Abstract
The sense of taste is a common ability shared by all organisms and is used to detect nutrients as well as potentially harmful compounds. Thus taste is critical to survival. Despite its importance, surprisingly little is known about the mechanisms generating and regulating responses to taste stimuli. All taste responses depend on calcium signals to generate appropriate responses which are relayed to the brain. Some taste cells have conventional synapses and rely on calcium influx through voltage-gated calcium channels. Other taste cells lack these synapses and depend on calcium release to formulate an output signal through a hemichannel. Beyond establishing these characteristics, few studies have focused on understanding how these calcium signals are formed. We identified multiple calcium clearance mechanisms that regulate calcium levels in taste cells as well as a calcium influx that contributes to maintaining appropriate calcium homeostasis in these cells. Multiple factors regulate the evoked taste signals with varying roles in different cell populations. Clearly, calcium signaling is a dynamic process in taste cells and is more complex than has previously been appreciated.
Keywords: taste, calcium homeostasis, calcium influx, calcium release, ryanodine receptors, mitochondria, sodium-calcium exchangers
INTRODUCTION
There are multiple sensory systems to detect environmental stimuli. These sensory systems provide our brains with information about our surroundings that is critical for our survival. Sensory systems vary with the needs of the organism, some organisms do not detect light or sound waves while others detect electrical signals and magnetic fields. All organisms, however, possess the ability to detect chemicals in their environment. Chemosensation is used to find nutrients and conspecifics as well as to identify predators and avoid potentially harmful compounds or environmental conditions. Some organisms, including single-celled organisms have a single chemosensory system; other groups, including vertebrates, have evolved two separate systems which are comprised of olfaction and taste. Olfaction and taste function independently and jointly to inform the brain about chemicals in the environment. The olfactory system detects odorants and is used to identify potential dangers in the environment as well as being involved in many behavioral interactions, including kin recognition and mate selection. Taste is used to detect nutrients for consumption as well as potentially toxic compounds that should be avoided. The taste system is required for survival and its impairment often causes malnutrition which can lead to death [1].
The cells that detect the chemicals for taste are called taste receptor cells (TRCs) and these cells are grouped together in taste buds found in the oral cavity. Taste buds consist of 50-150 TRCs; in some organisms, taste buds are scattered across the tongue. In the channel catfish, additional taste buds are located on the outer epithelium across their entire body, essentially making the catfish a “giant tongue” [2]. This is quite unusual and for most vertebrates, taste buds are contained within the oral cavity. In mammals, taste buds are housed in specialized bumps and grooves called papillae. There are three types of papillae: circumvallate (CV), foliate (Fol) and fungiform (Fun). The CV papillae are found on the back of the tongue and are comprised of crypts that are lined with hundreds of taste buds. The Fol papillae also house hundreds of taste buds and are found on the both sides of the tongue. The Fun papillae are small protrusions that house 1-2 taste buds each and are scattered across the anterior two thirds of the tongue. Additional taste buds are located on the palate and throat, including a row of taste buds found between the hard and soft palate called the geschmacksstreifen [1, 3-12].
TRCs are unique in that they have properties of both neurons and epithelial cells. Like epithelial cells, TRCs express certain keratinocyte markers and are routinely replaced throughout an organism's life, having a limited lifespan between two weeks and two months [4, 13]. Like neurons, TRCs are excitable cells, fire action potentials and form synapses with gustatory neurons. TRCs extend microvilli into the oral cavity where receptors on these microvilli detect chemicals that are being released during mastication. TRCs convert this chemical stimulus into an output signal that is sent to the brain for processing via the afferent gustatory neurons. Depending on this message, the food item will either be ingested or ejected. Compared to other sensory systems, the taste system is able to detect a large number of chemically diverse stimuli that vary in size, charge, pH, and lipophilicity. In order to detect all of these different types of stimuli, the TRCs use a variety of receptors and signaling pathways. A particular TRC does not express all of these signaling mechanisms and is sensitive to a subset of different taste stimuli [1, 3-12]. This review is focused on the role that calcium plays in the signaling pathways that are used by taste cells to formulate a signal that is sent to the brain for processing.
TASTE TRANSDUCTION
The sense of taste is comprised of five basic taste qualities that include bitter, sour, salty, sweet and umami. Bitter detects potentially toxic compounds that usually should be avoided; sour detects protons which indicate spoiled food; salty is used to identify ions that are needed to maintain ionic balance; sweet is used to detect energy rich carbohydrates and umami detects glutamate and other amino acids. Based on the general chemical nature of the different taste stimuli, we can divide them into two broad groups, ionic and chemically complex. Salty and sour is the detection of different ions and are called ionic stimuli while the other taste qualities, bitter, sweet and umami are more chemically complex. Due to their simple ionic nature and charge, salty and sour activate ionotropic receptors to cause a cell depolarization. This activates a voltage-gated calcium influx which generates an increase in intracellular calcium that subsequently initiates neurotransmitter release from a conventional chemical synapse [1, 4, 9, 10].
Bitter, sweet and umami stimuli activate G-protein coupled receptors (GPCRs) that initiate a common signaling pathway. Upon activation, these GPCRs activate a phospholipase C (PLC) signaling pathway which causes calcium release from stores via activation of inositol triphosphate receptors (IP3Rs). These cells do not have conventional synapses but still communicate to gustatory nerves using neurotransmitters. The calcium release from ER stores activates a TRP channel, TRPM5. TRPM5 is a sodium selective TRP channel which causes a membrane depolarization through a sodium influx. This depolarization activates a hemichannel which opens and releases ATP onto the gustatory nerve. The identity of this hemichannel is still being debated with several candidate proteins including connexins, pannexins, and the calcium homeostasis modulator 1 (CALHM1). Regardless of its identity, the neurotransmitter ATP is released which causes a signal to be sent to the brain. Thus, calcium release from stores is sufficient for neurotransmitter release to occur in these cells [3, 11, 12, 14-28]. For all taste stimuli, a calcium signal is required for normal taste transduction to occur.
