Abstract
Background
Patients with perihilar cholangiocarcinoma and gallbladder cancer extending into the hilum often present with jaundice and a small future liver remnant (FLR). If resectable, preoperative biliary drainage and portal vein embolization (PVE) are indicated. Classically, these measures have been performed sequentially, separated by 4–6 weeks.
Purpose
To report on a new regime where percutaneous transhepatic biliary drainage (PTBD) and PVE are performed simultaneously, shortening the preoperative process.
Material and Methods
Six patients were treated with concurrent PTBD and PVE under general anesthesia.
Results
Surgical exploration followed the combined procedure after 35 days (range, 28–51 days). The FLR ratio increased from 22% to 32%. Three patients developed cholangitis after the procedure.
Conclusion
The combined approach of PTBD and PVE seems feasible, but more studies on morbidity are warranted.
Keywords: Abdomen/GI, embolization, drainage
Introduction
Biliary cancer, including perihilar cholangiocarcinoma and gallbladder cancer, is an uncommon disease with a poor prognosis, often presenting with jaundice (1). The only potentially curative treatment option is surgical resection, frequently requiring an extended hepatectomy (1). Before resection, the issues of jaundice and the projected future liver remnant (FLR) must be addressed. Biliary obstruction is associated with body fluid disturbances, renal failure, and myocardial dysfunction (2). Additionally, preoperative jaundice has been shown to be associated with a poor outcome after resection (2). Preoperative biliary drainage of the FLR is advocated in order to decrease bilirubin level and optimize postoperative liver hypertrophy (3) and has been shown to reduce mortality after right-sided hepatectomy (4). A FLR <30% has been shown to be a predictor of postoperative hepatic dysfunction and death (5). In these cases, portal vein embolization (PVE) is considered to be a safe and effective method to preoperatively increase the FLR (6). Traditionally, biliary drainage is performed first, and is followed by PVE when jaundice has receded (7). The use of simultaneous percutaneous transhepatic biliary drainage (PTBD) and PVE has been stated in a previous report (2) but only one study, with preliminary experiences including four patients, exists analyzing this strategy (8). Therefore, further studies are needed to investigate the simultaneous approach. The aim of this study was to investigate the new concept where PTBD of the FLR is combined with simultaneous PVE.
Material and Methods
Between 2010 and 2014, six patients with biliary cancer, radiologically resectable by an extended right hepatectomy, underwent simultaneous left-sided PTBD and right-sided PVE at our tertiary hepatobiliary surgical units (Fig. 1). Preprocedural radiological workup included computed tomography (CT) and magnetic resonance cholangiopancreatography. All patients had histologically verified diagnoses. Patient characteristics for each patient are presented in Table 1. Five patients had hilar cholangiocarcinoma Bismuth type IIIA and one patient had gallbladder cancer with extension to the hilum (patient 2). Before presenting to our centers, three patients had undergone endoscopic retrograde biliary drainage (ERBD), with insertion of plastic endoprosthesis into the right biliary system, two patients had had PTBD with catheter placement in the right biliary tree, while one patient presented without previous biliary interventions. Thus, all included patients presented to our centers with an undrained left biliary system. The future liver remnant volume ratio, FLR%, was defined as FLR/Total liver volume before PVE. Indication for PVE was a FLR% <40%. No specific technique for estimation of FLR function was used. Patients were prepared to be able to withstand an extended right hepatectomy in case of intraoperative evidence of left hepatic duct involvement. Therefore, manual volumetric measurement of total liver volume and FLR, which in all cases consisted of Couinaud’s segments 2 and 3, was performed on CT scans as previously reported (9) before and approximately 4 weeks after PVE. If not stated otherwise, results are presented as median (range).
