water only. This was followed by a two week abstinence period during which half of the animals from each group were exposed to 4 consecutive 6-hr advancing phase shifts of the light:dark cycle, while the other half remained on the original light:dark cycle. Methamphetamine consumption was assessed in all rats following the deprivation period using a two-bottle choice paradigm.
Results
Methamphetamine consumption was initially lower in methamphetamine pre-exposed vs. not pre-exposed rats. However, during the second week following abstinence, consumption was significantly higher in phase shifted rats of the methamphetamine pre-exposed group compared to all other groups.
Conclusions
These data reveal an effect of circadian rhythm disturbance on methamphetamine consumption, and suggest that dysregulation of the circadian system be considered in the etiology of relapse and addiction.
Keywords: addiction, circadian, jet lag, methamphetamine, relapse, reward
Introduction
The circadian timing system maintains synchrony among an organism's 24-hour behavioral and physiological rhythms, including the sleep/wake cycle, and the external day. In humans, clinical studies have shown that disruption of normal circadian rhythmicity can adversely affect health. Exposure to non-traditional work schedules such as night or rotating shifts, and frequent international travel by airline crews have been linked to increased risk of various types of cancers (Davis et al. 2001; Kubo et al. 2006; Schernhammer et al. 2003), as well as obesity and diabetes (Karlsson et al. 2001; Morikawa et al. 2005), stroke (Virtanaen and Notkola 2002), and heart attack (Haupt et al. 2008; Knuttson et al. 1999). The association between sleep disruption and substance use has also recently become evident. Alcohol and drug abuse is more prevalent in patients with sleep complaints than in the general population (Teplin et al. 2006), and up to 50% of recovering alcoholics report sleep problems prior to alcohol dependence onset (Currie et al. 2003). In addition, sleep disruptions such as insomnia have been found to precipitate relapse to addiction (Brower et al. 2001; Pace-Schott et al. 2005). In fact, Brower et al. (2001) found that the presence of insomnia was the most significant factor for predicting relapse in alcoholic patients entering treatment. Two studies also implicate abnormal circadian function as a vulnerability factor for addiction: increased alcohol consumption has been reported in international business travelers experiencing jet lag, a circadian rhythm sleep disorder (Rogers and Reilly 2002), and nurses working rotating shifts and night shifts show increased smoking, alcohol and other drug use compared to those working normal schedules (Trinkoff and Storr 1998).
Despite these studies correlating sleep disruption and substance use, there is a lack of data directly examining the effects of systematic manipulation of the circadian system. Results from preclinical studies, however, suggest that the circadian system may play a critical role in drug addiction. For example, several studies have demonstrated that animals with circadian clock gene mutations show altered responses to drugs of abuse. Increases in cocaine sensitization, conditioned place preference, and self-administration have been observed in Clock and Per2 mutant mice (Abarca et al. 2002; McClung et al. 2005; Ozburn et al. 2012), and Per2 mutant mice show increased alcohol consumption compared to wild type mice (Spanagel et al. 2005). In contrast, loss of Per1 has been shown to result in loss of cocaine sensitization (Abarca et al. 2002). Furthermore, the existence of daily and circadian rhythms of cocaine self-administration in rats (Baird and Gauvin 2000; Bass et al. 2010; Roberts et al. 2002), effects of SCN lesions on cocaine seeking behavior in rats (Sleipness et al. 2007), and a prevalence of daily variation in the sensitivity to many drugs of abuse, (for review see Falcon and McClung 2009), argue for clock control of reward behaviors. The transition to short photoperiod (i.e. short day length) has been found to reduce cocaine-induced reinstatement of conditioned place preference in rats (Sorg et al. 2011). Several other studies, also conducted in rats, have examined the effects of constant darkness, constant light, and photoperiod on ethanol consumption (Burke and Kramer 1974; Geller 1971; Sinclair and Geller 1972), and Gauvin et al. (1997) reported increases in ethanol intake in response to phase shifts of the light cycle. However, although a considerable literature exists in the circadian field on the effect of methamphetamine in revealing an SCN-independent oscillator in rats and mice (i.e., the methamphetamine-sensitive circadian oscillator or MASCO; for review, see Honma and Honma 2009), the effect of circadian disruption on methamphetamine consumption has not been tested.
In this study, we disrupted the circadian system using a chronic jet lag (CJL) model in which animals are exposed to light cycles that are shifted every 4 days to mimic a 6-hour eastward time zone shift. The CJL model has been widely used to reproduce the effects of shift work and jet lag (Davidson et al. 2006; Filipski et al. 2004; Penev et al. 1998; Preuss et al. 2008). Rhythms of clock gene expression in the SCN and other tissues are altered or abolished in response to CJL (Castanon-Cervantes et al. 2010), indicating that CJL is an effective circadian disrupter. Moreover, CJL is advantageous in that, unlike bright constant light, another light manipulation frequently used to produce circadian arrhythmicity, CJL has only minimum effects on sleep, and does not induce other hormonal or behavioral measures of stress (Castanon-Cervantes et al. 2010; Iwamoto et al. 2014; Sei et al. 2003). Here, we examine the effect of chronic jet lag in methamphetamine-treated rats on resumption of methamphetamine drinking after abstinence. We test the hypothesis that animals exposed to CJL will show an increase in methamphetamine consumption following abstinence compared to non-shifted rats.
Methods and materials
Animals
Three month old adult male Sprague-Dawley rats (n=32) purchased from Charles River Labs and weighing 360-420 grams were used in this study. Rats were used based on their history of use in circadian studies of oral methamphetamine consumption, and the Sprague-Dawley strain was chosen based on the use of this strain in the previous work examining effects of phase shifting and manipulation of light cycles on ethanol consumption. Rats were divided into 4 groups (n=8 rats per group), and were housed in individual (20.3 × 25.4 × 45.7 cm) running-wheel cages on corn cob bedding in light-tight, temperature- and humidity-controlled boxes (21°C / 50% relative humidity) and maintained on a 12:12 light:dark cycle (lights on at 05:00, off at 17:00; mean light intensity 40 μW/cm2). Animals had unrestricted access to food (Harlan Teklad 8664 rodent diet) and water (or methamphetamine-water) throughout the study. Locomotor activity was measured continuously as the number of wheel revolutions recorded in 1-min bins, and was analyzed using Clocklab software (Actimetrics, Wilmette, IL). Food, water and methamphetamine intake were measured daily by weighing of bottles and food. Body weights were obtained two times per week throughout the study. All procedures were approved by the University of Virginia Animal Care and Use Committee and are in compliance with the NIH Guide for the Care and Use of Laboratory Animals.
