Abstract
Neem (Azadirachta indica A. Juss) oil (NO) was assayed against forty-eight isolates of Escherichia coli by standardised disc diffusion test and microdilution test. By molecular biology characterization, fourteen isolates resulted in diarrheagenic E. coli with sixteen primer pairs that specifically amplify unique sequences of virulence genes and of 16S rRNA. The NO showed biological activity against all isolates. The bacterial growth inhibition zone by disc diffusion method (100 µL NO) ranged between 9.50 ± 0.70 and 30.00 ± 1.00 mm. The antibacterial activity was furthermore determined at lower NO concentrations (1 : 10–1 : 10,000). The percent of growth reduction ranged between 23.71 ± 1.00 and 99.70 ± 1.53. The highest bacterial growth reduction was 1 : 10 NO concentration with 50 µL of bacterial suspension (ca. 1 × 106 CFU/mL). There is significant difference between the antibacterial activities against pathogenic and nonpathogenic E. coli, as well as NO and ciprofloxacin activities. Viable cells after the different NO concentration treatments were checked by molecular biology assay using PMA dye. On the basis of the obtained results, NO counteracts E. coli and also influences the virulence of E. coli viable cells after NO treatment. The NO metabolomic composition was obtained using fingerprint HPTLC.
1. Introduction
Zoonotic food- and waterborne pathogens began resistant to antibiotics. It is now evident that antimicrobial resistance is an environmental problem. Detectable antibiotic residues are present in waste water from water treatment plants [1], and antibiotic-resistant bacteria can be isolated from ground water and soil [2, 3]. The cause of contamination may be inter alia the consequence of farming practices. Use of antibiotics, as growth promoters or for prophylaxis in farm animals, selects resistant strains of enterobacteria in gastrointestinal tract. These resistant strains have been also isolated from food and consequently this represents the main way to spread in the human gastrointestinal tract [4, 5]. The increasing incidence of foodborne diseases, coupled with the resultant social and economic implications, causes a constant striving to produce safer feed and food, as to develop new natural antimicrobial agents [6–8].
Meat contamination by pathogen bacteria may have great health consequence and high impact on consumers. The most known cases are related to HUS, hemolytic uremic syndrome, that was first recognized in 1982 in USA and Canada, with outbreaks associated with fast food restaurants. People experienced gastroenteritis with bloody diarrhoea, caused by the lining of their microbiota. In 1993, a multistate outbreak generated international interest in this disease, popularized by the name “hamburger disease.” Hamburger disease is based on association with the consumption of ground beef patties containing a pathogen Escherichia coli. This should not be confused with the related benign E. coli that is in the gut of every mammal. Many strains of E. coli are part of the nonpathogenic facultative flora of intestinal tract of humans and other mammals. However, some of them induce diseases of the gastrointestinal and urinary tracts or may affect the central nervous system [9].
On the basis of their pathogenetic mechanism, diarrheagenic E. coli strains include ETEC (enterotoxigenic E. coli), EIEC (enteroinvasive E. coli), EHEC (enterohemorrhagic E. coli), EPEC (enteropathogenic E. coli), EAEC (enteroaggregative E. coli), and DAEC (diffusely adherent E. coli). All of them cause serious economic losses in farm animal herds and are widespread in newborns [10] in developed and developing countries. There is a wide range of transmission possibilities of these pathogens, including direct contact, food, drinks, environment, and others [11]. Epidemiology and clinical symptoms of the disease are similar in various animal species but the majority of strains are species-specific. They differ particularly in the type of the expressed surface “adherence” antigen (adhesin or pilus). These microorganisms produce two main types of virulence factors, that is, adhesins and enterotoxins.
In this work, a collection of E. coli isolates was considered. They were different in geographical origin and source of isolation and showed different pathogenetic characteristics.
Consumers look for meat products of upgraded sensory quality and increased functional and nutritional properties, as well as guaranteed safety but yet less processing, and fewer additives or “technological” interventions. Plant derived extracts, or phytocomplex, as effective antimicrobial agents, offer an alternative to synthetic food additives.
Neem (Azadirachta indica A. Juss) is considered one of the most promising trees of the 21st century, for its great potential in pest management, environment protection, and medicine [12]. Neem oil (NO) is the most important derived product with a great market worldwide. It contains about one hundred biologically active compounds. The most famous constituents are several nortriterpenes, named limonoids, that is, azadirachtin, nimbin, nimbidin, and nimbolide, besides the predominant oily constituents. NO is the most commercially relevant product obtained from the seeds. The neem cake is remaining after the extraction process.
In our previous studies, the antibacterial activity of NO against E. coli was investigated [13, 14]. The antibacterial activity resulted highest in comparison to the neem cake extract against meat spoilage microorganisms. The aim of the present work was to evaluate NO capability to cope with plastic genome of E. coli.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
Forty-eight strains of E. coli were considered. Among them, seventeen (FLC isolates) were from microorganism's collection of the Fodder and Dairy Productions Research Centre of Lodi (CRA FLC) of CRA. They were isolated from milk and cheese. All strains were typed both phenotypically and genotypically. Phenotyping was made by the PhenePlate system for E. coli (PhP-EC, PhPPlate Microplate Techniques AB, Stockholm, Sweden) and genetic characterization by RAPD PCR technique [15].
Seventeen CVVI isolates were from microorganism's collection of the Institute of Veterinary Research and Development of Central Vietnam, Vietnam. These microorganisms were isolated from faeces of calves affected by diarrhoea.
Ten NL isolates were from microorganism's collection of the Department of Bacteriology of Wageningen UR Livestock Research, Wageningen University & Research Centre, Netherlands. They were isolated from faeces of piglets and calves. They are antigenically different and detectable using specific monoclonal antibodies towards different fimbria antigens by in vitro agglutination test [16]. Four reference strains were also considered (DSMZ and ATCC isolates). They were from international culture collections.
The cultivation/assay medium for E. coli was Minca + 1% Iso Vitalex Agar/Broth (Sifin, Berlin, Germany). Bacterial cultures for antibacterial testing were prepared by picking colony from 24-hour-old plates and suspending them in the broth medium (5 mL). Cultures were grown aerobically for 18 h at 37°C and 100 rpm. For antibacterial activity assay, 1 mL of each culture was diluted to 105–106 CFU/mL. The reference strains were grown on media and at the growth conditions as reported on products sheets.
2.2. Plant Extract
A commercial neem oil produced by Neem Italia (Manerba (BS), Italy) was used as test starting material (0.35% azadirachtin A). Total composition of the neem oil was checked by high performance thin layer chromatography [17].