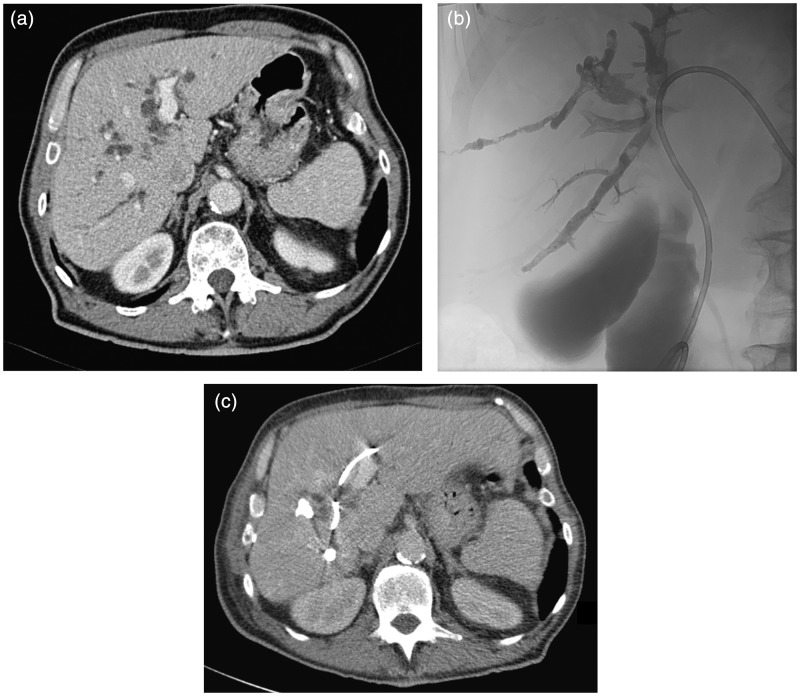
Fig. 1.
(a) Computed tomography (CT) image, before any intervention, of a patient with intrahepatic bile duct dilatation due to perihilar cholangiocarcinoma. (b) Image after placement of a left-sided percutaneous biliary drainage catheter and percutaneous portal vein embolization of the right portal tree with Histoacryl® and Lipiodol®. (c) Radiologic evaluation with CT 4 weeks after the simultaneous intervention showing hypertrophy of the left liver lobe and also depicting the radiopaque biliary catheter and embolization material.
Table 1.
Patient characteristics.
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Age (years) | 80 | 61 | 65 | 46 | 72 | 59 |
| Sex | Male | Female | Male | Male | Male | Male |
| ASA class | 1 | 2 | 2 | 1 | 3 | 1 |
| Bilirubin at diagnosis (µmol/L) | 162 | 200 | 251 | 328 | 135 | 279 |
| INR at diagnosis | 8.0 | NA | 1.3 | 1.5 | 1.0 | 1.2 |
| Biliary drainage before PVE + PTBD | ERBD | ERBD | ERBD | None | PTBD | PTBD |
| Bilirubin at PVE + PTBD (µmol/L) | 67 | 47 | 65 | 429 | 93 | 40 |
| INR at PVE + PTBD | 1.8 | 1.2 | 1.2 | 1.2 | 1.2 | 1.1 |
| Complications to PVE + PTBD | None | Cholangitis | None | Cholangitis | None | Cholangitis |
| Bilirubin at operation (µmol/L) | – | 12 | 39 | 43 | 19 | 13 |
| Resection | No | No | No | Yes | Yes | No |
ASA, American Society of Anesthesiologists; ERBD, endoscopic retrograde biliary draining; NA, not available; PTBD, percutaneous transhepatic biliary drainage; PVE, portal vein embolization.
The procedures were performed under general anesthesia after intravenous administration of antibiotic prophylaxis (4 g piperacillin-tazobactam). Access to peripheral bile ducts of the left lateral segment was obtained using a MAK NV set (Merit Medical Systems AB, Stockholm, Sweden) and the occluded central ducts were transversed. An 8 F biliary drainage catheter (Flexima, Boston Scientific, Helsingborg, Sweden) was placed with the loop in the duodenum.
Thereafter, a peripheral right portal vein branch was accessed under ultrasound and flouroscopic guidance using a MAK NV set (Merit Medical Systems AB, Stockholm, Sweden). Subsequently a 5 F introducer (Terumo, Gothia medical, Billdal, Sweden), followed by a 5 F pigtail catheter (Omniflush, Cordis, Waterloo, Belgium) was inserted and portography was performed. The pigtail catheter was exchanged to a 4 F SIM 1 catheter (Cordis, Waterlooo, Belgium), placed with the tip in the portal vein of the right liver lobe. The segmental right portal vein branches were thereafter superselectively catheterized with a co-axial microcatheter (Progreat, Terumo Sweden AB, Vastra Frolunda, Sweden) and embolized with a mixture 1:10 of Histoacryl (Braun, Tuttligen, Germany) and Lipiodol (Guerbet, Roissy, France) in a mixture. Successful embolization was confirmed by portography, which showed patent left branches and occluded branches in the right liver lobe.
Results
Total liver volume (TLV) and FLR before and after PVE + PTBD, in addition to time from diagnosis to PVE + PTBD and time to operation is presented in Table 2. Time between the first radiological examination suggestive of the diagnosis and PVE + PTBD was 36 days (range, 30–50 days). The endoscopically placed plastic stents (patients 1–3) were left in place after PVE + PTBD. The time from PVE + PTBD and radiological evaluation of volumetric regeneration was 33 days (range, 19–44 days). Time between PVE + PTBD and operation was 35 days (range, 28–51 days). The increase in FLR% was 10% units (range, 6–12% units). No case of recanalization of the embolized portal veins was encountered, and the left portal vein system was patent in all cases after embolization.