Drugs
Methamphetamine hydrochloride (Sigma Aldrich, St. Louis, MO) was dissolved to a concentration of 100 mg/L in tap water. The concentration of methamphetamine in water (0.01%) was held constant across all subjects. This concentration was selected based on its history of use in circadian studies (Honma et al. 1989; Ruis et al. 1990; Tataroglu et al. 2006), where it has been shown to induce robust behavioral effects on activity without affecting neuronal activity rhythms in the SCN (Moriya et al. 1996) or rhythms of clock gene expression in the SCN (Masubuchi et al. 2000). Methamphetamine solutions were made fresh weekly and were delivered in bottles identical to those containing water.
Experimental Procedure
Figure 1 depicts the experimental timeline and groups used in this study. After one week of baseline recording of feeding, drinking, and activity in all rats (n=32), half of the animals (groups 1 and 2) underwent two weeks of forced methamphetamine consumption during which 0.01% methamphetamine was added to the drinking water bottle to induce dependence. The other half of the animals (groups 3 and 4) received water only. A two week abstinence period followed, during which half of the methamphetamine pre-exposed and half of the not pre-exposed rats (groups 1 and 3) were exposed to 4 consecutive phase shifts of the 12:12 light:dark cycle, such that a 6-hr advance was effected on day 1 of abstinence, and every 4 days thereafter (days 5, 9, and 13 of abstinence). The fourth phase shift, which occurred 2 days before the end of the abstinence period, returned the shifted animals to the original light:dark cycle. A two week abstinence period was selected based on previous work demonstrating that the neuroadaptations leading to the development of an addicted phenotype, i.e. the enhanced motivation to obtain drug following abstinence in animals with extended versus short exposure, develop over a 10-14 day abstinence period (Roberts et al. 2007; Ramôa et al. 2013). We hypothesized that circadian disruption during this critical period might disrupt these neuroadaptations and lead to changes in subsequent methamphetamine consumption. At the end of this abstinence period, consumption of methamphetamine was measured in all animals for two weeks using a two-bottle choice paradigm. Each animal was presented with two bottles, one containing water and one containing 0.01% methamphetamine in water. The concentration of methamphetamine used for two-bottle choice testing was the same as was used for forced consumption in order to facilitate direct comparisons between forced and free consumption. Bottle positions were switched two times per week to control for position preferences. Sipper tubes were used that contained ball bearings at the tip to prevent leakage. In our preliminary testing these bottles dripped less than 0.75 ml per day. All procedures in the dark were carried out with the aid of infrared viewers.
Fig. 1.
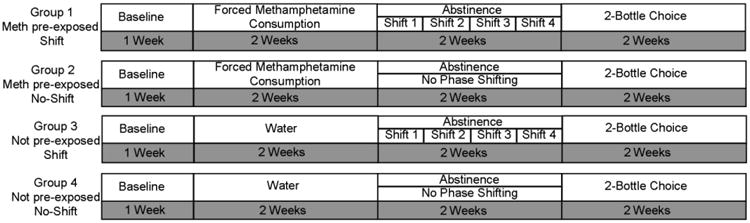
Timeline of experimental procedures. After one week of baseline recording of feeding, drinking, and activity, two groups of rats (1 and 2) underwent two weeks of forced methamphetamine consumption, while two groups (3 and 4) received water only. This was followed by a two-week abstinence period, during which 4 discrete phase shifts of the light:dark cycle were administered to half of the methamphetamine pre-exposed and not pre-exposed rats (groups 1 and 3) while the other half (groups 3 and 4) remained on the original light:dark cycle. All groups underwent two-bottle choice testing for two weeks following abstinence.
Withdrawal scoring
In order to establish that methamphetamine dependence had been induced by the forced access procedure, animals were screened for behavioral signs of withdrawal. Behavioral observations were conducted at 12, 24, 36, 48, 60, and 72 h following the removal of methamphetamine. Each rat was observed in its cage for 15 min. by two independent observers, blind to the animals' treatment. Inter-observer scores did not differ by more than 15% and thus were averaged. Observers counted occurrences of withdrawal signs utilizing a checklist adapted from a model of cocaine withdrawal scoring developed by Malin et al. (2000) for quantitating spontaneously emitted behaviors following termination of exposure to drug. Withdrawal signs included ptosis, gasps/body writhes, teeth chatter/chews, head shakes/tremors, and less frequent signs such as scratches and genital licks/ejaculations. The presence of rhinorrhea, epiphora, piloerection, and abnormal posture (hunching) were counted no more than once per five minutes. Other signs were counted at each occurrence. Each rat's overall withdrawal score was recorded as the total number of withdrawal signs summed across all categories.
Data Analyses
Periods of activity rhythms were determined by Chi Square periodogram analysis using Clocklab software, with a significance level of p = 0.01. The number of days to reach stable entrainment was calculated by counting the number of days of transients of the activity rhythm following the final shift of the light:dark cycle and preceding entrainment of the rhythm to a 24-hour period for at least 5 days. Student's t-test was used to compare group means. Methamphetamine consumption in milligrams per kilogram was calculated by using methamphetamine consumed and body weights. Preference was calculated for two-bottle choice consumption by dividing the volume of methamphetamine-containing solution consumed by total fluid intake. The not pre-exposed no-shift group was used as a comparison group to determine the effects of chronic jet lag with percent difference calculated by dividing the difference between the experimental and control groups by control values and multiplying by 100. Data were analyzed using the Statistical Package for Social Sciences (SPSS, Version 20). Repeated measures ANOVA was used to determine group differences in withdrawal signs, body weight, consumption, and preference, with exposure (methamphetamine pre-exposed, not-pre-exposed) and shift (shift, no-shift) as between-subjects factors and days as the within-subjects factor. Following a significant 3-way interaction of day x methamphetamine pre-exposure x shift, or a significant 2-way interaction of day x methamphetamine pre-exposure or day x shift, data were collapsed on exposure and/or shift and further examined using repeated measures ANOVA. Subsequent group comparisons across days were made using univariate ANOVA. Univariate ANOVA was also used for percent change from baseline analyses, with the not pre-exposed, no-shift group used as the control and set to 0. When appropriate, post-hoc comparisons were conducted using a Bonferroni test. Statistical significance was set at p < 0.05.