Neem oil was diluted in Tween 80 (1 : 1 V/V; VWR, PBI International, MI, Italy) under agitation and sterilised by filtration through a 0.22 μm Millipore express filter (Millex-GP, Bedford, OH, USA) before use in the experiment.
2.3. HPTLC Assay
2.3.1. HPTLC System and Materials
The HPTLC system (CAMAG, Muttenz, Switzerland) consisted of (i) Linomat 5 sample applicator using 100 μL syringes, connected to a nitrogen tank; (ii) ADC 2 chamber containing twin trough chamber 20 × 10 cm; (iii) immersion device III; (iv) TLC Plate Heater III; (v) TLC visualizer; (vi) TLC scanner 3 linked to winCATS software.
Solvents for extraction and HPLC grade solvents were purchased from Sigma-Aldrich and Carlo Erba (Milan, Italy). Glass plates 20 cm × 10 cm with glass-backed layers silica gel 60 (2 μm thickness) were from Merck (Darmstadt, Germany). Before use, plates were prewashed with methanol and dried for 3 min at 100°C. Standards used in the HPTLC analysis were isolated from neem cake (i.e., salannin, azadirachtin A, and unsaturated and saturated lipids) in previous research [18] and data concerning isolation and identification are not reported, but they are available per request. Limonoids standards concentration was 2 mM.
2.4. Sample Application
Filtered solutions were applied with nitrogen flow. Operating conditions were syringe delivery speed, 10 s μL−1 (100 nL s−1); injection volume, 2 μL; band width, 6 mm; distance from bottom, 15 mm.
2.5. Development
The HPTLC plates were developed in toluene : AcOEt 7 : 3 (v/v) as mobile phase (Figure 1), in the automatic and reproducibly developing chamber ADC 2, saturated with the same mobile phase for 20 min at room temperature. The developing solvents (i.e., type of solvents and ratios) were carefully optimized before the analyses. The length of the chromatogram run was 80 mm from the point of application. The developed layers were allowed to dry in air for 5 min, derivatized with a selected solution, including p-anisaldheyde (1.5 mL p-anisaldheyde, 2.5 mL H2SO4, and 1 mL AcOH in 37 mL EtOH), dried in the open air, and then dipped into Macrogol reagent (1 g polyethylene glycol 400 in 20 mL of dichloromethane). Finally, the plates were warmed for 5 min at 120°C before inspection. All treated plates were inspected by a CAMAG TLC visualizer under a UV light at 254 or 366 nm or under reflectance and transmission white light (WRT), respectively, before and after derivatization.
Figure 1.
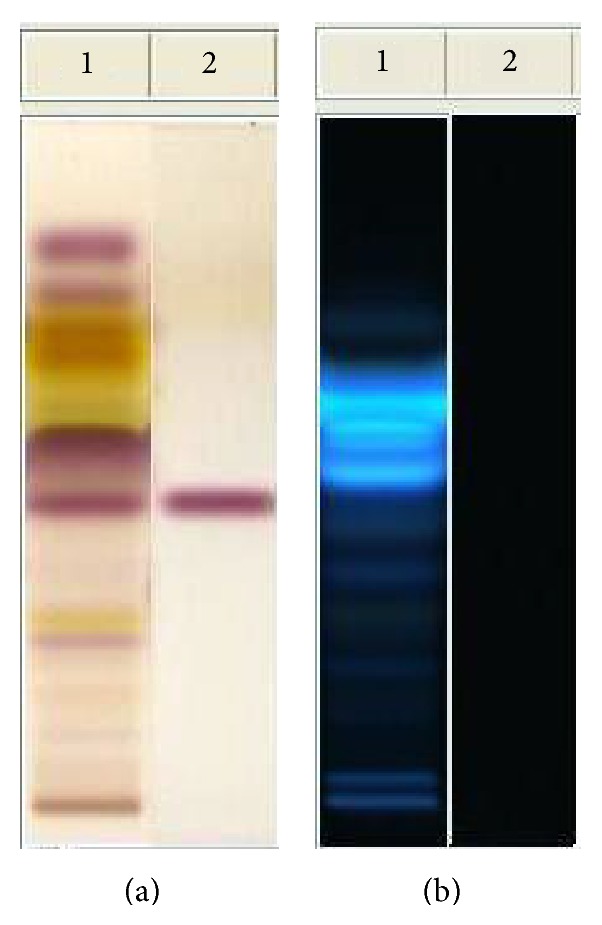
HPTLC analysis of neem oil EtOAc extract. Mobile phase: toluene : AcOEt 7 : 3 (v/v). Visualization: plate (a) (on the left) white light upper and lower; plate (b) (on the right) UV lamp at 366 nm. Derivatization: p-anisaldheyde. Track 1: neem oil; track 2: salannin.
2.6. Molecular Biology Characterization of the E. coli Isolates
Two primer pairs that amplify specific E. coli 16S rRNA sequences and fourteen primer pairs that specifically amplify target gene coding for virulence factors (adhesins and toxins) were employed to characterize the E. coli isolates considered in this study (Table 1). The PCR reaction mixtures and conditions are those as reported in the literature (Table 1).
Table 1.
Primer pairs used to specifically amplify target gene coding for virulence factors (1–9 = toxins; 9–15 fimbriae) of E. coli and 16S rRNA (16-17).