CALCIUM SIGNALING IN TASTE CELLS
Beyond this calcium signal requirement in taste transduction, very little is known about these signals. Analyses of the calcium release and calcium influx signals found, as expected, that these signals are significantly different from each other both in their timing as well as their magnitude. An additional PLC signaling pathway was also identified in some of the taste cells that have conventional synapses and express voltage-gated calcium channels (VGCCs). These TRCs can respond to multiple taste stimuli. The taste-evoked calcium signals in these cells are still different from each other in both their kinetics and their magnitude, further supporting the conclusion that unique calcium signals are generated for different taste stimuli [29].
Further studies have demonstrated that ryanodine receptors are expressed in a sub-population of taste cells and that these receptors are contributing to the calcium release signal in about 30% of the taste cells expressing the PLC signaling pathway and hemichannels for ATP neurotransmitter release. When ryanodine receptors are present, the taste-evoked calcium signal is significantly larger, which is predicted to increase the amount of neurotransmitter that is released. Interestingly, ryanodine receptors were also expressed in some TRCs with voltage-gated channels. In this population of taste cells, the ryanodine receptors were contributing to the calcium influx signal generated by VGCCs. Further analysis found that in the TRCs with both VGCCs and the PLC signaling pathway, the ryanodine receptors were exclusively contributing to the calcium influx signal generated by VGCCs and no longer contributed to the calcium release signal generated by activating the PLC signaling pathway [30]. Data indicated that the ryanodine receptors were associating with L-type VGCCs in these cells and that there is a functional, but not physical interaction, between these proteins [31]. The mechanism underlying this selective interaction has not been identified in taste cells but this specificity may be due to the localization of these different signaling components on the endoplasmic reticulum. Further work is needed to determine the mechanism that is directing the selectivity of the ryanodine receptor function in the different taste cell populations.
CALCIUM REGULATION IN TASTE CELLS
Since TRCs are detecting chemicals from the environment, they must be in contact with the environment, which dictates their position in the oral cavity. These cells extend microvilli into the oral cavity which makes them susceptible to damage and for this reason, TRCs are routinely replaced. In addition to their exposure to potential dangers such as bacteria, viruses, or significant temperature changes, TRCs must also be capable of dealing with a variable external environment. The environment in the oral cavity changes every time potential nutrients are being consumed. But within this context, TRCs must accurately respond to the chemicals that are introduced during consumption so it is absolutely critical that these cells maintain an appropriate intracellular ionic balance during feeding. As a result, TRCs are very actively regulating cytosolic calcium levels, even in the absence of any stimulation. TRCs can undergo a constitutive calcium influx even in the absence of stimulation but this influx is buffered by multiple mechanisms to maintain appropriate resting cytosolic calcium levels and thus allow the cell to respond appropriately when taste stimuli are presented.
Calcium clearance mechanisms in basal calcium regulation
While the evoked calcium signals are not particularly well-described in taste cells, even less is understood about how TRCs regulate their calcium levels. While TRCs maintain resting calcium levels around 100nM, there is evidence that these cells can have a constitutive calcium influx in the absence of cell stimulation which they actively regulate. In the absence of external calcium, the resting calcium levels in TRCs often decrease to a resting value between 30nM to 50nM which suggests that TRCs may also depend on this constitutive calcium influx to maintain their normal resting calcium levels. When the external calcium concentration is increased around TRCs, their baseline calcium levels also increase [32], further supporting an active relationship between the cytosolic calcium and the external calcium environment in these cells. This constitutive calcium influx is regulated by multiple calcium clearance mechanisms, including both the mitochondria and the sodium/calcium exchanger (NCX) [32, 33]. Inhibiting the mitochondria's ability to take up calcium using the protonophore, carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) in unstimulated TRCs caused an increase in cytosolic calcium that remained elevated until the mitochondria were once again able to buffer calcium [32] (Figure 1A). When FCCP is applied to TRCs, its acts as an uncoupler of the proton gradient in the mitochondria and disrupts ATP synthesis. In addition, it dispels the large negative potential inside the mitochondrial matrix which normally creates a strong driving force for calcium to leave the cytosol and enter the inner mitochondrial matrix. In the presence of FCCP, there is no longer a driving force on calcium to enter the mitochondria.
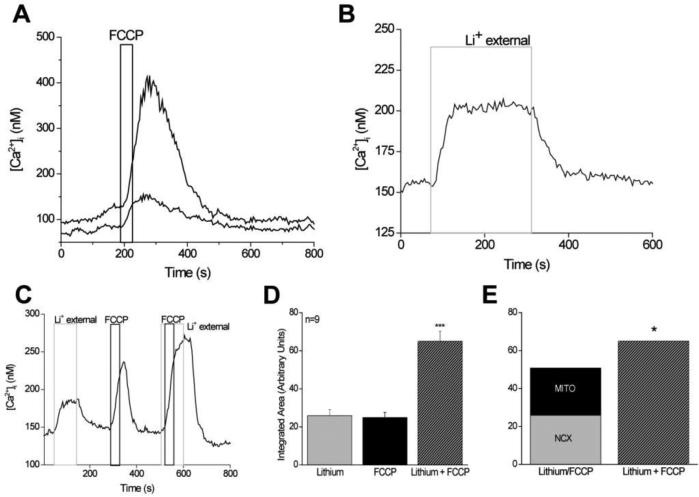
Figure 1. Basal calcium levels are actively regulated in taste cells even in the absence of stimulation.
