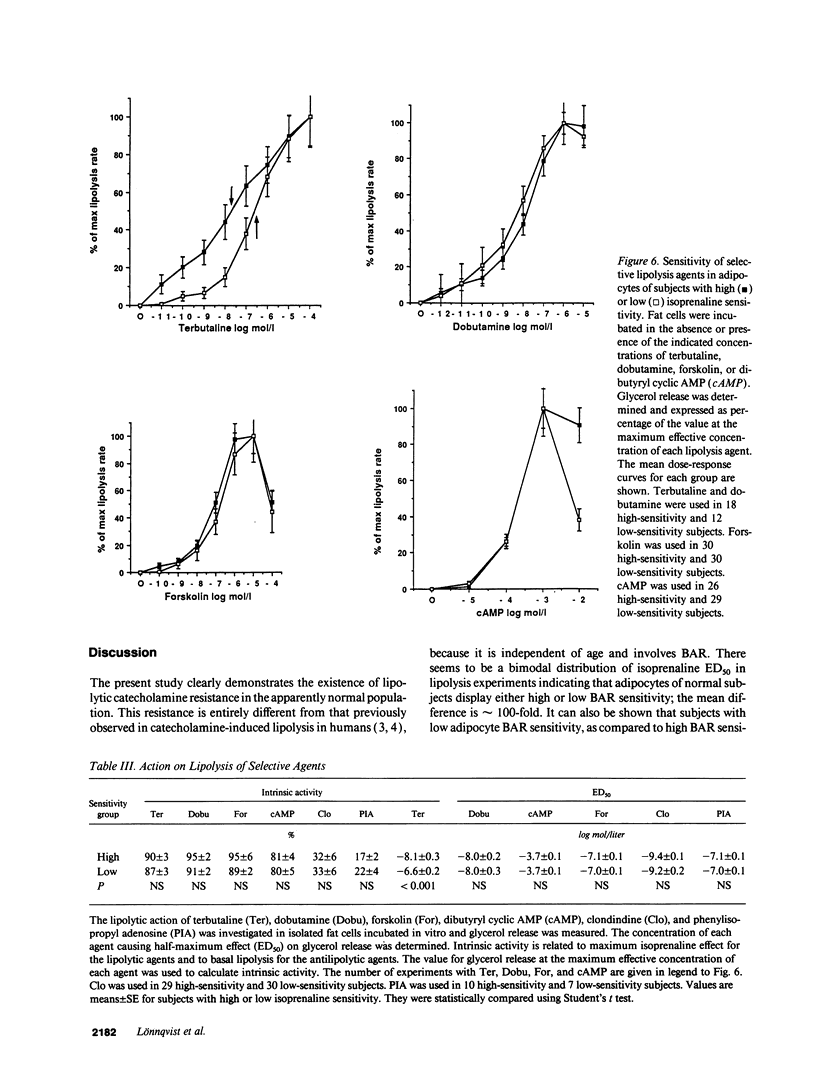
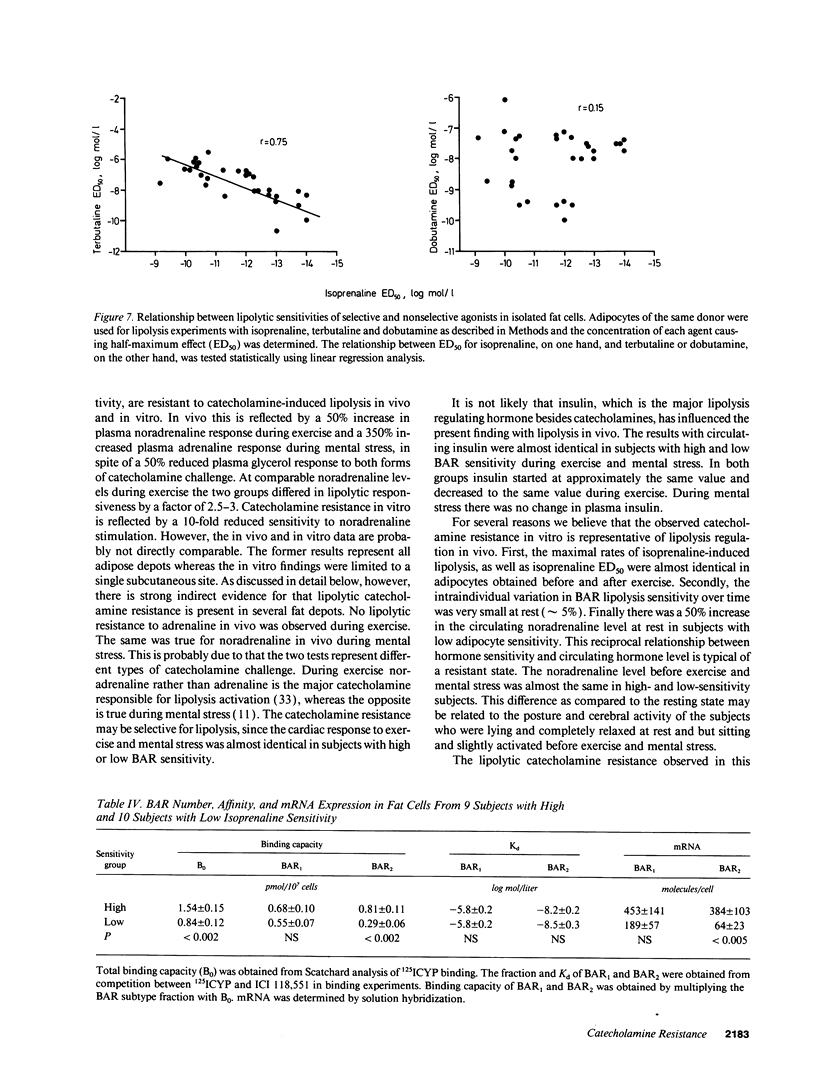
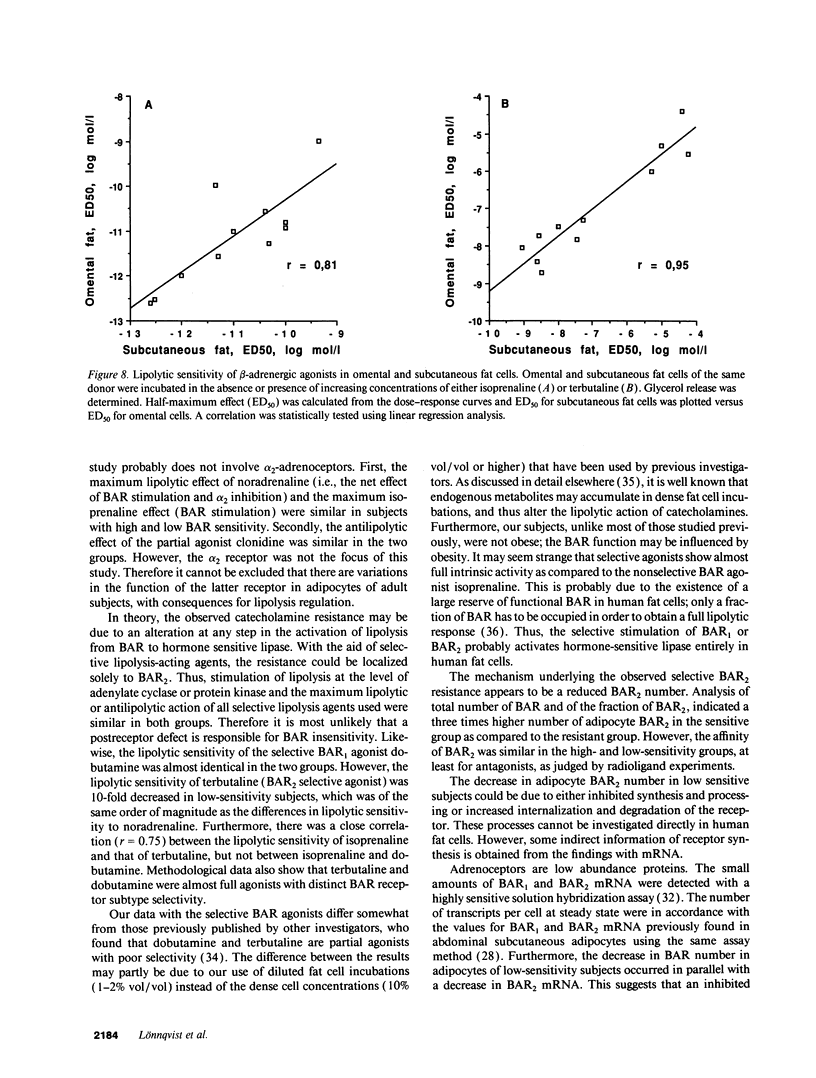
Abstract
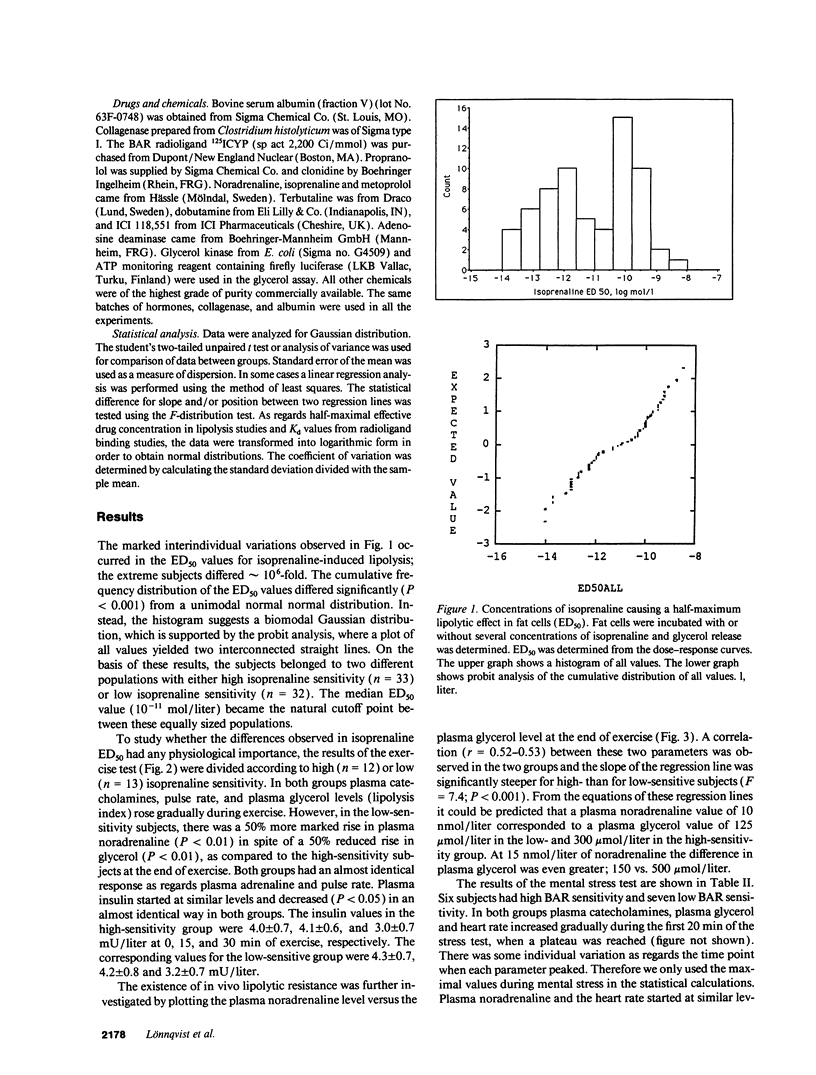
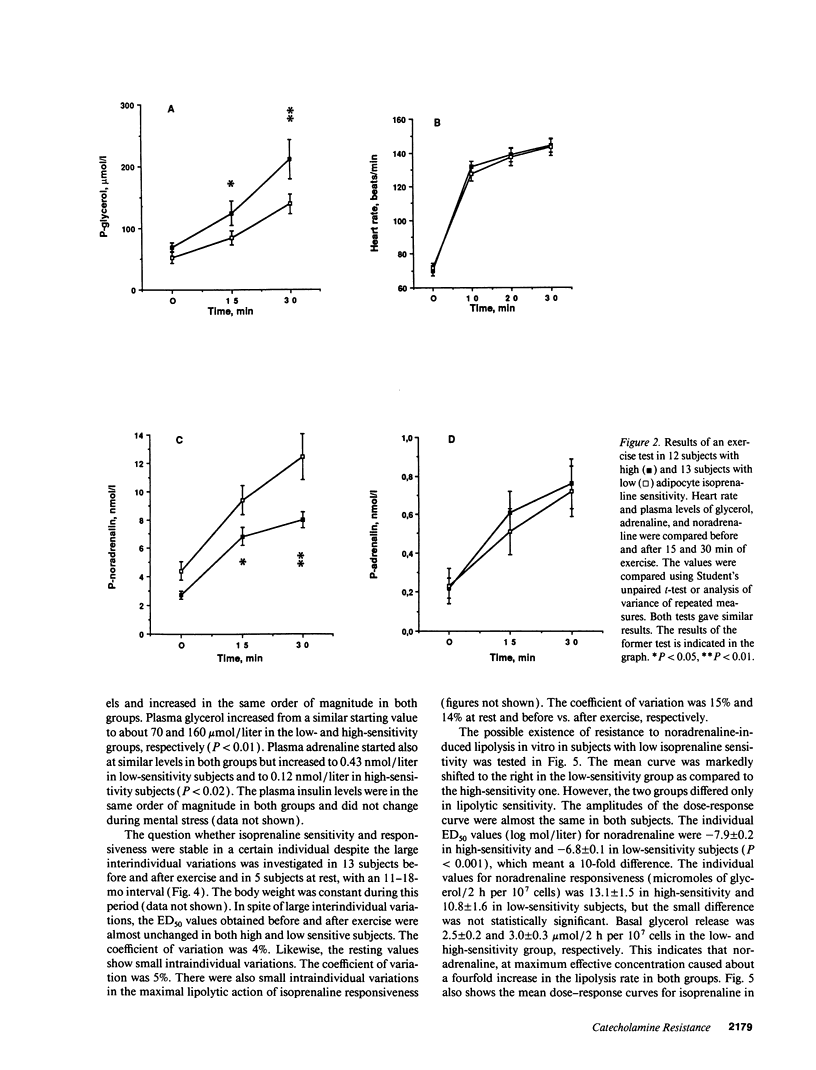
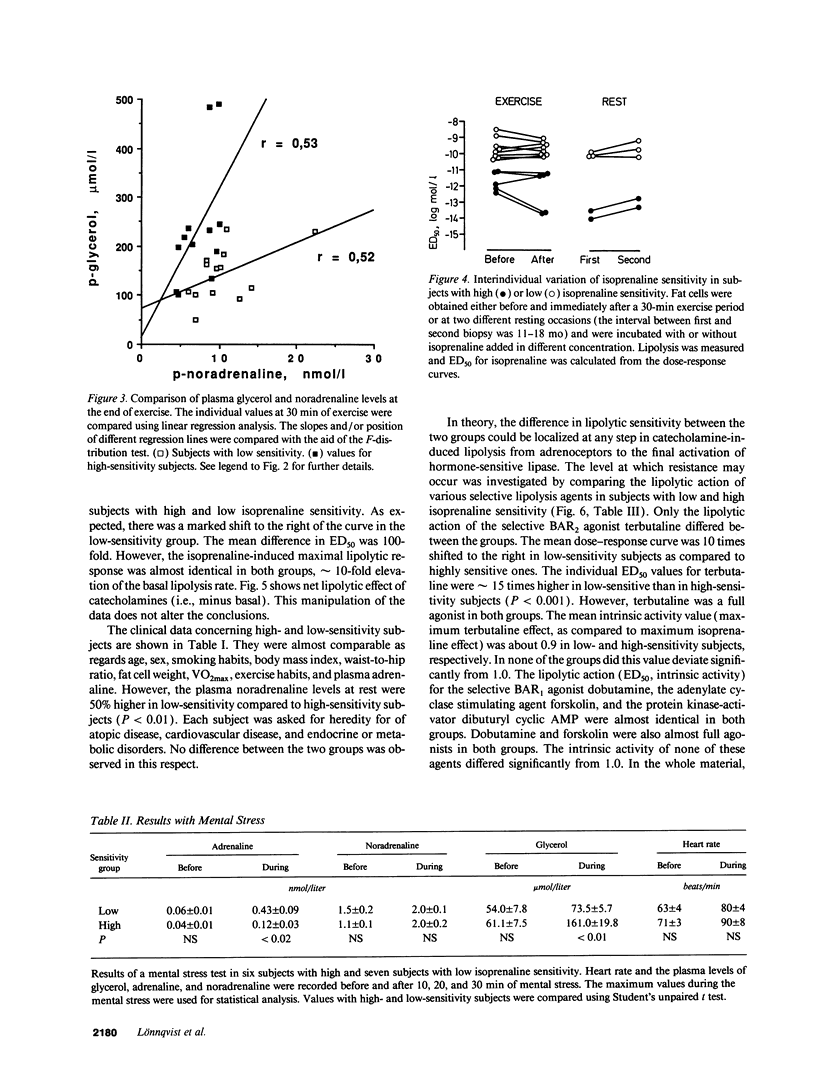
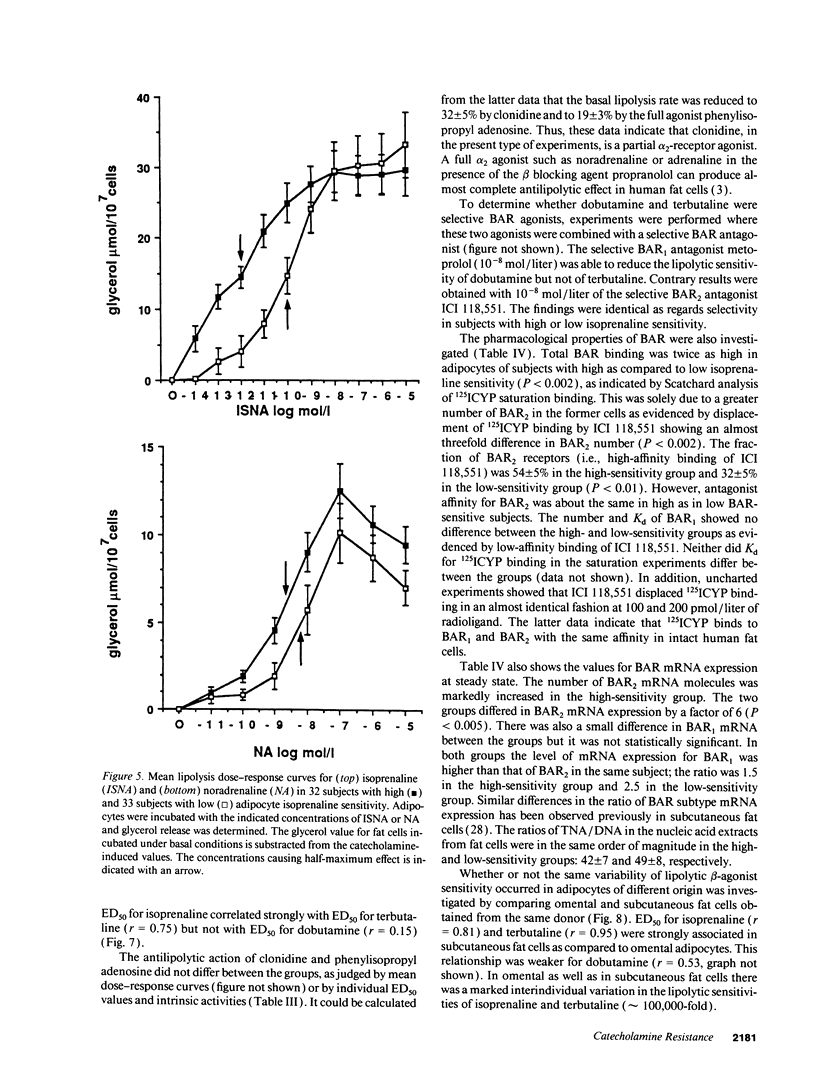
The existence of lipolytic beta-adrenoceptor (BAR) resistance was investigated in vivo and in isolated abdominal subcutaneous adipocytes in 65 healthy and drug-free subjects. The concentration of isoprenaline (nonselective BAR agonist) causing half-maximum lipolysis effect (ED50) varied bimodally and 10(6)-fold between individuals but was almost constant in the same subject when measured two times at rest or before and 30 min after exercise. The subjects were categorized as having either high or low isoprenaline sensitivity. The former group had a 50% reduced in vivo lipolytic response to exercise and mental stress, despite a 50% increased plasma