| Target gene coding for virulence factors | Oligonucleotide sequences of primers | Reference |
|---|---|---|
| (1) LT | F 5′-ATT TAC GGC GTT ACT ATC CTC-3′ R 5′-TTT TGG TCT CGG TCA GAT ATG-3′ |
[25] |
|
| ||
| (2) Sta | F 5′-TCC GTG AAA CAA CAT GAC GG-3′ R 5′-ATA ACA TCC AGC ACA GGC AG-3′ |
[26] |
|
| ||
| (3) STb | F 5′-GCC TAT GCA TCT ACA CAA TC-3′ R 5′-TGA GAA ATG GAC AAT GTC CG-3′ |
[26] |
|
| ||
| (4) Stx1all | F 5′-CGC TGA ATG TCA TTC GCT CTG C-3′ R 5′-CGT GGT ATA GCT ACT GTC ACC-3′ |
[27] |
|
| ||
| (5) Stx2all | F 5′-CTT CGG TAT CCT ATT CCC GG-3′ R 5′-CTG CTG TGA CAG TGA CAA AAC GC-3′ |
[27] |
|
| ||
| (6) Stx2e | F 5-ATG AAG AAG ATG TTT ATA GCG-3′ R 5′-TCA GTT AAA CTT CAC CTG GGC-3′ |
[25] |
|
| ||
| (7) EAST1 | F 5′-CCA TCA ACA CAG TAT ATC CGA-3′ R 5′-GGT CGC GAG TGA CGG CTT TGT-3′ |
[28] |
|
| ||
| (8) eae | F 5′-GGA ACG GCA GAG GTT AAT CTGCAG-3′ R 5′-GGC GCT CAT CAT AGT CTTTC-3′ |
[27] |
|
| ||
| (9) hlyA | F 5′-AGCTGCAAGTGCGGGTCTG-3′ R 5′-TACGGGTTATGCCTGCAAGTTCAC-3′ |
[29] |
|
| ||
| (10) F4 (K88) | F 5′-GCT GCA TCT GCT GCA TCT GGTATG G-3′ R 5′-CCA CTG AGT GCT GGTAGT TAC AGC C-3′ |
[30] |
|
| ||
| (11) F5 (K99) | F 5′-TGC GAC TAC CAA TGC TTC TG-3′ R 5′-TAT CCA CCA TTA GAC GGA GC-3′ |
[26] |
|
| ||
| (12) F6 (P987) | F 5′-TCT GCT CTT AAA GCT ACT GG-3′ R 5′-AAC TCC ACC GTT TGT ATC AG-3′ |
[25] |
|
| ||
| (13) F17 | F 5′-GGG CTG ACA GAG GAG GTG GGGC-3′ R 5′-CCC GGC GAC AAC TTC ATCACC GG-3′ |
[30] |
|
| ||
| (14) F18 | F 5′-GTG AAA AGA CTA GTG TTT ATT TC-3′ R 5′-CTT GTA AGT AAC CGC GTA AGC-3′ |
[31] |
|
| ||
| (15) F41 | F 5′-GAG GGA CTT TCA TCT TTT AG-3′ R 5′-AGT CCA TTC CAT TTA TAG GC-3′ |
[26] |
|
| ||
| (16) E16SI | F 5′-CCCCCTGGACGAAGACTCAC-3′ R 5′-ACCGCTGGCAACAAAGGATA -3′ |
[29] |
|
| ||
| (17) E16SII | F 5′-AGAGTTTGATGGCTCAG-3′ R 5′-GGACTACCAGGGTATCTAAT-3′ |
[31] |
The amplification products' sizes, coordinates, and accession numbers of each primer pair are shown in Table 2. Amplified products (7 μL) were analyzed by electrophoresis in 2% or 3% agarose gels buffered in 0.5x TBE (TBE buffer: 90 mM tris(hydroxymethyl)aminomethane, 90 mM boric acid, and 3 mM ethylenediaminetetraacetate Na salt, pH 8.3, Sigma-Aldrich, Milano, Italy) against a 50 bp, 100 bp, and 1 Kb ladder used as size marker (Invitrogen, Milano, Italia) and visualized by UV light at 260 nm (Fotodine 3-3102 Celbio, Milano, Italy) after staining with ethidium bromide (3,8-diamino-5-ethyl-6-phenylphenanthridinium bromide, EtBr, Sigma-Aldrich, Milano, Italy).
Table 2.
List of primer pairs' amplification products, coordinates, and accession numbers.
| Target gene coding for virulence factors | Amplicon (bp) | Primer coordinates | Accession number |
|---|---|---|---|
| (1) LT | 281 | 27–47, 287–307 | S60731 |
| (2) STa | 244 | 267–286, 492–510 | M58746 |
| (3) STb | 279 | 515–534, 773–793 | AY028790 |
| (4) Stx1all | 302 | 113–134, 394–414 | M17358 |
| (5) Stx2all | 516 | 50–69, 543–565 | M59432 |
| (6) Stx2e | 264 | 1176–1196, 1419–1439 | M36727 |
| (7) EAST1 | 111 | 2–24, 94–114 | S81691 |
| (8) eae | 775 | 1441–1460, 2193–2215 | AF022236 |
| (9) hylA | 569 | 867-885, 1435–1412 | X79839 |
| (10) F4 (K88) | 792 | 31–54, 798–822 | M29374 |
| (11) F5 (K99) | 450 | 45–64, 475–494 | M35282 |
| (12) F6 (P987) | 333 | 193–212, 506–525 | M35257 |
| (13) F17 | 411 | 289–310, 677–699 | AF055313 |
| (14) F18 | 510 | 1–23, 490–510 | M61713 |
| (15) F41 | 431 | 154–173, 565–584 | X14354 |
| (16) E16SI | 401 | 1628-170, 2063–2082 | AB035924 |
| (17) E16SII | 798 | 8-27, 798–805 | J01859 |
2.7. Assessment of Antibacterial Activity
The antibacterial activity of the NO was assayed using standardized disc diffusion agar and microdilution methods. Disc diffusion method was carried out according to the standard method by Bauer et al. [19]. Bacteria cultures adjusted to 0.5 McFarland standard were used to lawn Muller Hinton agar plates evenly using a sterile swab. The agar plates were dried for 15 minutes. The discs impregnated with NO (100 μL) were placed on the agar surface. Each test plate comprises three discs. The discs were placed equidistant to each other. Muller Hinton agar plates were set also up with positive control, which is the antibiotic ciprofloxacin (CFX) (100 μL wt/v) (hydrochloride monohydrate 1 mg/mL, Bayer, Milano, Italy) and Tween 80 (TWN) (VWR International PBI Srl, Milano, Italy, 1 mg/mL) as negative control. The plates were then incubated at 37°C for 18 h. After the incubation, the plates and those considered as controls were examined for inhibition zone. The inhibition zones were then measured using calipers and were recorded. The plates were done in triplicate for each bacterial isolate and the experiment was performed twice. The results were recorded as mean ± S.D. of the duplicate experiment. Differences between means of data were compared by LSD calculated using the SAS.
The antibacterial activity of NO was also evaluated using microdilution method in conventional sterile polystyrene microplates (Corning, Euroclone SpA, Milan, Italy). Each well of the microplate was filled with 100 μL of sterile suitable liquid media for each bacterial isolate considered, 50 μL of inoculums and amounts of extract at lower concentrations (1 : 10–1 : 10,000) were added. Control treatment without NO was used in the experiment. The microplates were incubated at 37°C for 24 h. Bacterial growth was determined by OD reading at 630 nm/10 mm pathlength with an ELISA microplate reader (Dynatech ML-3000, Pina de Ebro, Spain). Bacterial cell concentration was transformed to cells/mL using the reference curve equation.