A, Application of the protonophore, FCCP (black bar), reversibly eliminated the mitochondria's ability to buffer calcium. Application of FCCP caused increases in cytosolic calcium levels in 94% of previously un-stimulated taste cells (n=536). The amplitude of the response varied among different cells as shown here. Two taste cells simultaneously stimulated by FCCP evoked very different amplitudes in their calcium responses. Reprinted from Hacker and Medler (2008). B,Replacing external sodium with lithium (4 min, light gray column) inhibited sodium/calcium exchanger activity and caused a sustained increase in cytosolic calcium that was maintained until sodium was replaced and the exchangers were again functional. C, Replacement of external sodium with lithium (gray column) for 100 s caused an elevation in cytosolic calcium. Applying FCCP for 40 s (1 μM, dark gray column) to inhibit mitochondrial calcium uptake also elevated cytosolic calcium. Adding FCCP when NCXs were inhibited caused a much larger increase in cytosolic calcium compared to FCCP or lithium alone. D, The area under the curve for each condition was integrated and compared using the one-way ANOVA. FCCP + lithium was significantly larger than either FCCP or lithium alone (n=9, p<0.0001, ***). E, The integrated area under the FCCP + lithium curve was significantly larger than the combination of the integrated areas reported in D for the calcium elevations that occurred when FCCP and lithium were applied individually (n=9, p<0.05, *). Adding the area of the FCCP-dependent calcium response to the integrated area of the calcium response when NCXs were inhibited was only 78% of the integrated area of the calcium response that was generated when FCCP was applied while NCXs were inhibited. Reprinted from Laskowski and Medler (2009).
One of the primary functions of mitochondria is to produce ATP and support the metabolic needs of the taste cell, so it was possible that inhibiting the mitochondria's ability to make ATP was causing the observed changes in the cytosolic calcium. Since both PMCAs and SERCA pumps require ATP to function, it was possible that the mitochondria were not directly buffering cytosolic calcium but were indirectly inhibiting the calcium ATPases through ATP depletion. However, control experiments demonstrated that ATP depletion does not significantly contribute to the observed calcium elevations within the time frame of the experiments and thus, inhibiting mitochondrial calcium uptake was the only process responsible for the measured effect [32]. Therefore, the mitochondria are actively regulating cytosolic calcium in taste cells, even in absence of stimulation.
The amplitudes of the calcium elevations due to mitochondrial inhibition were variable but correlated significantly with the signaling mechanisms used by the taste cells. TRCs with stimulus-induced calcium release from internal stores had significantly smaller changes in cytosolic calcium levels when the mitochondria were inhibited compared to taste cells that expressed VGCCs [32]. These data suggest a relationship between the amplitude of the FCCP-evoked calcium responses and the signaling mechanisms used by the taste cells. While the reason for this correlation is not known, it is possible that numbers of mitochondria vary by cell type either due to the different metabolic needs of the cells or due to the different calcium buffering requirements needed to appropriately control the evoked calcium signals. It is also possible that these responses are due to differences in mitochondrial localization within the cells. While TRCs are polarized cells and have distinct apical and basal orientations, they have a very simple morphology compared to many neurons. It is possible that mitochondrial localization within the cell help restrict calcium responses to specific areas of the cytosol as has been seen in other cell types [34, 35]. Mitochondria may preferentially localize near the plasma membrane in taste cells with voltage-gated calcium channels while clustering close to the ER in taste cells that depend solely on calcium release from stores to increase cytosolic calcium. Localizing near the ER could result in fewer mitochondria contributing to the regulation of calcium influx signals, including a constitutive calcium influx. It is equally possible that there are different channels contributing to the constitutive calcium influx in the different cell types and it is the magnitude of the calcium influx that is changing rather than any difference in the mitochondrial calcium buffering.
One potential variance across cell types that might significantly affect how mitochondrial calcium buffering regulates calcium signaling in TRCs is the presence of atypical mitochondria in some TRCs. Atypical mitochondria are much larger than normal mitochondria, are near sites of nerve contact, and often associate with sub-surface cisternae [36]. Sub-surface cisternae have been reported to be predominantly found in the taste cells which respond to bitter, sweet or umami taste stimuli via calcium release from internal stores [37, 38]. These data suggest a potential connection between the presence of atypical mitochondria in taste cells that use calcium release from stores and the role of mitochondrial calcium buffering in these taste cells. While the role of these atypical mitochondria is currently unknown, it is possible they are contributing to the differences in the calcium response amplitudes that occur when mitochondria are inhibited.
Since mitochondria function as temporary calcium sinks and cannot take up calcium indefinitely, there has to be at least one calcium extrusion mechanism to remove excess calcium due to this constitutive calcium influx. Since this influx can generate a relatively large calcium load, sodium/calcium exchangers (NCXs) may be important in the regulation of basal calcium levels in taste cells. NCXs are localized on the plasma membrane in cells and have a low calcium affinity but high-capacity for calcium removal [39-41].
In un-simulated TRCs, inhibiting NCX activity by replacing external sodium with lithium caused an increase in cytosolic calcium which was maintained as long as the NCXs were not functional (Figure 1B). While lithium can substitute for sodium as an ion carrier through some channels, it cannot substitute for sodium through the exchanger. Therefore, replacement of external sodium with lithium maintains the appropriate ionic balance for the cells but inhibits NCX activity [42]. When the exchangers were inhibited, the cytosolic calcium increased and then usually plateaued where it was maintained until the external lithium was replaced with sodium and the exchangers were once again functioning. This allowed the calcium elevation to return to baseline values, presumably through NCX activity. In these experiments, there was also variability in the magnitude of the calcium elevation due to NCX inhibition that correlated with the different calcium signaling mechanisms used by the taste cells (either calcium release or calcium influx via VGCCs). As seen with the mitochondria, the taste cells that rely on calcium release from stores had significantly smaller calcium elevations as a result of inhibiting NCX activity compared to the magnitude of the cytosolic calcium elevation when the exchangers were inhibited in taste cells expressing VGCCs [33]. These data also suggest that each taste cell population differs in the way they regulate cytosolic calcium. The reason for this difference is unknown but since exchangers are restricted to the plasma membrane, their cellular localization is less likely to be contributing to these differences. However, taste cells express multiple exchanger isoforms [33] and there may be some specificity in exchanger isoform expression that is driving these differences. In addition, there may be a correlation between the channel(s) responsible for the calcium influx and the calcium signaling mechanism used by the taste cell.