noradrenaline response (P < 0.01) and a 350% increased plasma adrenaline response (P < 0.02). In fat cells the lipolytic ED50 values for noradrenaline and terbutaline (BAR2 agonist) were 10 times lower (P < 0.001) in low-sensitive subjects, but the maximum lipolytic actions of these agents (and of isoprenaline) were similar in both groups. The action on lipolysis of dobutamine (BAR1 agonist), forskolin (stimulating adenylate cyclase), dibutyryl cyclic AMP (activating protein kinase), clonidine (alpha 2-adrenergic agonist), or phenyl isopropyladenosine (adenosine receptor agonist) were almost identical in high- and low-sensitivity subjects. ED50 for isoprenaline correlated with ED50 for terbutaline (r = 0.75), but not with ED50 for dobutamine. In high-sensitivity subjects the number of BAR2 was almost three-fold increased (P < 0.002) and the steady-state adipocyte mRNA level for BAR2 was sixfold increased (P < 0.005). BAR2 affinity as well as BAR1 number, affinity and mRNA expression were similar in both groups. In 11 cholecystectomy patients (otherwise healthy) lipolytic ED50 for beta agonists correlated in omental and subcutaneous fat cells (r = 0.85 for isoprenaline; r = 0.95 for terbutaline). In conclusion, lipolytic resistance to catecholamines is present in vivo in apparently healthy subjects due to reduced expression of BAR2 in adipocytes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P., Engfeldt P., Hellström L., Lönnqvist F., Wahrenberg H., Sonnenfeld T., Brönnegård M. Beta-adrenoreceptor subtype expression in human liver. J Clin Endocrinol Metab. 1990 Nov;71(5):1119–1126. doi: 10.1210/jcem-71-5-1119. [DOI] [PubMed] [Google Scholar]
- Arner P., Hellmér J., Wennlund A., Ostman J., Engfeldt P. Adrenoceptor occupancy in isolated human fat cells and its relationship with lipolysis rate. Eur J Pharmacol. 1988 Jan 27;146(1):45–56. doi: 10.1016/0014-2999(88)90485-2. [DOI] [PubMed] [Google Scholar]