Results
Behavioral and physiological effects of forced methamphetamine consumption
Forced methamphetamine consumption
Fig. 2a illustrates mean (±SEM) methamphetamine intake (mg/kg) during the 14-day period of forced consumption. As expected, methamphetamine intake escalated over the 14-day period, with repeated measures ANOVA revealing a significant main effect of day [F(13,182)= 7.02, p < 0.001]. Importantly, there was no significant difference in methamphetamine intake in the groups designated to receive the shift or no-shift manipulation during abstinence, nor was there a day by shift interaction, indicating that methamphetamine consumption was equivalent between the two methamphetamine pre-exposed groups during the period of forced consumption prior to abstinence and phase-shifting.
Fig. 2.
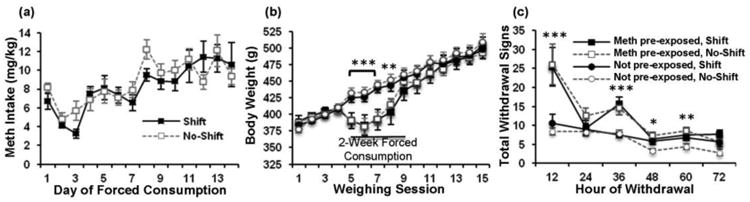
Physiological and behavioral responses to forced methamphetamine consumption and abstinence. (a) Mean (± SEM) daily methamphetamine intake during the 2-week period of forced methamphetamine consumption prior to abstinence, showing escalation of intake. (b) Mean (± SEM) body weights, measured every 3-4 days over the course of the 6-week long experiment. n = 8 animals/group. (c) Mean (± SEM) overall withdrawal signs, recorded every 12h for 72h following removal of methamphetamine bottles. * p < 0.05, ** p < 0.01, *** p < 0.001, indicate significant differences between methamphetamine pre-exposed and not-pre-exposed groups.
Effects on body weight
Body weights, measured approximately twice a week over the course of the 7-week long experiment, are shown in Fig. 2b. Repeated measures ANOVA revealed significant overall effects of day [F(14,392)= 185.44, p < 0.001], and methamphetamine pre-exposure [F(1, 28)= 4.59, p < 0.05], and a significant interaction of day by methamphetamine pre-exposure [F(14,392) = 11.62, p < 0.001]. No overall or interactive effects of shift were observed (p's>0.05), and thus subsequent analysis of the effect of methamphetamine pre-exposure on body weight was examined across days collapsed across shift. This analysis revealed significant overall effects of day [F(14,420) = 189.53, p < 0.001] and methampehetamine pre-exposure [F(1,30) = 10641.15, p < 0.05], and a significant interaction of day by methamphetamine pre-exposure [F(14,420) = 11.85, p < 0.001]. Futher analysis of differences between methamphetamine pre-exposed groups on each day using univariate ANOVA revealed significant differences between methamphetamine pre-exposed and not pre-exposed groups during weighing sessions 5 through 8 (sessions 5, 6, and 7, p's < 0.001; session 8, p < 0.01), with trends for a significant difference for sessions 9 through 11 (session 9 and 10, p < 0.1; session 11, p = 0.06). Thus, during the period of forced methamphetamine consumption (i.e. weighing sessions 5 through 8), body weights of the methamphetamine pre-exposed animals were significantly lower than those of the not pre-exposed controls, demonstrating a clear physiological effect of the drug. At the end of the forced consumption period, body weights of the methamphetamine pre-exposed animals rapidly rebounded and did not differ from those of controls for the remainder of the experiment.
Withdrawal
In order to examine signs of physical dependence induced by the forced consumption procedure, we scored withdrawal behaviors every 12 hours for 72 hours after removal of methamphetamine bottles (Fig. 2c). Repeated measures ANOVA revealed significant overall effects of time [F(5,140) = 24.48, p <0.001] and methamphetamine pre-exposure [F(1,28)= 35.94, p <0.001], and a significant interaction of time by methamphetamine pre-exposure [F(5,140) = 8.68, p < 0.001]. No overall or interactive effects of shift were observed (p's>0.05), and thus subsequent analysis of the effect of methamphetamine pre-exposure on withdrawal behaviors was examined over time collapsed across shift. Results from the repeated measures ANOVA revealed significant overall effects of time [F(5,150) = 25.61, p < 0.001], and methamphetamine pre-exposure [F(1,30) = 36.76, p < 0.001], and a significant interaction of time by methamphetamine pre-exposure [F(5,150) = 9.07, p < 0.001]. Further analyses of differences between methamphetamine pre-exposed groups at each withdrawal time using univariate ANOVA revealed significant differences between methamphetamine pre-exposed and not pre-exposed groups at both 12 hours (p < 0.001) and 36 hours (p < 0.05) after removal of methamphetamine bottles. However, at the 24-hour timepoint, withdrawal signs in the methamphetamine pre-exposed groups did not differ from the not pre-exposed groups. The number of withdrawal signs in the methamphetamine pre-exposed groups remained higher than in the not pre-exposed groups at 48 hours (p < 0.05) and 60 hours (p < 0.01), but did not differ from the not pre-exposed groups by 72 hours after removal of methamphetamine bottles.