Since inhibiting either the mitochondria or the NCX caused similar correlations in the response amplitudes, we postulated that these two calcium regulatory mechanisms work together to control cytosolic calcium. To address this question, the effect on cytosolic calcium when each calcium regulatory mechanism was individually inhibited, was analyzed and then compared to the effect on cytosolic calcium when they were inhibited together. If these two mechanisms are working together to regulate cytosolic calcium and if one mechanism is inhibited, the other mechanism could at least partially compensate for the additional calcium load. If both mechanisms are inhibited, the overall calcium response would increase even more because neither mechanism would be available to compensate for the other. When both mitochondria and NCX were inhibited, the calcium load was significantly greater than the sum of the individual calcium loads from inhibiting either NCX or mitochondrial calcium uptake alone (Figure 1C-E). Therefore, in TRCs, the mitochondria and NCXs appear to work together to regulate cytosolic calcium, even in the absence of cell stimulation [33].
Calcium clearance mechanisms in regulating evoked calcium signals
Taste cells also require mechanisms to terminate evoked calcium signals. When 50mM KCl is applied to taste cells, they depolarize and calcium enters the cell through VGCCs. This process causes an initial fast signal that quickly peaks and is then followed by a plateau phase that slowly returns to pre-stimulus calcium levels (Figure 2A). The return to baseline calcium can be quite extended and is likely due to the slow release of cytosolic calcium by the mitochondria and calcium buffering proteins [43-45]. When NCXs are inhibited, the initial peak amplitude of the response does not change but the duration of the initial response significantly increases (Figure 2A), suggesting that NCXs are important in limiting the length of time that the TRCs are exposed to high cytosolic calcium when VGCCs open; however, NCXs do not appear to affect the amplitude of the peak calcium response [46].
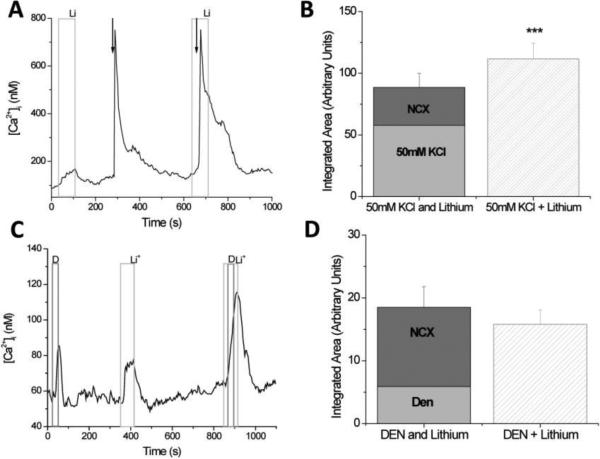
Figure 2. Sodium/calcium exchangers significantly regulate evoked calcium influx signals but do not contribute to the reduction of the evoked calcium release from internal stores.
A, Replacing external sodium with lithium (light gray column) caused a reversible increase in cytosolic calcium levels while a 10s application of 50mM KCl (arrow) depolarized the taste cells and opened VGCCs. The calcium influx through the VGCCs generated a large increase in cytosolic calcium that slowly returned to baseline levels. B, The integrated area under the 50mM KCl + lithium elevation was significantly larger than the combination of the integrated areas for the calcium elevations that occurred when 50mM KCl and lithium were applied individually (***p<0.001). Adding the area of the 50mM KCl-evoked calcium response (light gray column) to the integrated area of the calcium response when NCXs were inhibited (dark gray column) was only 79% of the integrated area of the calcium response that was generated when VGCCs were open while NCXs were inhibited (striped column). C, Replacing external sodium with lithium (Li) caused a reversible increase in cytosolic calcium levels while a 30s application of the bitter compound, 10mM denatonium benzoate (D) caused calcium release from internal stores. When bitter receptors were activated while NCXs were inhibited, the cytosolic calcium response increased. D, The integrated area under the lithium + denatonium response (Li + Den, striped column) was approximately equal to the combination of the integrated areas for the calcium signals that occurred when denatonium (light gray column) and lithium (dark gray column) were applied individually. Reprinted from Szenbenyi et al., (2010).
One possible explanation for why the peak amplitudes are not changing when VGCCs channels open and NCXs are inhibited is that most of the calcium influx is coming through L-type calcium channels which are inactivated by calcium [47]. The additional calcium load due to NCX inhibition may be sufficient to at least partially inactivate these L-type calcium channels and thus inhibit the peak calcium response that would otherwise occur during cell depolarization and NCX inhibition. The distribution of VGCCs in taste cells has not been well-characterized but studies have established that multiple VGCCs are expressed in taste cells, including L-type calcium channels. It is clear that L-type calcium channels are not the only VGCCs in taste cells [20, 31, 48, 49] and the Roberts et al. (2009) study indicates that there is some segregation in the expression of VGCC isoforms within taste cells. If L-type calcium channel inactivation does significantly affect the amplitude of the response peak, then NCXs may actually have an important role in controlling the initial peak response magnitude that was not measured in the earlier study [46].