- Arner P., Hellström L., Wahrenberg H., Brönnegård M. Beta-adrenoceptor expression in human fat cells from different regions. J Clin Invest. 1990 Nov;86(5):1595–1600. doi: 10.1172/JCI114880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P., Bolinder J. Adrenergic regulation of lipolysis in situ at rest and during exercise. J Clin Invest. 1990 Mar;85(3):893–898. doi: 10.1172/JCI114516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P. In situ studies of catecholamine-induced lipolysis in human adipose tissue using microdialysis. J Pharmacol Exp Ther. 1990 Jul;254(1):284–288. [PubMed] [Google Scholar]
- Arner P., Kriegholm E., Engfeldt P. In vivo interactions between beta-1 and beta-2 adrenoceptors regulate catecholamine tachyphylaxia in human adipose tissue. J Pharmacol Exp Ther. 1991 Oct;259(1):317–322. [PubMed] [Google Scholar]
- Berrettini W. H., Hoehe M. R. A polymorphism of the beta 1 adrenergic receptor gene (BADR) detected with Bgl I. Nucleic Acids Res. 1988 Aug 11;16(15):7754–7754. doi: 10.1093/nar/16.15.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodde O. E. The functional importance of beta 1 and beta 2 adrenoceptors in the human heart. Am J Cardiol. 1988 Aug 11;62(5):24C–29C. doi: 10.1016/s0002-9149(88)80063-8. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Emorine L. J., Marullo S., Briend-Sutren M. M., Patey G., Tate K., Delavier-Klutchko C., Strosberg A. D. Molecular characterization of the human beta 3-adrenergic receptor. Science. 1989 Sep 8;245(4922):1118–1121. doi: 10.1126/science.2570461. [DOI] [PubMed] [Google Scholar]
- Engfeldt P., Hellmér J., Wahrenberg H., Arner P. Effects of insulin on adrenoceptor binding and the rate of catecholamine-induced lipolysis in isolated human fat cells. J Biol Chem. 1988 Oct 25;263(30):15553–15560. [PubMed] [Google Scholar]