Locomotor activity
Activity records from one rat of each experimental group, and showing median data records in order to best represent the most common characteristic of each group, are shown in Fig. 3. In rats of groups 1 and 2, methamphetamine treatment during the two-week period of forced consumption led to changes in the daily activity rhythm that are typical for rodents treated with this moderate-to-high dose of methamphetamine (Ruis et al. 1990; Tataroglu et al. 2006; Honma, Honma and Hiroshige 1986). In particular, significant increases in the period length of the daily activity rhythm were observed in 13 out of 16 of the animals (Table 1), with 11 out of these 16 demonstrating two activity components; a component that remained synchronized with the light:dark cycle (i.e. the light-entrained SCN circadian oscillator), and another component free-running with a long period similar to that reported for the methamphetamine-sensitive circadian oscillator (Tataroglu et al., 2006). Such variability is to be expected due to interactions between competing oscillators. In contrast, activity rhythms of not pre-exposed control rats given water during this same two-week period (groups 3 and 4) remained stably entrained by the light:dark cycle, with periods close to 24 hours, and activity largely restricted to the dark phase. Upon removal of methamphetamine, the period of the activity rhythm returned to 24 hours within 2-3 days in animals that were not phase shifted. In animals subjected to chronic jet lag (groups 1 and 3), activity rhythms either advanced in the direction of the light:dark cycle shift (n=5 of 16) or delayed in the opposite direction (n=2 of 16), as evidenced by either short or long period rhythms respectively (Table 1), and rhythms in 9 of 16 animals split into both a short and a long period component, but in all cases failed to entrain to the 24-hour period of the light:dark cycles at any point. Following the final shift of the light:dark cycle, the number of days required to achieve stable entrainment (i.e. the number of days of transients in the activity rhythm) did not differ between methamphetamine pre-exposed (9.6 ± 0.98) and not pre-exposed (7.8 ± 0.76) rats.
Fig. 3.
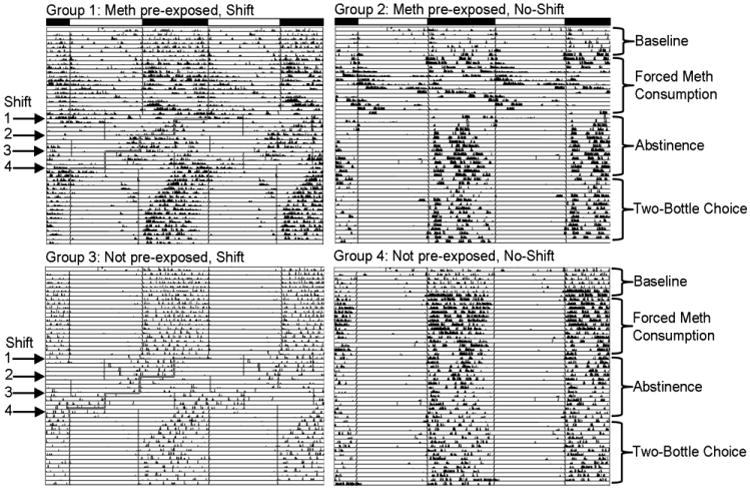
Running wheel activity records of one representative animal from each experimental group. Records are double-plotted so that each horizontal line represents 48 hours, and successive days are plotted below and to the right of the preceding day. The main light:dark cycle (lights on from 0500 – 1700) is represented by black and white bars above the top two records. For animals that received phase shifts, the advance of the onset of the light phase for each shift is highlighted in red within the records.
Table 1.
Mean circadian period (± SEM) as determined by chi square periodogram analysis (significance level p=0.01) for each experimental group during different phases of the experiment. In the two groups of animals pre-exposed to methamphetamine (Meth pre-exposure), most rats displayed activity rhythms during methamphetamine pre-exposure that were split into two distinct components; a 24 hour component (∼24:) and a long period (L:) component. The other animals displayed either a 24-hour period or a long period. Likewise, in the two groups exposed to chronic jet lag (CJL), activity rhythms were often split into two components; a short period component (S:) and a long period component, while the remaining animals displayed either a short period or long period rhythms. Values under the Transients heading show free-running circadian period during the time following the chronic jet lag treatment when the activity rhythm is in the process of entraining to the final light:dark cycle. Values under Post-Transient show the activity rhythm period following stable entrainment to the final light:dark cycle.
| Baseline | Meth pre-exposure | Chronic Jet Lag (CJL) | Transients | Post-Transient | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Meth CJL | 24.00±0.01 (n=8/8) | 24.07 (n=1/8) | L: none | S: 23.38 ±0.70* (n=2/8) | L: 24.42±0.17* (n=2/8) | 23.67 ±0.26 (n=8/8) | 23.98 ±0.01 (n=8/8) |
|
| |||||||
| ∼24: 23.99±0.03 | S: 22.76 ±0.39* | ||||||
| L: 26.67 ±0.31* | L: 25.15 ±0.26* | ||||||
| (n=7/8) | (n=4/8) | ||||||
|
| |||||||
| Meth No CJL | 23.99±0.01 (n=8/8) | 24.15±0.15 (n=2/8) | L: 24.67 ±0.08* (n=2/8) | 23.97 ±0.02 (n=8/8) | n/a | n/a | 23.98 ±0.01 (n=8/8) |
|
| |||||||
| ∼24: 24.07±0.07 | |||||||
| L: 27.81 ±1.11* | |||||||
| (n=4/8) | |||||||
|
| |||||||
| Water CJL | 24.01±0.01 (n=8/8) | 23.97±0.01 (n=8/8) | n/a | S: 23.10 ±0.06* (n=3/8) | L: none | 23.36 ±0.21 (n=8/8) | 24.01 ±0.01 (n=8/8) |
|
| |||||||
| S: 23.04 ±0.19* | |||||||
| L: 25.12 ±0.07* | |||||||
| (n=5/8) | |||||||
|
| |||||||
| Water No CJL | 23.99±0.01 (n=8/8) | 24.01+0.01 (n=8/8) | n/a | 24.00 ±0.01 (n=8/8) | n/a | n/a | 24.00 ±0.00 (n=8/8) |
indicates significant difference from baseline period, Student's t-test, p <0.05.
Two-Bottle Choice Testing
The effects of chronic phase shifting on methamphetamine consumption and preference were tested in all groups using a two-bottle choice paradigm (Fig. 4). For consumption, results from the repeated measures ANOVA (for consumption) revealed significant effects of day [F(13,364) = 28.09, p < 0.001], methampethamine pre-exposure [F(1,28) = 3.39, p < 0.05], day by methamphetamine pre-exposure [F(39,364) = 5.83, p < 0.001], and day by shift [F(13,364) = 2.43, p < 0.01]. Following these significant interactions of day by methamphetamine pre-exposure and day by shift, data were collapsed on either methamphetamine pre-exposure or shift and further examined. Repeated measures ANOVA examining effects of pre-exposure on methamphetamine consumption revealed significant overall effects of day [F(13,390) = 26.54, p < 0.001], and methamphetamine pre-exposure [F(1,30) = 3.31, p < 0.05], and a significant interaction of day by pre-exposure [F(13,390) = 5.50, p < 0.001]. Subsequent univariate analysis of the effect of methamphetamine pre-exposure on each of the days revealed significant differences between the pre-exposed and not pre-exposed groups on days 1 (p <0.001), 3 (p < 0.01), 5 (p < 0.001), 8 (p < 0.01), 10 (p < 0.05), 11 (p < 0.01), and 13 (p < 0.05). Repeated measures ANOVA examining effects of shift on consumption revealed significant effects of day [F(13,390) = 23.98, p < 0.001] and day by shift [F(13,390) = 2.071, p < 0.05]. Subsequent analysis of differences between shift groups across days using univariate ANOVA revealed significant differences between shift and no-shift groups on days 9 (p < 0.001), 11 (p < 0.05), and 12 (p = 0.01).