Table 2.
Volumetric and time data.
| Patient no. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| TLV at diagnosis (mL) | 1623 | 2186 | 1885 | 2292 | 1723 | 2519 |
| FLR% at diagnosis (%) | 21 | 22 | 22 | 28 | 27 | 20 |
| Time from diagnosis to PVE + PTBD (days) | 37 | 30 | 42 | 31 | 34 | 50 |
| TLV after PVE + PTBD (mL) | 1726 | 2267 | 1913 | 2304 | 1845 | 2583 |
| FLR% after PVE + PTBD (%) | 33 | 28 | 30 | 39 | 39 | 27 |
| Time from PVE+PTBD to operation (days) | – | 28 | 46 | 51 | 35 | 33 |
FLR%, Future liver remnant volume ratio; PVE, portal vein embolization; TLV, total liver volume.
Although no deterioration in general health during the interventions occurred, one patient was eventually not explored after general health reassessment, which was in the end judged too poor to withstand a major hepatectomy (patient 1). Of the five patients undergoing surgical exploration three were not resected. One patient was found to require a combined Whipple’s procedure for tumor clearance which was considered unsafe (patient 2 with gallbladder cancer), one patient had tumor engagement of the left hepatic artery not apparent on preoperative CT scans (patient 3) and one patient had local peritoneal carcinomatosis at exploration (patient 6). The two resected patients had R0 resections.
Discussion
Provided that both PTBD and PVE are performed under general anesthesia, an advantage of a concept where biliary drainage of the FLR is combined with simultaneous PVE is consequently that one period of general anesthesia is avoided. A further advantage of a simultaneous approach is that the time to surgery is minimized. Although there is no proof that the time to final treatment affects resectability or survival (10), we believe that it is worth reducing this time not least from a human perspective. Guiu et al. (8) recently performed a feasibility report on this strategy including four patients. In our study, the time from PVE and PTBD to exploration was 35 days, which is comparable to the mean time of 27 days as reported by Guiu (8).
Patients were diagnosed at their regional hospitals, and in all cases except one, biliary interventions were performed at the regional hospitals before referral to our tertiary units. The patients were then discussed in a weekly multidisciplinary team meeting, where an individual treatment plan is proposed. In total, this resulted in a time from radiological diagnosis to intervention with PVE and PTBD of 36 days (range, 30–50 days), which is comparable to what has been reported from The Netherlands (10).
The FLR% increase after simultaneous PVE and PTBD was 10%-units. This is in exact agreement to the result of a recent meta-analysis on the hypertrophy response after PVE in patients with perihilar cholangiocarcinoma (11). Biliary drainage of the FLR before PVE is generally advocated to increase FLR hypertrophy although clear clinical evidence of the benefit is lacking (12).
To perform liver resection in jaundiced patients represents a risk factor for postoperative mortality (13). We have chosen the strategy of biliary decompression by PTBD of the FLR before resection of perihilar cholangiocarcinoma due to our experiences of difficulties of sufficiently draining the left biliary tree by endoscopic methods. Farges et al. (4) found preoperative biliary drainage to be significantly associated with reduced mortality after right-sided hepatectomy and suggested that jaundiced patients planned for right hepatectomy should undergo biliary drainage and await surgery until serum bilirubin level is less than 50 µmol/L. Not all agree on which method is preferable and ERBD is a less invasive technique than PTBD. However, it has been shown that morbidity rates are lower in PTBD than ERBD (14) and therapeutic success rate is higher with PTBD (15). Kloek et al. (14) compared the different methods and found the most frequent complication in ERBD to be cholangitis resulting in a higher number of procedures leading to delayed surgery. On the other hand, catheter tract implantation metastasis has been reported after PTBD (16).
It has been shown that preoperative cholangitis is an independent predictor of mortality (1). In our study, three out of six patients developed cholangitis after the combined procedure of PTBD and PVE, however responding promptly to antibiotics. This result differs from Guiu et al. (8) who reported no complications in their four patients. One difference between the studies is that two of our patients with cholangitis had received right-sided biliary drainage at their local hospital before admittance to our unit. To compare, Farges et al. (4) reported a 25% biliary drainage-related rate of cholangitis, and a 33% overall biliary drainage-related morbidity in a study not reporting the number of patients subjected to PVE. It is conceivable that the approach of simultaneous PVE and PTBD will not have a lower morbidity rate than biliary drainage alone. The safety of the combined procedure with respect to complications needs to be investigated in further studies.