To accurately evaluate the role of NCXs in regulating evoked calcium loads, analyses must consider the calcium load that is generated by inhibiting NCXs in the absence of cell stimulation. In taste cells, this calcium load is significant and could potentially confound the analysis (see Figure 2A, lithium external alone). To evaluate the role of NCXs in the termination of the calcium influx signal, the area under the response curve due to 50mM KCl application was integrated to derive a proportional measure of the amount of calcium that enters during cell stimulation. The area under the curve when NCXs were inhibited in the absence of cell stimulation was also measured. Finally, the calcium signal with NCXs are inhibited (due to external lithium) and the cell is depolarized (application of 50mM KCl) was measured. By adding the calcium signal due to inhibiting NCX activity and the calcium signal due to opening VGCCs alone, this added value can be compared to the magnitude of the calcium signal that occurs when both VGCCs are open and NCXs are inhibited. If this added value (Hi K alone + NCX inhibition alone) is equal to the calcium signal when both events are occurring simultaneously, then we would conclude that these two events are occurring independently inside the cell. However, when these values were added, the overall calcium response was significantly less than the magnitude of the calcium signal that occurred when both events happened at the same time (Figure 2B). This supports the hypothesis that the NCXs significantly regulate the calcium influx signal through VGCCs as well as their role in regulating basal calcium levels, because when NCXs were non-functional, the calcium load was significantly larger even after the constitutive calcium influx due to inhibiting NCX activity was taken into account [46].
Similar experiments were performed to determine if NCXs regulate the calcium signals due to calcium release from internal stores. TRCs were stimulated to activate the PLC signaling pathway and cause calcium release from internal stores. Adding the integrated calcium loads for the taste-evoked calcium signal and the NCX-dependent calcium signal resulted in an overall calcium signal that was approximately equal to the calcium signal in the TRCs when both events occurred simultaneously. These data suggest that the NCXs do not significantly contribute to the termination of these taste-evoked calcium release signals (Figure 2C,D) [46].
Since NCXs have a relatively low affinity for calcium [50], one potential reason for the differences in the role of NCXs between these calcium signals is that the calcium load due to calcium release from stores is not large enough to activate the NCXs while the calcium influx signal is sufficient. The other likely reason is that the initiation site of the calcium signal is driving NCX activity since calcium influx occurs at the plasma membrane where NCXs are located while calcium release occurs at the internal stores. Follow-up experiments determined that signal location rather than calcium load underlies the selectivity of the NCX response. Weak depolarizations that generate much smaller calcium loads on the cell were still affected by NCX activity while comparable calcium loads due to calcium release from stores were not [46].
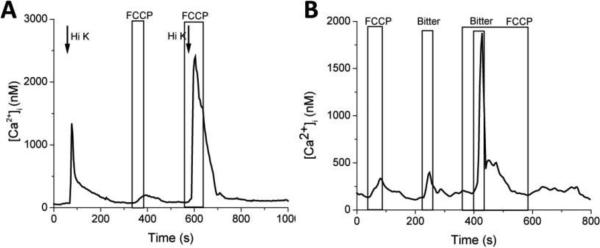
Further studies evaluated the role of the mitochondria in regulating evoked calcium signals. Isolated TRCs were loaded with Fura-2AM to measure how cytosolic calcium levels changed in response to cell stimulation in the presence and absence of the protonophore, FCCP. The effect of inhibiting mitochondrial calcium uptake with FCCP was measured for evoked calcium signals in TRCs, including both depolarizing the cell with 50mM KCl and measuring the calcium influx signal through VGCCs as well as generating the taste-evoked calcium release signal from internal stores. When the mitochondria were inhibited with FCCP, calcium influx signals significantly increased in both amplitude and duration. This increase was significantly larger than the sum of the calcium influx signal and the calcium load due to inhibiting mitochondria in the absence of cell stimulation (Figure 3A). Similar effects were seen on the calcium release signals as well (Figure 3B). These data indicate that mitochondria contribute to the regulation of evoked calcium signals in addition to their role in regulating cytosolic calcium in the absence of cell stimulation.
Figure 3. Mitochondria significantly regulate both evoked calcium influx and calcium release signals in TRCs.
A, Taste cells were depolarized with 50mM KCl (Hi K) and the calcium influx through VGCCs was measured. Inhibiting mitochondrial calcium uptake with the protonophore, FCCP, increased the amplitude and duration of the calcium influx signal through VGCCs. B, When mitochondrial calcium uptake was inhbitied with FCCP, the amplitude and duration of the taste-evoked calcium release signal increased.
For the calcium influx signal, mitochondria appear to be important in reducing the initial amplitude of the signal as well as shortening the duration of the initial peak of the calcium signal. When mitochondrial calcium uptake was inhibited with FCCP, the plateau phase that often develops as the calcium influx signal returns to pre-stimulus levels was lost. These data support the hypothesis that the mitochondria play important roles in regulating the magnitude and duration of the calcium influx signals through VGCCs in TRCs. Similar results were found when the mitochondria were inhibited during taste-evoked calcium release from stores. Both the initial peak amplitude and the duration of the response increased when mitochondria were not buffering calcium and the constitutive calcium influx was taken into account. Unlike NCXs, the mitochondria appear to have a very important role in regulating both types of evoked calcium signals. This is likely because the mitochondria are not spatially restricted to the membrane as is the case for NCXs.