Fig. 4.
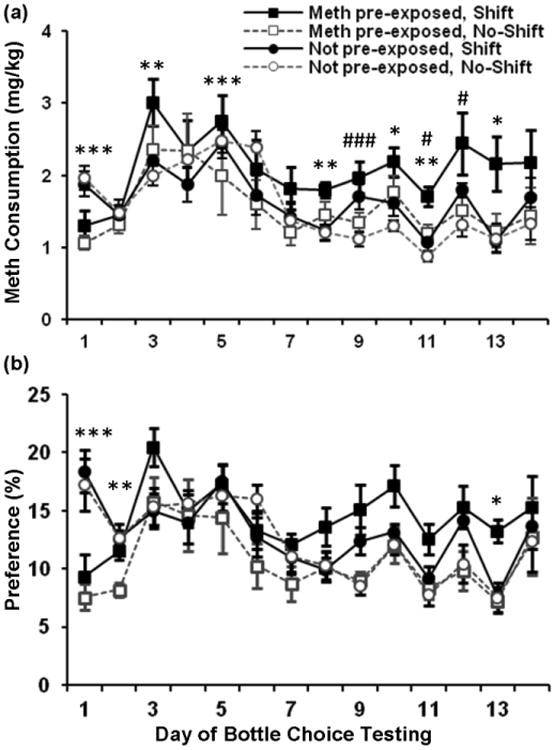
Mean (± SEM) daily methamphetamine intake (a) and daily percent preference (b) over 14 days of two-bottle choice testing. n = 8 animals/group. * p < 0.05, ** p < 0.01, *** p < 0.001, indicate significant differences between methamphetamine pre-exposed and not pre-exposed groups. # p < 0.05, ### p < 0.001, indicate significant differences between shift and no-shift groups.
For percent preference (Fig 4b), repeated measures ANVOA revealed significant overall effects of day [F(13,364) = 13.12 p < 0.001], and shift [F(1,28) = 4.39, p < 0.05], and a significant interaction of day by methamphetamine pre-exposure [F(13,364) = 6.12, p < 0.001]. Following a significant interaction of day by methamphetamine pre-exposure, data were collapsed on shift and the effect methamphetamine pre-exposure on preference was examined. ANOVA revealed significant effects of day [F(13,390) = 13.18, p < 0.001] and day by methamphetamine pre-exposure [F(13,290) = 6.15, p < 0.001]. Further univariate analyses of the effect of methamphetamine pre-exposure on each of the days revealed significant differences between methamphetamine pre-exposed and not pre-exposed groups on days 1 (p < 0.001), 2 (p < 0.01) and 13 (p < 0.05). Thus, both methamphetamine pre-exposure and phase shifting during abstinence had significant effects on subsequent methamphetamine consumption. Percent preference measures, however, were affected only by methamphetamine pre-exposure.
In order to further examine the effects of day on consumption in the different groups over the two-week two bottle choice period, and to further explore a priori hypothesized differences in the effect of circadian disruption in methamphetamine pre-exposed versus not pre-exposed rats, the data were plotted as percent change in methamphetamine consumption from not pre-exposed no-shift controls on day 1, during the first week, and during the second week of two-bottle choice testing (Fig. 5). On day 1 of testing (Fig. 5a), both methamphetamine pre-exposed groups consumed significantly lower levels of methamphetamine as compared to the two not pre-exposed groups (group effect F(3,28) = 7.27, p = 0.001; post hoc comparison between groups showing significant differences between methamphetamine pre-exposed and not pre-exposed groups, p < 0.05). Over days 1-7 (Fig. 5b), consumption in the methamphetamine pre-exposed groups increased and was no longer significantly different from that of not pre-exposed controls.
Fig. 5.
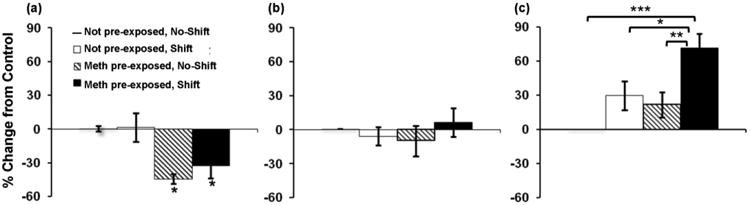
Mean (± SEM) percent change in daily mg/kg methamphetamine consumption from not pre-exposed, no-shift controls on day 1 (a), days 1-7 (b), and days 8-14 (c) of two-bottle choice testing. Not pre-exposed, no-shift control level is set at 0. * p < 0.05 in (b) indicates a significant difference from not pre-exposed no-shift and not pre-exposed shift groups. * p < 0.05, ** p < 0.01, *** p < 0.001. n = 8 animals/group.
During days 8-14 (Fig. 5c), however, methamphetamine consumption in phase shifted rats of the methamphetamine pre-exposed group was higher than in all other groups, with ANOVA revealing a significant effect of group (F(3,28) = 8.16, p < 0.001), and post hoc comparisons between groups showing that levels of consumption were significantly higher in phase shifted methamphetamine pre-exposed rats as compared to methamphetamine pre-exposed animals that had not been been subjected to phase shifts (p < 0.01), not pre-exposed animals that had been phase shifted (p <0.05) and not pre-exposed animals that had not received phase shifts (p < 0.001). Similar results were found when data were analyzed using percent change in preference from not pre-exposed, no-shift controls (Fig. 6) with ANOVA revealing a significant group effect [F(3,28) = 6.11, p = 0.01], and post hoc comparison between groups showing significant differences between not pre-exposed and methamphetamine pre-exposed groups on day 1 (Fig. 6a, p's < 0.05), and between the methamphetamine pre-exposed shift and both the methamphetamine pre-exposed no-shift group (p < 0.05) and the not pre-exposed no-shift control group (p < 0.05) on days 8-14 (Fig. 6c).