The reference curve was constructed by diluting at 1 : 100 each bacterial isolate. Counting the number of bacterial cells of an aliquot of this dilution was done using a Neubauer chamber (Celeromics, Vedano al Lambro, MI, Italy). Finally, cell concentrations were transformed to a percentage of bacterial inhibition. The percentage of bacterial growth reduction (GR%) was estimated using as reference the control treatment (T = without extract) as
| (1) |
Three replicates were considered. The results were recorded as mean ± S.D. of the duplicate experiment. Differences between means of data were compared by least significant difference (LSD) calculated using the SAS.
3. Results and Discussion
3.1. Molecular Biology Characterization of the E. coli Isolates
The molecular biology characterization of the forty-eight E. coli isolates showed that fourteen isolates were diarrheagenic E. coli. They were ten E. coli isolated from feces of calves and piglets and four from calves collected, respectively, in Netherlands and Central Vietnam. Their virulence characteristics are reported in Table 3.
Table 3.
Molecular characterisation of enteropathogenic E. coli and reference strains considered in this study.
| E. coli isolate collection's designation | Surface antigen | Toxins | Fimbriae |
|---|---|---|---|
| (1) CVVI K10B | nd | STb, LT, EAST1 | F4 |
| (2) CVVI KH10 | nd | STa, STb | F18 |
| (3) NLK99 | O8K85K99 | nr1 | F5 |
| (4) NLP987 | O64 : K; 9877 | STa+ | F6 |
| (5) CVVI E12b | nd | STa | F5, F41 |
| (6) CVVI E10 | nd | STa | F5, F41 |
| (7) NLK99-1 | O8 : K25 : K99 | nr | F5 |
| (8) NLK99-3 | O101 : K28 : K99 | nr | F5 |
| (9) NLK99-5 | O9 : K30 : K99 | nr | F5 |
| (10) NLK99-7 | O101 : K32 : K99 | nr | F5 |
| (11) NLK99-9 | O9 : K35 : K99 | nr | F5 |
| (12) NLK99-11 | O9 : K37 : K99 | nr | F5 |
| (13) NLK99-15 | O20 : K? : K99 | nr | F5 |
| (14) NLK99-19 | O101 : K? : K99 | nr | F5 |
| (15) DSMZ8696 | O55 : H6 | nr | Nr |
| (16) DSMZ9025 | — | — | — |
| (17) DSMZ10973 | O6 | nr | nr |
| (18) ATCC33559 | — | — | — |
CVVI: Central Vietnam Veterinary Institute; NL: Department of Bacteriology and Animal Science, University of Wageningen, Netherlands; DSMZ: Leibniz-Institut DSMZ—Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH; ATCC: American Type Culture Collection.
3.2. HPTLC Assay
The NO metabolomic fingerprint shows characteristic sequence of metabolites according to the polarity of constituents. The identification of the raw material was assured by the presence of salannin (Rf = 0.42), which is a typical maker of neem. In comparison with the spot of azadirachtin (Rf = 0.23), salannin appears as the main limonoid spot. Spots concerning lipids are present at Rf values at ca. 0.80, due to unsaturated fatty acids and fatty alcohols, and at Rf ca. 0.50, due to saturated and unsaturated triglycerides. The most interesting feature of the plate concerns the presence of compounds with high fluorescent reaction at between Rf 0.55 and 0.66, which are perfectly visible at 366 nm after derivatization with p-anisaldheyde. These spots can be attributed to compounds with high conjugated unsaturation in polycyclic aromatic structures, very different from those of the nortriterpenes limonoids, so far considered responsible for the activity. Therefore, more studies are necessary to decide about the importance of antibacterial activity of these substances in the phytocomplex.
3.3. NO Antibacterial Activity
The results obtained show that NO has a broad spectrum of antibacterial activities against the tested E. coli isolates. As shown in Table 4, the antibacterial activity was evaluated based on the diameters of clear inhibition zone surrounding the paper discs soaked with 100 μL of neem oil. The NO average GIZ mm range from 9.50 ± 0.70 to 30.00 ± 1.00. The NO GIZ varies between enteropathogenic and nonenteropathogenic E. coli being, respectively, 24.33 ± 0.58–30.00 ± 1.00 and 9.50 ± 0.70–21.53 ± 1.53. It is significantly (P < 0.05) different with respect to the antibiotic activity. However, the E. coli isolate FLC1167 (from milk) resulted to be less susceptible and the E. coli isolate NLP097/F5 (from piglet feces) the most susceptible to NO treatment (100 μL) among all tested bacteria using the disc diffusion method.