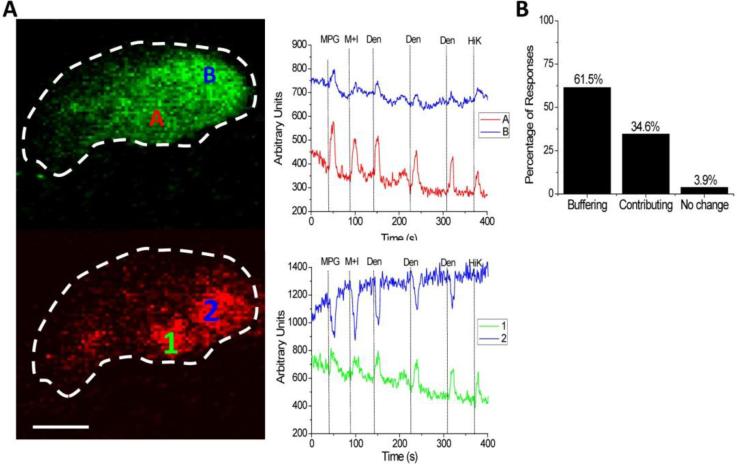
These initial mitochondrial studies were expanded by the development of a technique in taste cells to simultaneously measure mitochondrial calcium changes and cytosolic calcium changes directly. In these experiments, TRCs were loaded with Rhod-2AM and Fluo-4AM and calcium changes in both the cytosol and mitochondria were measured. Direct measurements of mitochondrial calcium changes in response to taste evoked calcium signals in TRCs revealed some unexpected findings. In these experiments, most mitochondrial calcium levels increased (approximately 60%) as the cytosolic calcium levels increased, indicating that the mitochondria are removing excess calcium to regulate the size of the evoked cytosolic calcium signal. A small percentage (approximately 4%) of mitochondria had no change in response to elevated cytosolic calcium. However, a significant number of mitochondria (approximately 35%) had an acute reduction in their calcium levels in response to taste-evoked calcium signals, indicating that they are releasing calcium into the cytosol in response to cell stimulation. The channel or channels that are releasing mitochondrial calcium in response to the taste-evoked calcium signals have not been identified. Preliminary studies suggest that at least some of this calcium release is coming through the mitochondrial sodium/calcium exchanger, but further studies are needed to confirm that finding.
It is not clear what determines if mitochondria are going to take up calcium or release calcium in response to a taste-evoked calcium increase. Initial findings suggest that the localization of the mitochondria relative to the site of the calcium increase has an effect in determining their response to elevated calcium. Localized recordings of evoked cytosolic calcium signals found that the size of the evoked calcium signal was not uniform across the entire TRC. While this is not surprising given what has been seen in other systems, this has not previously been reported in TRCs. Much of signal localization in other systems is thought to depend on anatomical specializations that physically restrain the ability of the calcium signal to diffuse to other areas. However, TRCs have very simple anatomy with little to no specialization that would restrict calcium diffusion within the cell. It is possible, indeed even likely, that the TRCs use mitochondria to restrict and localize the calcium signals within the cytoplasm. Mitochondria have been shown to spatially restrict calcium signals in pancreatic acinar cells which also lack anatomical specializations to physically restrict calcium diffusion. In these cells, mitochondria appear to form a physical barrier that prevents calcium from diffusing across the cell and this effectively localizes the calcium signal [34, 35, 51]. Further studies are required to determine the extent of the mitochondria's role in localizing calcium signals in TRCs, but the current data support a role for them in this process. These data also suggest that the mitochondria, in some cases, can contribute to the calcium signal.
CONCLUSIONS
TRCs have the critical role of detecting chemicals in potential food items and translating that initial chemical signal into an output signal that the brain can understand. They must perform this function in the backdrop of an external environment that is constantly changing, very often while the chemicals from the food are being detected. In response to this need, TRCs evolved unique mechanisms to maintain an appropriate intracellular environment, so the evoked taste signals are correctly translated into signals that are sent to the brain. While our understanding of the mechanisms used by TRCs to regulate their internal environment is quite limited, initial studies have shown that TRCs exhibit a constitutive calcium influx that is routinely regulated by both the mitochondria and NCXs [32, 33]. Other calcium regulatory mechanisms may also control this calcium influx in the absence of stimulation but their roles have not been identified.
The relative roles of the mitochondria and NCXs in regulating this constitutive influx appears to vary by taste cell type [32, 33] as do their relative roles in regulating evoked calcium signals. There is also some selectivity in the expression of calcium buffering proteins that correlates with taste cell type [52] further supporting the hypothesis that calcium regulation in taste cells varies with taste cell type. These variances in the roles of the different calcium regulatory mechanisms are likely due to the different calcium loads that these taste cell populations are exposed to, but further work is needed to determine if this is why their roles vary.
Initial studies indicate a very active role in NCXs in regulating evoked calcium influx signals but not calcium release signals while the mitochondria appear to regulate both types of signals in TRCs. Indeed, these studies demonstrate that mitochondria have a central role in localizing the evoked calcium signal within the taste cell and in some instances, the mitochondria appear to contribute to the evoked calcium signal. Thus, even though TRCs have a very simple anatomy that does not appear to contribute to the localization of evoked calcium signals, these cells still have a sophisticated system in place to precisely control cytosolic calcium. Since neurotransmitter release is proportional to the changes in intracellular calcium, the calcium regulatory mechanisms in these cells play critical roles in the translation of the evoked signals to the brain.
Highlights.
Taste receptor cells that detect chemicals in food use multiple signaling pathways.
Taste cells have evolved mechanisms to cope with changing environments.
Taste cells can have a constitutive Ca2+ influx in the absence of stimulation.
Both the mitochondria and sodium/calcium exchanger regulate this Ca2+ influx.
The mitochondria and exchanger also regulate evoked Ca2+ signals in distinct ways.
Figure 4. Mitochondria contribute to the localization of calcium signals within TRCs.