In our study only two out of six patients were eventually resected. Three patients were found unresectable at exploration due to peritoneal carcinomatosis, tumor engagement of the left hepatic artery, and extensive tumor disease requiring a hepatopancreatoduodenectomy. Hepatopancreatoduodenectomy is technically demanding and although the procedure can be performed with low mortality, it is still associated with a high rate of morbidity (17). One can speculate that staging laparoscopy should be used in order to prevent unnecessary laparotomies. Staging laparoscopy after modern radiological workup has been shown to prevent unnecessary laparotomies in 14–45% of patients (18,19). In our centers, we do not routinely perform staging laparoscopies.
The greatest limitation of the study is the small number of patients included due to the rarity of the disease of perihilar cholangiocarcinoma.
In conclusion, based on six patients that underwent PTBD of the FLR combined with simultaneous PVE, this approach seems feasible. This strategy results in a short time to surgical exploration. The increase in FLR volume appears to be comparable to the traditional sequential method; however three out of six patients developed cholangitis. Further studies are required to make definite conclusions.
Conflict of interest
None declared.
References
- 1.Nagino M, Ebata T, Yokoyama Y, et al. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg 2013; 258: 129–140. [DOI] [PubMed] [Google Scholar]
- 2.Grandadam S, Compagnon P, Arnaud A, et al. Role of preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma type III. Ann Surg Oncol 2010; 17: 3155–3161. [DOI] [PubMed] [Google Scholar]
- 3.Belghiti J, Ogata S. Preoperative optimization of the liver for resection in patients with hilar cholangiocarcinoma. HPB (Oxford) 2005; 7: 252–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farges O, Regimbeau JM, Fuks D, et al. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg 2013; 100: 274–283. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy TJ, Yopp A, Qin Y, et al. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford) 2009; 11: 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong YK, Choi SB, Lee KH, et al. The efficacy of portal vein embolization prior to right extended hemihepatectomy for hilar cholangiocellular carcinoma: a retrospective cohort study. Eur J Surg Oncol 2011; 37: 237–244. [DOI] [PubMed] [Google Scholar]
- 7.Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg 2006; 243: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guiu B, Bize P, Demartines N, et al. Simultaneous biliary drainage and portal vein embolization before extended hepatectomy for hilar cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol 2014; 37: 698–704. [DOI] [PubMed] [Google Scholar]
- 9.Sturesson C, Keussen I, Tranberg KG. Prolonged chemotherapy impairs liver regeneration after portal vein occlusion - an audit of 26 patients. Eur J Surg Oncol 2010; 36: 358–364. [DOI] [PubMed] [Google Scholar]
- 10.Ruys AT, Heuts SG, Rauws EA, et al. Delay in surgical treatment of patients with hilar cholangiocarcinoma: does time impact outcomes? HPB (Oxford) 2014; 16: 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higuchi R, Yamamoto M. Indications for portal vein embolization in perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci 2014; 21: 542–549. [DOI] [PubMed] [Google Scholar]
- 12.van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013; 36: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000; 191: 38–46. [DOI] [PubMed] [Google Scholar]
- 14.Kloek JJ, van der Gaag NA, Aziz Y, et al. Endoscopic and percutaneous preoperative biliary drainage in patients with suspected hilar cholangiocarcinoma. J Gastrointest Surg 2010; 14: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walter T, Ho CS, Horgan AM, et al. Endoscopic or percutaneous biliary drainage for Klatskin tumors? J Vasc Interv Radiol 2013; 24: 113–121. [DOI] [PubMed] [Google Scholar]
- 16.Sakata J, Shirai Y, Wakai T, et al. Catheter tract implantation metastases associated with percutaneous biliary drainage for extrahepatic cholangiocarcinoma. World J Gastroenterol 2005; 11: 7024–7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebata T, Yokoyama Y, Igami T, et al. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg 2012; 256: 297–305. [DOI] [PubMed] [Google Scholar]
- 18.Barlow AD, Garcea G, Berry DP, et al. Staging laparoscopy for hilar cholangiocarcinoma in 100 patients. Langenbecks Arch Surg 2013; 398: 983–988. [DOI] [PubMed] [Google Scholar]
- 19.Ruys AT, Busch OR, Gouma DJ, et al. Staging laparoscopy for hilar cholangiocarcinoma: is it still worthwhile? Ann Surg Oncol 2011; 18: 2647–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]