Table 4.
Antibacterial activity of neem oil (NO) against forty-eight Escherichia coli isolates revealed as growth inhibition zone (mm).
| E. coli isolates | Growth inhibition zone (mm)* | |||
|---|---|---|---|---|
| NSO (100 µL ) | TWN (100 µL) | WTR (100 µL) | CFX (100 µL) | |
| (1) FLC 1056 | 11.33 ± 0.58 b | — | — | 30.41 ± 0.20 a |
| (2) FLC 1247 | 16.13 ± 1.15 b | — | — | 30.52 ± 1.07 a |
| (3) FLC 1059 | 15.83 ± 1.13 b | — | — | 29.62 ± 1.00 a |
| (4) FLC 1243 | 19.00 ± 1.00 b | — | — | 31.53 ± 0.67 a |
| (5) FLC 1048 | 12.33 ± 0.58 b | — | — | 29.42 ± 0.58 a |
| (6) FLC 1167 | 9.50 ± 0.70 b | — | — | 30.61 ± 1.21 a |
| (7) FLC 1249 | 13.33 ± 0.58 b | — | — | 29.61 ± 1.11 a |
| (8) FLC 1055 | 14.53 ± 1.25 b | — | — | 31.75 ± 0.82 a |
| (9) FLC 1054 | 16.23 ± 1.18 b | — | — | 31.41 ± 0.76 a |
| (10) FLC 1085 | 18.00 ± 1.00 b | — | — | 30.53 ± 1.17 a |
| (11) FLC 1244 | 15.33 ± 0.48 b | — | — | 28.86 ± 1.00 a |
| (12) FLC 1165 | 19.50 ± 0.70 b | — | — | 31.33 ± 0.67 a |
| (13) FLC 1086 | 11.33 ± 0.58 b | — | — | 29.82 ± 0.48 a |
| (14) FLC 1053 | 14.53 ± 1.15 b | — | — | 32.65 ± 1.39 a |
| (15) FLC 1095 | 16.83 ± 1.18 b | — | — | 29.05 ± 1.22 a |
| (16) FLC 1219 | 10.70 ± 1.00 b | — | — | 32.75 ± 0.55 a |
| (17) FLC 1235 | 13.23 ± 0.88 b | — | — | 30.15 ± 0.55 a |
| (18) DSM8696 | 13.50 ± 0.50 b | — | — | 26.21 ± 1.00 a |
| (19) DSM9025 | 13.33 ± 0.58 b | — | — | 21.64 ± 0.94 a |
| (20) DSM10973 | 13.53 ± 1.25 b | — | — | 29.14 ± 1.75 a |
| (21) ATCC33559 | 13.83 ± 1.18 b | — | — | 32.12 ± 1.09 a |
| (22) CVVI E210 | 13.00 ± 1.00 a | — | — | 25.83 ± 1.65 a |
| (23) CVVI E173 | 12.23 ± 0.58 b | — | — | 32.35 ± 1.49 a |
| (24) CVVI E12b | 27.50 ± 0.50 b | — | — | 11.25 ± 0.68 a |
| (25) CVVI E16 | 14.33 ± 0.88 b | — | — | 27.54 ± 1.45 a |
| (26) CVVI E320 | 21.53 ± 1.35 b | — | — | 28.75 ± 1.86 a |
| (27) CVVI E130 | 11.83 ± 1.78 b | — | — | 29.64 ± 0.87 a |
| (28) CVVI E48 | 10.00 ± 1.40 a | — | — | 29.31 ± 0.27 a |
| (29) CVVI KH10 | 26.33 ± 0.53 b | — | — | 15.34 ± 0.66 a |
| (30) CVVI K10B | 27.50 ± 0.56 b | — | — | 0 |
| (31) CVVI E298 | 11.33 ± 0.48 b | — | — | 23.90 ± 1.69 a |
| (32) CVVI E273 | 13.53 ± 1.75 b | — | — | 29.59 ± 1.77 b |
| (33) CVVI K436 | 13.14 ± 1.68 b | — | — | 30.21 ± 1.38 a |
| (34) CVVI E98 | 11.00 ± 1.00 a | — | — | 26.91 ± 1.56 a |
| (35) CVVI E77 | 14.33 ± 0.58 b | — | — | 23.93 ± 0.59 a |
| (36) CVVI E148 | 13.50 ± 0.50 b | — | — | 28.71 ± 0.87 a |
| (37) CVVI E10 | 24.33 ± 0.58 b | — | — | 10.35 ± 1.11 a |
| (38) CVVI E215 | 16.53 ± 1.15 b | — | — | 26.41 ± 1.40 a |
| (39) NLK99/F5 | 29.83 ± 1.18 b | — | — | 16, 21 ± 0.89 a |
| (40) NLP987/F5 | 30.00 ± 1.00 a | — | — | 13.21 ± 1.15 a |
| (41) NLK99-1* | 21.73 ± 1.35 b | — | — | 15.34 ± 1.37 a |
| (42) NLK99-3* | 28.33 ± 1.50 b | — | — | 0 |
| (43) NLK99-5* | 25.00 ± 1.10 a | — | — | 9.54 ± 1.11 a |
| (44) NLK99-7* | 26.33 ± 0.58 b | — | — | 14,25 ± 1.11 a |
| (45) NLK99-9* | 27.50 ± 0.50 b | — | — | 11.26 ± 1.78 a |
| (46) NLK99-11* | 29.53 ± 1.25 b | — | — | 16.24 ± 1.68 a |
| (47) NLK99-15* | 28.83 ± 1.38 b | — | — | 16.35 ± 1.11 a |
| (48) NLK99-19* | 25.00 ± 1.70 a | — | — | 15.53 ± 0.84 a |
Three paper discs per plate and three plates for each bacterium were considered. The experiment was repeated twice. Values are given as mean ± S.D. Values in a row followed by different lowercased letters are significantly different at P ≤ 0.05.
The CFX GIZ range is 0.00 ± 0.00–32.65 ± 75. The enteropathogenic E. coli resulted to be resistant or less susceptible to CFX than the nonenteropathogenic E. coli, showing a GIZ range of, respectively, 0.00 ± 0.00–18.24 ± 1.68 mm and 21.64 ± 0.94–32.65 ± 75 mm. The isolates CVVIK10B (from calve feces) and NLK99-3* (from calve feces) both revealed resistance to CFX. No GIZ was detected in plates treated with negative controls (TWN and WTR).
As shown in Table 5, the percent bacterial GR revealed at 100 μL, 10 μL, 1 μL, and 0.1 μL NO concentrations was in the range 23.71 ± 1.00–99.70 ± 1.53; 21.61 ± 0.56–91.63 ± 0.08; 17.58 ± 1.33–69.57 ± 0.00; and 11.18 ± 0.89–67.58 ± 0.89.
Table 5.
Bacterial growth reduction (%) at 24 h in liquid medium with different concentrations of NSO, using as reference the control treatment (without NSO).