Fig. 6.
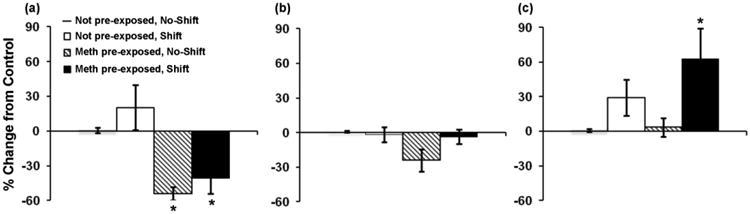
Mean (± SEM) percent change in preference for methamphetamine from not pre-exposed, no-shift controls on (a) day 1, (b) days 1-7, and (c) days 8-14 of two-bottle choice testing. * p < 0.05 in (a) indicates a significant difference from not pre-exposed shift and not-pre-exposed no-shift groups; * p < 0.05 in (b) indicates a significant difference from methamphetamine pre-exposed no-shift and not-pre-exposed shift groups. n = 8 animals/group.
Discussion
In this study, we used a rat model to examine the effects of a two-week chronic jet lag paradigm on methamphetamine consumption following abstinence. During the second week following abstinence rats previously exposed to methamphetamine and subjected to circadian disruption during abstinence showed a significantly higher percent change in methamphetamine consumption from not pre-exposed no-shift controls, compared to the methamphetamine pre-exposed no-shift and the not pre-exposed shifted groups. These results suggest that disruption of normal circadian rhythmicity may increase drug-taking behavior, and that normal clock function may be important for maintaining homeostasis within the reward system.
Methamphetamine pre-exposure was provided with two-weeks of forced consumption of 0.01% methamphetamine in the drinking water. This treatment resulted in clear physiological and behavioral effects, including weight loss, changes in activity patterns, and escalation of intake during forced consumption. These effects, in combination with the presence of withdrawal symptoms, suggest physical dependence in the methamphetamine pre-exposed groups. However, although withdrawal behaviors were higher in the methamphetamine pre-exposed groups, we did not find a significant association between levels of withdrawal behavior and methamphetamine consumption (data not shown). This may have been due to an insufficient sensitivity of our visual withdrawal scoring technique and/or the high concentration of methamphetamine used during forced consumption. Further experiments are necessary to definitively show dependence in the methamphetamine pre-exposed rats under this paradigm. Surprisingly, the number of withdrawal signs was significantly lower at the 24-hour time point compared to the 12- and 36-hour time points, suggesting the possibility of a circadian rhythm of withdrawal symptoms. Although the demonstration of circadian rhythms of withdrawal symptoms in constant conditions has not, to our knowledge, been explored for any drug, Damaggio and Gorman (2014) recently reported that physiological responses to ethanol withdrawal do vary as a function of circadian phase in animals entrained to a skeleton photoperiod.
We found that methamphetamine pre-exposed rats subjected to a two-week chronic jet lag procedure showed significantly higher percent change in methamphetamine consumption from not pre-exposed no-shift controls, compared to the other experimental groups (i.e. methamphetamine pre-exposed no-shift and not pre-exposed shifted groups) during the second week following abstinence. In a limited access maintenance model of ethanol drinking in rats, Gauvin et al. (1997) reported transient increases in ethanol consumption when single phase advances or delays of the light:dark cycle were administered during the self-administration period. In the same study, alternating high and low ethanol intake was observed during a more slowly changing 7-day ‘shift work’ schedule, depending on which shift was in effect. These alternating levels of drinking may have been due to partial entrainment of the circadian clock to the light:dark cycle during some shifts and not others, and/or differing effects of circadian disruption in non-dependent animals. In the current study we used a high concentration of methamphetamine that is likely to have induced dependence, and shifted the light:dark cycle at a rapid rate that did not permit clock entrainment. Importantly, with the exception of the first day of abstinence, percent change in methamphetamine consumption and preference for the methamphetamine pre-exposed no-shift and the not pre-exposed shifted rats was not significantly higher than that of the not pre-exposed non-shifted control group. These results indicate that neither prior exposure to this concentration of methamphetamine nor the two-week long chronic jet lag procedure were in themselves sufficient to produce increases in consumption following abstinence in the methamphetamine pre-exposed shifted rats, and raise the possibility that there may be a combined effect of circadian disruption and prior methamphetamine exposure on enhancing drug-induced neuroadaptations. Such neuroadaptations are believed to lead to increased drug-seeking and to mediate prolonged relapse vulnerability following abstinence (Grimm et al. 2001). However, it is also possible that additional mechanisms such as physiological stresses induced by forced methamphetamine exposure may produce downstream effects that interact with circadian disruption to increase subsequent methamphetamine consumption. The circadian clock regulates daily rhythms of glucocorticoids, and is itself influenced by them (for review, see Dickmeis et al. 2013). Thus alterations in glucocorticoid levels resulting from forced methamphetamine consumption prior to phase shifting may have enhanced the level of circadian disruption in the methamphetamine pre-exposed, phase shifted group. In addition, the effects of phase shifting during the period of rapid body weight gain immediately following forced methamphetamine consumption might produce effects that alter later methamphetamine intake. Future studies will be necessary to elucidate the neurobiological mechanism(s) underlying the possible effects of circadian disruption on methamphetamine consumption.