A, Dual loading of TRCs with Fluo4 (green) and Rhod 2 (red) allowed for the direct measurement of changes in both cytosolic (upper panel) and mitochondrial calcium changes (lower panel). In this representative experiment, two cytosolic measurements (A, B) and two mitochondrial measurements (1,2) were taken. Measurements of the taste-evoked changes in cytosolic calcium revealed a localization of the calcium changes within different regions of the taste cell (upper panel). Some mitochondria took up calcium in response to cytosolic calcium increases while other mitochondria had a transient decrease in their calcium levels (lower panel). MPG-umami, M+I-umami, Den-bitter, HiK-50mM KCl to depolarize the cell and open VGCCs. Scale bar = 5 μM. B, Approximately 60% of the mitochondria buffer calcium in response to stimulus induced calcium increases while about 35% of mitochondria contribute to the evoked calcium signal. Another 4% of mitochondria did not change when the cells were stimulated. 182 mitochondria tested from 72 cells.
ACKNOWLEDGMENTS
Work from my lab was funded by the National Institute of Deafness and Other Communication Disorders Grant DC-006358, and by National Science Foundation grants 0917893, 1256950 to K. Medler.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Finger TE, Simon SA. Cell Biology of taste epithelium. In: Finger TE, Silver WL, Restrepo D, editors. The Neurobiology of Taste and Smell. Wiley-Liss; 2000. pp. 287–314. Place Published. [Google Scholar]
- 2.Caprio J, Brand JG, Teeter JH, Valentincic T, Kalinoski DL, Kohbara J, Kumazawa T, Wegert S. The taste system of the channel catfish: from biophysics to behavior. Trends Neurosci. 1993;16:192–197. doi: 10.1016/0166-2236(93)90152-c. [DOI] [PubMed] [Google Scholar]
- 3.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 4.Chaudhari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 6.Iwata S, Yoshida R, Ninomiya Y. Taste Transductions in Taste Receptor Cells: Basic Tastes and Moreover. Current pharmaceutical design. 2013 doi: 10.2174/13816128113199990575. [DOI] [PubMed] [Google Scholar]
- 7.Kinnamon JC. Organization and innervation of taste buds. In: Finger t.E., Silver WL., editors. The Neurobiology of Taste and Smell. Wiley, Place Published; 1987. pp. 277–297. [Google Scholar]
- 8.Kinnamon SC, Margolskee RF. Mechanisms of taste transduction. Curr Opin Neurobiol. 1996;6:506–513. doi: 10.1016/s0959-4388(96)80057-2. [DOI] [PubMed] [Google Scholar]
- 9.Lindemann B. Taste reception. Physiol Rev. 1996;76:718–766. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- 10.Lindemann B. Receptors and transduction in taste. Nature. 2001;413:219–225. doi: 10.1038/35093032. [DOI] [PubMed] [Google Scholar]
- 11.Medler K. Signaling mechanisms controlling taste cell function. Crit Rev Eukaryot Gene Expr. 2008;18:125–137. doi: 10.1615/critreveukargeneexpr.v18.i2.20. [DOI] [PubMed] [Google Scholar]
- 12.Medler KF, Kinnamon SC. Transduction Mechanisms in Taste Cells. In: Stephan Frings JB, editor. Transduction Channels in Sensory Cells. Wiley-VCH, Place Published; 2004. pp. 153–177. [Google Scholar]
- 13.Perea-Martinez I, Nagai T, Chaudhari N. Functional cell types in taste buds have distinct longevities. PLoS One. 2013;8:e53399. doi: 10.1371/journal.pone.0053399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caicedo A, Pereira E, Margolskee RF, Roper SD. Role of the G-protein subunit alpha-gustducin in taste cell responses to bitter stimuli. J Neurosci. 2003;23:9947–9952. doi: 10.1523/JNEUROSCI.23-30-09947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba NJ. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhari N, Maruyama Y, Roper S, Trubey K. Multiple pathways for signaling glutamate taste in rodents. Chem Senses. 2005;30(Suppl 1):i29–i30. doi: 10.1093/chemse/bjh097. [DOI] [PubMed] [Google Scholar]
- 17.Clapp TR, Medler KF, Damak S, Margolskee RF, Kinnamon SC. Mouse taste cells with G protein-coupled taste receptors lack voltage-gated calcium channels and SNAP-25. BMC Biol. 2006;4:7. doi: 10.1186/1741-7007-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clapp TR, Stone LM, Margolskee RF, Kinnamon SC. Immunocytochemical evidence for co-expression of Type III IP3 receptor with signaling components of bitter taste transduction. BMC Neurosci. 2001;2:6. doi: 10.1186/1471-2202-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Perez CA, Shigemura N, Yoshida R, Mosinger B, Jr., Glendinning JI, Ninomiya Y, Margolskee RF. Trpm5 null mice respond to bitter, sweet, and umami compounds. Chem Senses. 2006;31:253–264. doi: 10.1093/chemse/bjj027. [DOI] [PubMed] [Google Scholar]
- 20.DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, Chaudhari N. Separate populations of receptor cells and presynaptic cells in mouse taste buds. J Neurosci. 2006;26:3971–3980. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 22.Huang YA, Roper SD. Intracellular Ca2+ and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol. 2010;588:2343–2350. doi: 10.1113/jphysiol.2010.191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kataoka S, Baquero A, Yang D, Shultz N, Vandenbeuch A, Ravid K, Kinnamon SC, Finger TE. A2BR adenosine receptor modulates sweet taste in circumvallate taste buds. PLoS One. 2012;7:e30032. doi: 10.1371/journal.pone.0030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyoshi MA, Abe K, Emori Y. IP(3) receptor type 3 and PLCbeta2 are co- expressed with taste receptors T1R and T2R in rat taste bud cells. Chem Senses. 2001;26:259–265. doi: 10.1093/chemse/26.3.259. [DOI] [PubMed] [Google Scholar]