| E. coli isolates | Percent growth reduction zone (%) | |||
|---|---|---|---|---|
| NSO (100 µL) | NSO (10 µL) | NSO (1 µL) | NSO (0.1 µL) | |
| (1) FLC 1056 | 39.25 ± 1.43 c | 35.61 ± 1.00 b | 21.67 ± 1.33 a | 11.31 ± 2.08 a |
| (2) FLC 1247 | 25.51 ± 1.15 c | 24.70 ± 1.00 b | 21.88 ± 1.33 a | 11.86 ± 1.00 a |
| (3) FLC 1059 | 31.51 ± 1.15 c | 25.70 ± 1.00 b | 24.58 ± 1.33 a | 14.86 ± 1.00 a |
| (4) FLC 1243 | 38.90 ± 1.00 d | 28.79 ± 1.00 c | 29.40 ± 0.00 b | 16.68 ± 1.20 a |
| (5) FLC 1048 | 25.60 ± 1.53 d | 23.73 ± 2.08 b | 23.69 ± 2.00 b | 12.83 ± 1.73 a |
| (6) FLC 1167 | 23.71 ± 1.00 b | 21.61 ± 0.58 a | 21.27 ± 0.00 a | 11.18 ± 0.89 a |
| (7) FLC 1249 | 29.65 ± 1.53 b | 28.61 ± 1.00 a | 28.77 ± 1.33 a | 18.51 ± 2.08 a |
| (8) FLC 1055 | 34.51 ± 1.15 c | 29.70 ± 1.00 c | 24.38 ± 1.33 a | 14.86 ± 1.00 a |
| (9) FLC 1054 | 31.51 ± 1.15 d | 29.70 ± 1.00 c | 25. 78 ± 1.33 a | 14.86 ± 1.00 a |
| (10) FLC 1085 | 39.90 ± 1.00 c | 28.79 ± 1.00 b | 28.10 ± 0.00 b | 15.58 ± 1.20 a |
| (11) FLC 1244 | 35.70 ± 1.53 d | 28.73 ± 2.08 b | 28.59 ± 2.00 b | 12.63 ± 1.73 a |
| (12) FLC 1165 | 39.71 ± 1.00 c | 29.61 ± 0.58 c | 29.17 ± 0.00 b | 16.48 ± 0.89 a |
| (13) FLC 1086 | 39.55 ± 1.53 c | 28.61 ± 1.00 c | 27.87 ± 1.33 b | 12.21 ± 2.08 a |
| (14) FLC 1053 | 34.51 ± 1.15 c | 89.70 ± 1.00 c | 27.58 ± 1.33 b | 14.86 ± 1.00 a |
| (15) FLC 1095 | 31.51 ± 1.15 c | 29.70 ± 1.00 b | 25.18 ± 1.33 a | 14.86 ± 1.00 a |
| (16) FLC 1219 | 38.95 ± 1.00 b | 28.79 ± 1.00 b | 28.10 ± 0.00 b | 16.68 ± 1.20 a |
| (17) FLC 1235 | 36.70 ± 1.53 d | 27.73 ± 2.08 c | 27.79 ± 2.00 b | 12.83 ± 1.73 a |
| (18) DSM8696 | 39.71 ± 1.00 c | 29.61 ± 0.58 c | 29.67 ± 0.00 b | 17.58 ± 0.89 a |
| (19) DSM9025 | 39.65 ± 1.53 c | 28.61 ± 1.00 c | 26.17 ± 1.33 b | 18.10 ± 2.08 a |
| (20) DSM10973 | 34.51 ± 1.15 c | 29.70 ± 1.00 c | 23.28 ± 1.33 b | 14.86 ± 1.00 a |
| (21) ATCC33559 | 31.51 ± 1.15 d | 29.70 ± 1.00 c | 21.58 ± 1.33 b | 14.86 ± 1.00 a |
| (22) CVVI E210 | 88.90 ± 1.00 c | 88.79 ± 1.00 c | 69.20 ± 0.00 b | 14.78 ± 1.20 a |
| (23) CVVI E173 | 36.50 ± 1.53 d | 27.73 ± 2.08 c | 27.99 ± 2.00 b | 22.83 ± 1.73 a |
| (24) CVVI E126 | 89.81 ± 1.00 c | 89.61 ± 0.58 c | 69.37 ± 0.00 b | 44.58 ± 0.89 a |
| (25) CVVI E16 | 38.55 ± 1.53 c | 28.61 ± 1.00 c | 27.57 ± 1.33 b | 11.51 ± 2.08 a |
| (26) CVVI E320 | 38.51 ± 1.15 c | 29.70 ± 1.00 c | 27.18 ± 1.33 b | 14.86 ± 1.00 a |
| (27) CVVI E130 | 30.71 ± 1.15 c | 29.70 ± 1.00 c | 27.68 ± 1.33 a | 14.46 ± 1.00 a |
| (28) CVVI E48 | 38.60 ± 1.00 c | 28.79 ± 1.00 c | 29.20 ± 0.00 a | 16.68 ± 1.20 a |
| (29) CVVI KH10 | 99.60 ± 1.53 d | 81.73 ± 2.08 c | 68.39 ± 2.00 b | 62.33 ± 1.73 a |
| (30) CVVI K10B | 89.81 ± 1.00 c | 89.61 ± 0.58 c | 69.37 ± 0.00 b | 60.58 ± 0.89 a |
| (31) CVVI E298 | 39.75 ± 1.53 c | 28.61 ± 1.00 c | 27.57 ± 1.33 b | 20.11 ± 2.08 a |
| (32) CVVI E273 | 34.51 ± 1.15 c | 29.70 ± 1.00 c | 26.58 ± 1.33 b | 22.86 ± 1.00 a |
| (33) CVVI K436 | 31.85 ± 1.15 d | 29.70 ± 1.00 c | 27.18 ± 1.33 b | 15.86 ± 1.00 a |
| (34) CVVI E98 | 37.93 ± 1.00 c | 28.79 ± 1.00 c | 29.50 ± 0.00 b | 16.68 ± 1.20 a |
| (35) CVVI E77 | 33.69 ± 1.53 d | 21.73 ± 2.08 c | 27.29 ± 2.00 b | 19.83 ± 1.73 a |
| (36) CVVI E148 | 39.71 ± 1.00 c | 29.61 ± 0.58 c | 29.57 ± 0.00 b | 22.58 ± 0.89 a |
| (37) CVVI E10 | 89.65 ± 1.53 c | 88.61 ± 1.00 c | 61.67 ± 1.33 b | 50.79 ± 2.08 a |
| (38) CVVI E215 | 34.51 ± 1.15 c | 29.70 ± 1.00 c | 17.58 ± 1.33 b | 21.86 ± 1.00 a |
| (39) NLK99/F5 | 91.51 ± 1.15 d | 89.70 ± 1.00 c | 67.68 ± 1.33 b | 61.86 ± 1.00 a |
| (40) NL12B/F5 | 88.90 ± 1.00 c | 88.79 ± 1.00 c | 69.60 ± 0.00 b | 63.68 ± 1.20 a |
| (41) NLK99-1* | 97.70 ± 1.53 d | 81.73 ± 2.08 c | 68.69 ± 2.00 b | 62.83 ± 1.73 a |
| (42) NLK99-3* | 89.71 ± 1.00 c | 79.61 ± 0.58 c | 69.57 ± 0.00 b | 67.58 ± 0.89 a |
| (43) NLK99-5* | 89.65 ± 1.53 c | 78.61 ± 1.00 c | 67.67 ± 1.33 b | 50.81 ± 2.08 a |
| (44) NLK99-7* | 84.51 ± 1.15 c | 79.70 ± 1.00 c | 67.58 ± 1.33 b | 44.86 ± 1.00 a |
| (45) NLK99-9* | 91.51 ± 1.15 c | 79.70 ± 1.00 c | 67.28 ± 1.13 b | 64.86 ± 1.00 a |
| (46) NLK99-11* | 88.90 ± 1.00 c | 78.79 ± 1.00 c | 69.60 ± 0.00 b | 66.68 ± 1.20 a |
| (47) NLK99-15* | 99.70 ± 1.53 d | 91.63 ± 0.28 c | 68.69 ± 2.00 b | 62.83 ± 1.73 a |
| (48) NLK99-19* | 89.71 ± 1.00 c | 79.61 ± 0.58 c | 69.57 ± 0.00 b | 67.58 ± 0.89 a |
Three plates for each bacterium were considered. The experiment was repeated twice. Values are given as mean ± S.D. Values in a row followed by different lowercased letters are significantly different at P ≤ 0.05.