Repeated exposure to methamphetamine is known to result in hypersensitivity, or sensitization, to the psychomotor and incentive motivational effects of the drug following an abstinence period (Ito et al. 1997; McDaid et al. 2006), and sensitization is believed to reflect neurobiological changes related to addiction. In the current study, our measures of mg/kg consumption, percent preference, and percent change show that both methamphetamine pre-exposed groups consumed significantly less methamphetamine than not pre-exposed groups on the first day of two-bottle choice testing. Although this initial low level of consumption is consistent with a sensitized response to methamphetamine in which a lower dose would be required to elicit the same level of psychomotor stimulation, it is also possible that it indicates a developed aversion for methamphetamine, an interpretation supported by the low preference scores. There were no significant differences in percent change in methamphetamine consumption from not pre-exposed no-shift controls between the two methamphetamine pre-exposed groups on the first day and during the first week of two-bottle choice testing. However, during the second week of testing, the methamphetamine pre-exposed shifted rats showed significantly higher percent change in methamphetamine consumption from not pre-exposed no-shift controls, compared to the other experimental groups. The mechanism behind this delayed effect is unclear but, interestingly, this period of time corresponds to the time when the rats had stably re-entrained to the 12-hour light:12-hour dark cycle. It is possible that the transient clock phase during the first week of testing obscured group differences, and only after stabilization of clock phase did these differences become apparent. The combined effects of methamphetamine exposure and circadian disruption in maintaining a high level of methamphetamine drinking may have been due to either an increase in the rewarding effects or a decrease in the aversive effects of methamphetamine compared to the control groups. In either case, our findings support the idea that circadian disruption may enhance the drug-induced neurobiological changes that lead to addiction.
Numerous clinical studies indicate a correlation between the presence of sleep/and or circadian disorders and the onset of substance use and abuse (Currie et al. 2003; Rogers and Reilly 2002; Teplin et al. 2006; Trinkoff and Storr 1998; Wong et al. 2009). In addition, sleep problems have been shown to be a predominant factor predicting relapse (Brower et al. 2001; Pace-Schott et al. 2005). The current study indicates that methamphetamine pre-exposed rats consume greater amounts of methamphetamine following abstinence when subject to disruptions of their circadian system during the abstinence period. These results underscore the importance of maintaining circadian regularity in human substance abuse patients, and suggest that circadian therapies may significantly decrease relapse vulnerability in substance abuse patients. They further suggest that alterations in the circadian timing system, such as those occurring in the shift workers who constitute almost 15% of full-time workers in the U.S. (Bureau of Labor Statistics 2005), may be a vulnerability factor in the development of addiction.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 DA024716 (WJL), and through support from the University of Virginia (MM).
Footnotes
Conflict of interest disclosure All authors report no potential conflicts of interest.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci USA. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TJ, Gauvin D. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacol Biochem Behav. 2000;65:289–299. doi: 10.1016/s0091-3057(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Bass CE, Jansen HT, Roberts DC. Free-running rhythms of cocaine self-administration in rats held under constant lighting conditions. Chronobiol Int. 2010;27:535–548. doi: 10.3109/07420521003664221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ, Aldrich MS, Robinson EA, Zucker RA, Greden JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158:399–404. doi: 10.1176/appi.ajp.158.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. [Accessed 26 October 2012];Workers on flexible and shift schedules in May 2004. 2005 Available at: http://www.bls.gov/news.release/flex.nr0.htm.
- Burke LP, Kramer SZ. Effects of photoperiod, melatonin and pinealectomy on ethanol consumption in rats. Pharmacol Biochem Behav. 1974;2:459–463. doi: 10.1016/0091-3057(74)90004-5. [DOI] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie SR, Clark S, Rimac S, Malhotra S. Comprehensive assessment of insomnia in recovering alcoholics using daily sleep diaries and ambulatory monitoring. Alcohol Clin Exp Res. 2003;27:1262–1269. doi: 10.1097/01.ALC.0000081622.03973.57. [DOI] [PubMed] [Google Scholar]
- Damaggio AS, Gorman MR. Circadian phase determines effects of repeated ethanol vapor exposure and withdrawal on body temperature and activity rhythms of male mice. Alcohol Clin Exp Res. 2014;38:879–888. doi: 10.1111/acer.12297. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol. 2006;16:R914–916. doi: 10.1016/j.cub.2006.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93:1557–1562. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- Dickmeis T, Weger BD, Weger M. The circadian clock and glucocorticoids--interactions across many time scales. Mol Cell Endocrinol. 2013;380:2–15. doi: 10.1016/j.mce.2013.05.012. [DOI] [PubMed] [Google Scholar]
- Falcon E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56(Suppl 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Holloway FA. Effects of time-of-day and photoperiod phase shifts on voluntary ethanol consumption in rats. Alcohol Clin Exp Res. 1997;21:817–825. [PubMed] [Google Scholar]
- Geller I. Ethanol preference in the rat as a function of photoperiod. Science. 1971;173:456–459. doi: 10.1126/science.173.3995.456. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Neuroadaptation. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt CM, Alte D, Dorr M, Robinson DM, Felix SB, John U, Völzke H. The relation of exposure to shift work with atherosclerosis and myocardial infarction in a general population. Atherosclerosis. 2008;201:205–211. doi: 10.1016/j.atherosclerosis.2007.12.059. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S. The SCN-independent clocks, methamphetamine and food restriction. Eur J Neurosci. 2009;30:1707–1717. doi: 10.1111/j.1460-9568.2009.06976.x. [DOI] [PubMed] [Google Scholar]
- Honma K, Honma S, Hiroshige T. Disorganization of the rat activity rhythm by chronic treatment with methamphetamine. Physiol Behav. 1986;38:687–695. doi: 10.1016/0031-9384(86)90265-9. [DOI] [PubMed] [Google Scholar]