- 26.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. Embo J. 2007;26:657–667. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romanov RA, Rogachevskaja OA, Khokhlov AA, Kolesnikov SS. Voltage dependence of ATP secretion in mammalian taste cells. J Gen Physiol. 2008;132:731–744. doi: 10.1085/jgp.200810108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hacker K, Laskowski A, Feng L, Restrepo D, Medler K. Evidence for two populations of bitter responsive taste cells in mice. J Neurophysiol. 2008;99:1503–1514. doi: 10.1152/jn.00892.2007. [DOI] [PubMed] [Google Scholar]
- 30.Rebello MR, Medler KF. Ryanodine receptors selectively contribute to the formation of taste-evoked calcium signals in mouse taste cells. Eur J Neurosci. 2010;32:1825–1835. doi: 10.1111/j.1460-9568.2010.07463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebello MR, Maliphol AB, Medler KF. Ryanodine Receptors Selectively Interact with L Type Calcium Channels in Mouse Taste Cells. PLoS One. 2013;8:e68174. doi: 10.1371/journal.pone.0068174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hacker K, Medler KF. Mitochondrial calcium buffering contributes to the maintenance of Basal calcium levels in mouse taste cells. J Neurophysiol. 2008;100:2177–2191. doi: 10.1152/jn.90534.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laskowski AI, Medler KF. Sodium/calcium exchangers contribute to the regulation of cytosolic calcium levels in mouse taste cells. The Journal of physiology. 2009;587(16):4077–4089. doi: 10.1113/jphysiol.2009.173567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson PR, Tepikin AV, Erdemli G. Role of mitochondria in Ca(2+) homeostasis of mouse pancreatic acinar cells. Cell Calcium. 2002;32:59–69. doi: 10.1016/s0143-4160(02)00091-x. [DOI] [PubMed] [Google Scholar]
- 35.Petersen OH, Burdakov D, Tepikin AV. Polarity in intracellular calcium signaling. Bioessays. 1999;21:851–860. doi: 10.1002/(SICI)1521-1878(199910)21:10<851::AID-BIES7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 36.Royer SM, Kinnamon JC. Ultrastructure of mouse foliate taste buds: synaptic and nonsynaptic interactions between taste cells and nerve fibers. J Comp Neurol. 1988;270:11–24, 58-19. doi: 10.1002/cne.902700103. [DOI] [PubMed] [Google Scholar]
- 37.Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. J Comp Neurol. 2004;468:311–321. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, Ryba NJ. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 39.Berridge M, Bootman M. Calcium Signaling. Chapman and Hall; 1996. Place Published. [Google Scholar]
- 40.Monteith GR, Roufogalis BD. The plasma membrane calcium pump--a physiological perspective on its regulation. Cell Calcium. 1995;18:459–470. doi: 10.1016/0143-4160(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 41.Sedova M, Blatter LA. Dynamic regulation of [Ca2+]i by plasma membrane Ca(2+)-ATPase and Na+/Ca2+ exchange during capacitative Ca2+ entry in bovine vascular endothelial cells. Cell Calcium. 1999;25:333–343. doi: 10.1054/ceca.1999.0036. [DOI] [PubMed] [Google Scholar]
- 42.Le Guennec JV, Noble D. Effects of rapid changes of external Na+ concentration at different moments during the action potential in guinea-pig myocytes. The Journal of physiology. 1994;478(Pt 3):493–504. doi: 10.1113/jphysiol.1994.sp020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medler K, Gleason EL. Mitochondrial Ca(2+) buffering regulates synaptic transmission between retinal amacrine cells. J Neurophysiol. 2002;87:1426–1439. doi: 10.1152/jn.00627.2001. [DOI] [PubMed] [Google Scholar]
- 44.White RJ, Reynolds IJ. Mitochondria and Na+/Ca2+ exchange buffer glutamate- induced calcium loads in cultured cortical neurons. J Neurosci. 1995;15:1318–1328. doi: 10.1523/JNEUROSCI.15-02-01318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White RJ, Reynolds IJ. Mitochondria accumulate Ca2+ following intense glutamate stimulation of cultured rat forebrain neurones. J Physiol. 1997;498(Pt 1):31–47. doi: 10.1113/jphysiol.1997.sp021839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szebenyi SA, Laskowski AI, Medler KF. Sodium/calcium exchangers selectively regulate calcium signaling in mouse taste receptor cells. J Neurophysiol. 2010;104:529–538. doi: 10.1152/jn.00118.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hille B. Ion Channels of Excitable Membranes. 3rd ed. Sinauer Associates, Inc.; 2001. Place Published. [Google Scholar]
- 48.Behe P, DeSimone JA, Avenet P, Lindemann B. Membrane currents in taste cells of the rat fungiform papilla. Evidence for two types of Ca currents and inhibition of K currents by saccharin. J Gen Physiol. 1990;96:1061–1084. doi: 10.1085/jgp.96.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts CD, Dvoryanchikov G, Roper SD, Chaudhari N. Interaction between the second messengers cAMP and Ca2+ in mouse presynaptic taste cells. J Physiol. 2009;587:1657–1668. doi: 10.1113/jphysiol.2009.170555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuda T, Takuma K, Baba A. Na(+)-Ca2+ exchanger: physiology and pharmacology. Jpn J Pharmacol. 1997;74:1–20. doi: 10.1254/jjp.74.1. [DOI] [PubMed] [Google Scholar]
- 51.Kopach O, Kruglikov I, Pivneva T, Voitenko N, Fedirko N. Functional coupling between ryanodine receptors, mitochondria and Ca(2+) ATPases in rat submandibular acinar cells. Cell Calcium. 2008;43:469–481. doi: 10.1016/j.ceca.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Rebello MR, Aktas A, Medler KF. Expression of calcium binding proteins in mouse type II taste cells. J Histochem Cytochem. 2011;59:530–539. doi: 10.1369/0022155411402352. [DOI] [PMC free article] [PubMed] [Google Scholar]