There is a significant difference of antibacterial activity among the isolates and the NO concentrations tested (Table 5). The highest percent bacterial GRs were detected at 100 μL NO and they concerned mainly enteropathogenic E. coli isolates. Amplicons of the expected sizes from virulence genes of enteropathogenic E. coli isolates were not detected when bacterial viable cells were checked in samples treated with 100 μL and 10 μL NO concentrations. On the contrary, amplicons of the expected size were revealed in the same samples using primer pair numbers 16 and 17, as reported in Table 1, that specifically amplify unique sequences of E. coli 16S rRNA.
The antibiotic activity of ciprofloxacin is to bind and inhibit bacterial topoisomerase types II and IV, thus being able to interfere with the bacterial processes of replication, transcription, and DNA repair.
An increasing ciprofloxacin resistance of E. coli isolates was reported [20] according several epidemiological studies. E. coli, the most commonly isolated bacterium in clinical samples from patients affected by different severity of diarrheal symptoms, shows high antibiotic resistance [21, 22]. Diarrheagenic E. coli, considered in the experiment, showed a resistance or less susceptibility to ciprofloxacin, in comparison with the other nonenteropathogenic isolates tested.
The viable cells of the fourteen diarrheagenic E. coli were checked after NO treatment with primer pairs listed in Table 1 and PMA dye. The dye propidium monoazide (PMA Biotium Inc., Hayward, CA, USA) is a photoreactive dye with high affinity for DNA. The dye intercalates into DNA and forms a covalent linkage upon exposure to intense visible light. It is cell membrane impermeable. When a sample comprising both live and dead bacteria is treated with PMA, only dead cells are susceptible to DNA modification due to their compromised cell membranes [23, 24]. Therefore, selective detection of the sole live cells is achieved.
The fourteen ciprofloxacin resistant/less susceptible diarrheagenic E. coli seem to lose their virulence after NO treatment, because amplicons were obtained only with the primer pairs numbers 16 and 17 (Table 1). This let us suppose that antibacterial activity acts on adhesion factor and membrane and its permeability with possible loss of extrachromosomal DNA.
4. Conclusions and Future Implications
Studies of new antimicrobials from plant derived extracts and agroindustrial byproducts as antimicrobials and preservatives are important issues in applied microbiology and biotechnology, for both implementing and improving effective alternative technologies to tackle antimicrobial resistance. The potential use of plant natural antimicrobials would require amendments of several different legal texts involving areas such as food additives, food packaging, and hygiene. Anyway, the applications could concern either the natural preservation in the food industries or an accessible and safe alternative to synthetic antimicrobial drugs.
Acknowledgments
The authors thank Dr. D. Carminati of the Fodder and Dairy Productions Research Centre (CRA FLC) of Lodi, Agricultural Research Council (CRA), Italy, Dr. vu Kack of Central Vietnam Veterinary Institute, and Dr. F. G. Van Zijderveld of the University of Wageningen, The Netherland, for kindly providing the E. coli isolates.
Abbreviations
- ATCC:
American Type Culture Collection
- CFX:
Antibiotic: hydrochloride monohydrate
- CRA:
Agricultural Research Council, Italy
- CVVI:
Central Vietnam Veterinary Institute
- DAEC:
Diffusely adherent E. coli
- DSMZ:
Leibniz-Institut DSMZ—Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH
- EAEC:
Enteroaggregative E. coli
- EHEC:
Enterohemorrhagic E. coli
- EIEC:
Enteroinvasive E. coli
- ELISA:
Enzyme-linked immunosorbent assay
- EPEC:
Enteropathogenic E. coli
- ETEC:
Enterotoxigenic E. coli
- GIZ:
Growth inhibition zone
- HPTLC:
High performance thin layer chromatography
- HUS:
Hemolytic uremic syndrome
- LSD:
Least significant difference
- NCE:
Neem cake extract
- NL:
Department of Bacteriology and Animal Science, University of Wageningen, Netherlands
- NO:
Neem oil
- OD:
Optical density
- PMA:
Propidium monoazide
- RAPD PCR:
Random amplified polymorphic DNA polymerase chain reaction
- Rf:
Ratio between the migration distance of substance and the migration distance of solvent front
- SAS:
Statistical Analysis System (SAS Institute, Inc., Cary, NC, USA)
- SD:
Standard deviation
- TWN:
Tween 80
- WTR:
Sterile distilled water.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Paola Del Serrone carried out research concept and design, collection and/or assembly of biological assay data, collection and/or assembly of molecular biology assay data, data analysis and interpretation, statistical analysis, writing the paper, critical revision of the paper, and the final approval of the paper. Chiara Toniolo was responsible for collection and/or assembly of chemical data, data analysis and interpretation, critical revision of the paper, and final approval of the paper. Marcello Nicoletti carried out collection and/or assembly of chemical data, data analysis and interpretation, writing the paper, critical revision of the paper, and final approval of the paper.