- Honma S, Honma K, Hiroshige T. Methamphetamine induced locomotor rhythm entrains to restricted daily feeding in SCN lesioned rats. Physiol Behav. 1989;45:1057–1065. doi: 10.1016/0031-9384(89)90237-0. [DOI] [PubMed] [Google Scholar]
- Ito C, Onodera K, Watanabe T, Sato M. Effects of histamine agents on methamphetamine-induced stereotyped behavior and behavioral sensitization in rats. Psychopharmacology (Berl) 1997;130:362–367. doi: 10.1007/s002130050251. [DOI] [PubMed] [Google Scholar]
- Iwamoto A, Kawai M, Furuse M, Yasuo S. Effects of chronic jet lag on the central and peripheral circadian clocks in CBA/N mice. Chronobiol Int. 2014;31:189–198. doi: 10.3109/07420528.2013.837478. [DOI] [PubMed] [Google Scholar]
- Karlsson B, Knutsson A, Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people. Occup Environ Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson A, Hallquist J, Reuterwall C, Theorell T, Akerstedt T. Shiftwork and myocardial infarction: A case-control study. Occup Environ Med. 1999;56:46–50. doi: 10.1136/oem.56.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Ozasa K, Mikamai K, Wakia K, Fujino Y, Watanabe Y, Miki T, Nakao M, Hayashi K, Suzuki K, Mori M, Washio M, Sakauchi F, Ito Y, Yoshimura T, Tamakoshi A. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: Findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- Malin DH, Moon WD, Moy ET, Jennings RE, Moy DM, Warner RL, Wilson OB. A rodent model of cocaine abstinence syndrome. Pharmacol Biochem Behav. 2000;66:323–328. doi: 10.1016/s0091-3057(00)00181-7. [DOI] [PubMed] [Google Scholar]
- Masubuchi S, Honma S, Abe H, Ishizaki K, Namihira M, Ikeda M, Honma K. Clock genes outside the suprachiasmatic nucleus involved in manifestation of locomotor activity rhythm in rats. Eur J Neurosci. 2000;12:4206–4214. [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the clock gene. Proc Natl Acad Sci USA. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, Graham MP, Napier TC. Methamphetamine-induced sensitization differentially alters pCREB and DeltaFosB throughout the limbic circuit of the mammalian brain. Mol Pharmacol. 2006;70:2064–2074. doi: 10.1124/mol.106.023051. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Nakagawa H, Miura K, Soyama Y, Ishizaki M, Kido T, Nogawa K. Shift work and the risk of diabetes mellitus among japanese male factory workers. Scand J Work Environ Health. 2005;31:179–183. doi: 10.5271/sjweh.867. [DOI] [PubMed] [Google Scholar]
- Moriya T, Fukushima T, Shimazoe T, Shibata S, Watanabe S. Chronic administration of methamphetamine does not affect the suprachiasmatic nucleus-operated circadian pacemaker in rats. Neurosci Lett. 1996;208:129–132. doi: 10.1016/0304-3940(96)12565-9. [DOI] [PubMed] [Google Scholar]
- Ozburn AR, Larson EB, Self DW, McClung CA. Cocaine self-administration behaviors in ClockDelta19 mice. Psychopharmacology (Berl) 2012;223:169–177. doi: 10.1007/s00213-012-2704-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott EF, Stickgold R, Muzur A, Wigren PE, Ward AS, Hart CL, Clarke D, Morgan A, Hobson JA. Sleep quality deteriorates over a binge--abstinence cycle in chronic smoked cocaine users. Psychopharmacology (Berl) 2005;179:873–883. doi: 10.1007/s00213-004-2088-z. [DOI] [PubMed] [Google Scholar]
- Penev PD, Kolker DE, Zee PC, Turek FW. Chronic circadian desynchronization decreases the survival of animals with cardiomyopathic heart disease. Am J Physiol. 1998;275:H2334–2337. doi: 10.1152/ajpheart.1998.275.6.H2334. [DOI] [PubMed] [Google Scholar]
- Preuss F, Tang Y, Laposky AD, Arble D, Keshavarzian A, Turek FW. Adverse effects of chronic circadian desynchronization in animals in a “challenging” environment. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2034–2040. doi: 10.1152/ajpregu.00118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Morgan D, Liu Y. How to make a rat addicted to cocaine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1614–1624. doi: 10.1016/j.pnpbp.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramôa CP, Doyle SE, Lycas MD, Chernau AK, Lynch WJ. Diminished role of dopamine D1-receptor signaling with the development of an addicted phenotype in rats. Biol Psychiatry. 2014;76:8–14. doi: 10.1016/j.biopsych.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HL, Reilly SM. A survey of the health experiences of international business travelers. part one--physiological aspects. AAOHN J. 2002;50:449–459. [PubMed] [Google Scholar]
- Ruis JF, Buys JP, Cambras T, Rietveld WJ. Effects of T cycles of light/darkness and periodic forced activity on methamphetamine-induced rhythms in intact and SCN-lesioned rats: Explanation by an hourglass-clock model. Physiol Behav. 1990;47:917–929. doi: 10.1016/0031-9384(90)90020-5. [DOI] [PubMed] [Google Scholar]
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- Sei H, Fujihara H, Ueta Y, Morita K, Kitahama K, Morita Y. Single eight-hour shift of light-dark cycle increases brain-derived neurotrophic factor protein levels in rat hippocampus. Life Sci. 2003;73:53–59. doi: 10.1016/s0024-3205(03)00251-0. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Geller I. Ethanol consumption by rats under different lighting conditions. Science. 1972;175:1143–1144. doi: 10.1126/science.175.4026.1143. [DOI] [PubMed] [Google Scholar]
- Sleipness EP, Sorg BA, Jansen HT. Contribution of the suprachiasmatic nucleus to day:night variation in cocaine-seeking behavior. Physiol Behav. 2007;91:523–530. doi: 10.1016/j.physbeh.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Sorg BA, Stark G, Sergeeva A, Jansen HT. Photoperiodic suppression of drug reinstatement. Neuroscience. 2011;176:284–295. doi: 10.1016/j.neuroscience.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Tataroglu O, Davidson AJ, Benvenuto LJ, Menaker M. The methamphetamine-sensitive circadian oscillator (MASCO) in mice. J Biol Rhythms. 2006;21:185–194. doi: 10.1177/0748730406287529. [DOI] [PubMed] [Google Scholar]
- Teplin D, Raz B, Daiter J, Varenbut M, Tyrrell M. Screening for substance use patterns among patients referred for a variety of sleep complaints. Am J Drug Alcohol Abuse. 2006;32:111–120. doi: 10.1080/00952990500328695. [DOI] [PubMed] [Google Scholar]
- Trinkoff AM, Storr CL. Work schedule characteristics and substance use in nurses. Am J Ind Med. 1998;34:266–271. doi: 10.1002/(sici)1097-0274(199809)34:3<266::aid-ajim9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Virtanen SV, Notkola V. Socioeconomic inequalities in cardiovascular mortality and the role of work: A register study of Finnish men. Int J Epidemiol. 2002;31:614–621. doi: 10.1093/ije/31.3.614. [DOI] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Zucker RA. Childhood sleep problems, early onset of substance use and behavioral problems in adolescence. Sleep Med. 2009;10:787–796. doi: 10.1111/j.1530-0277.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