References
- 1.Raloff J. Drugged waters. Science News. 1998;153:187–189. doi: 10.2307/4010314. [DOI] [Google Scholar]
- 2.McKeon D. M., Calabrese J. P., Bissonnette G. K. Antibiotic resistant gram-negative bacteria in rural groundwater supplies. Water Research. 1995;29(8):1902–1908. doi: 10.1016/0043-1354(95)00013-B. [DOI] [Google Scholar]
- 3.Dröge M., Pühler A., Selbitschka W. Phenotypic and molecular characterization of conjugative antibiotic resistance plasmids isolated from bacterial communities of activated sludge. Molecular and General Genetics. 2000;263(3):471–482. doi: 10.1007/s004380051191. [DOI] [PubMed] [Google Scholar]
- 4.Manges A. R., Smith S. P., Lau B. J., et al. Retail meat consumption and the acquisition of antimicrobial resistant Escherichia coli causing urinary tract infections: a case-control study. Foodborne Pathogens and Disease. 2007;4(4):419–431. doi: 10.1089/fpd.2007.0026. [DOI] [PubMed] [Google Scholar]
- 5.Del Serrone P., Contillo R., Failla S., et al. Assessment of the microbiological quality of retail fresh pork meat in central Italy. Italian Journal of Food Science. 2006;18(4):397–408. [Google Scholar]
- 6.Palaniappan K., Holley R. A. Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. International Journal of Food Microbiology. 2010;140(2-3):164–168. doi: 10.1016/j.ijfoodmicro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Rawani A., Pal S., Chandra G. Evaluation of antimicrobial properties of four plant extracts against human pathogens. Asian Pacific Journal of Tropical Biomedicine. 2011;1(1):S71–S75. doi: 10.1016/S2221-1691(11)60127-5. [DOI] [Google Scholar]
- 8.Del Serrone P., Nicoletti M. Antimicrobial activity of a neem cake extract in a broth model meat system. International Journal of Environmental Research and Public Health. 2013;10(8):3282–3295. doi: 10.3390/ijerph10083282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EFSA. The Community summary report on trends and sources of zoonoses, zoonotic agents and foodborne outbreaks in European Union in 2008. The EFSA Journal. 2010;8(1) [Google Scholar]
- 10.Comunicazione della Commissione al Parlamento Europeo e al Consiglio. Piano d’azione di lotta ai crescenti rischi di resistenza antimicrobica (AMR) 2011;(COM 2011-748)
- 11.Conclusioni del Consiglio, del 22 giugno 2012, sull’impatto della resistenza antimicrobica nel settore della salute umana e nel settore veterinario—una prospettiva di tipo ‘One Health’. Gazzetta Ufficiale. 2012;(2012/C 211/02)
- 12.National Research Council. Report of an Ad Hoc Panel of the Board on Science and Technology for International Development. Washington, DC, USA: National Academy Press; 1992. Neem: a tree for solving global problems. [Google Scholar]
- 13.Baswa M., Rath C. C., Dash S. K., Mishra R. K. Antibacterial activity of Karanj (Pongamia pinnata) and Neem (Azadirachta indica) seed oil: a preliminary report. Microbios. 2001;105(412):183–189. [PubMed] [Google Scholar]
- 14.SaiRam M., Ilavazhagan G., Sharma S. K., et al. Anti-microbial activity of a new vaginal contraceptive NIM-76 from neem oil (Azadirachta indica) Journal of Ethnopharmacology. 2000;71(3):377–382. doi: 10.1016/S0378-8741(99)00211-1. [DOI] [PubMed] [Google Scholar]
- 15.Zago M., Bonvini B., Platero A. M. M., Mucchetti G., Carminati D., Giraffa G. Characterisation of Escherichia coli isolated from raw milk cheeses. Annals of Microbiology. 2007;57(1):49–54. doi: 10.1007/BF03175049. [DOI] [Google Scholar]
- 16.van Zijderveld F. G., Overdijk E. Experiences with the ELISA for detection of the E. coli K99 antigen in calf faeces. Annales de Recherches Veterinaires. 1983;14(4):395–399. [PubMed] [Google Scholar]
- 17.Nicoletti M., Mariani S., MacCioni O., Coccioletti T., Murugan K. Neem cake: chemical composition and larvicidal activity on Asian tiger mosquito. Parasitology Research. 2012;111(1):205–213. doi: 10.1007/s00436-012-2819-8. [DOI] [PubMed] [Google Scholar]
- 18.Nicoletti M. HPTLC fingerprint: a modern approach for the analytical determination of botanicals. Brazilian Journal of Pharmacognosy. 2011;21(5):818–823. doi: 10.1590/S0102-695X2011005000131. [DOI] [Google Scholar]
- 19.Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology. 1966;36:493–496. [PubMed] [Google Scholar]
- 20.Abdullah F. E., Memon A. A., Bandukda M. Y., Jamil M. Increasing ciprofloxacin resistance of isolates from infected urines of a cross-section of patients in Karachi. BMC Research Notes. 2012;5, article 696 doi: 10.1186/1756-0500-5-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Momtaz H., Dehkordi F. S., Hosseini M. J., Sarshar M., Heidari M. Serogroups, virulence genes and antibiotic resistance in Shiga toxin-producing Escherichia coliisolated from diarrheic and non-diarrheic pediatric patients in Iran. Gut Pathogens. 2013;5(1, article 39) doi: 10.1186/1757-4749-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trung V. N., Phung V. L., Chinh H. L., Weintraub A. Antibiotic resistance in diarrheagenic Escherichia coli and Shigella strains isolated from children in Hanoi, Vietnam. Antimicrobial Agents and Chemotherapy. 2005;49(2):816–819. doi: 10.1128/AAC.49.2.816-819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nocker A., Cheung C.-Y., Camper A. K. Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. Journal of Microbiological Methods. 2006;67(2):310–320. doi: 10.1016/j.mimet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Chen S., Wang F., Beaulieu J. C., Stein R. E., Ge B. Rapid detection of viable Salmonellae in produce by coupling propidium monoazide with loop-mediated isothermal amplification. Applied and Environmental Microbiology. 2011;77(12):4008–4016. doi: 10.1128/AEM.00354-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osek J. Multiplex polymerase chain reaction assay for identification of enterotoxigenic Escherichia coli strains. Journal of Veterinary Diagnostic Investigation. 2001;13(4):308–311. doi: 10.1177/104063870101300405. [DOI] [PubMed] [Google Scholar]
- 26.Ojeniyi B., Ahrens P., Meyling A. Detection of fimbrial and toxin genes in Escherichia coli and their prevalence in piglets with diarrhea. The application of colony hybridization assay, polymerase chain reaction and phenotypic assays. Journal of Veterinary Medicine. 1994;41(1):49–59. doi: 10.1111/j.1439-0450.1994.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 27.Blanco M., Padola N. L., Krüger A., et al. Virulence genes and intimin types of Shiga-toxin-producing Escherichia coli isolated from cattle and beef products in Argentina. International Microbiology. 2004;7(4):269–276. [PubMed] [Google Scholar]
- 28.Yamamoto T., Nakazawa M. Detection and sequences of the enteroaggregative Escherichia coli heat- stable enterotoxin 1 gene in enterotoxigenic E. coli strains isolated from piglets and calves with diarrhea. Journal of Clinical Microbiology. 1997;35(1):223–227. doi: 10.1128/jcm.35.1.223-227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang G., Clark C. G., Rodgerst F. G. Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. Journal of Clinical Microbiology. 2002;40(10):3613–3619. doi: 10.1128/JCM.40.10.3613-3619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vu Khac H. V., Holoda E., Pilipcinec E., et al. Serotypes, virulence genes, and PFGE profiles of Escherichia coli isolated from pigs with postweaning diarrhoea in Slovakia. BMC Veterinary Research. 2006;2, article 10 doi: 10.1186/1746-6148-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imberechts H., van Pelt N., de Greve H., Lintermans P. Sequences related to the major subunit gene fedA of F107 fimbriae in porcine Escherichia coli strains that express adhesive fimbriae. FEMS Microbiology Letters. 1994;119(3):309–314. doi: 10.1016/0378-1097(94)90433-2. [DOI] [PubMed] [Google Scholar]