- Fain J. N., Garcĩa-Sáinz J. A. Adrenergic regulation of adipocyte metabolism. J Lipid Res. 1983 Aug;24(8):945–966. [PubMed] [Google Scholar]
- Frielle T., Collins S., Daniel K. W., Caron M. G., Lefkowitz R. J., Kobilka B. K. Cloning of the cDNA for the human beta 1-adrenergic receptor. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7920–7924. doi: 10.1073/pnas.84.22.7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellmér J., Arner P., Lundin A. Automatic luminometric kinetic assay of glycerol for lipolysis studies. Anal Biochem. 1989 Feb 15;177(1):132–137. doi: 10.1016/0003-2697(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Hellmér J., Wahrenberg H., Arner P. Stability over time of adrenergic sensitivity in isolated human fat cells. Int J Obes Relat Metab Disord. 1992 Jan;16(1):23–28. [PubMed] [Google Scholar]
- Hirsch J., Gallian E. Methods for the determination of adipose cell size in man and animals. J Lipid Res. 1968 Jan;9(1):110–119. [PubMed] [Google Scholar]
- Hjemdahl P. Catecholamine measurements in plasma by high-performance liquid chromatography with electrochemical detection. Methods Enzymol. 1987;142:521–534. doi: 10.1016/s0076-6879(87)42065-x. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P., Freyschuss U., Juhlin-Dannfelt A., Linde B. Differentiated sympathetic activation during mental stress evoked by the Stroop test. Acta Physiol Scand Suppl. 1984;527:25–29. [PubMed] [Google Scholar]
- Kather H. Pathways of purine metabolism in human adipocytes. Further evidence against a role of adenosine as an endogenous regulator of human fat cell function. J Biol Chem. 1990 Jan 5;265(1):96–102. [PubMed] [Google Scholar]
- Kobilka B. K., Dixon R. A., Frielle T., Dohlman H. G., Bolanowski M. A., Sigal I. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lai E., Rosen O. M., Rubin C. S. Dexamethasone regulates the beta-adrenergic receptor subtype expressed by 3T3 L1 preadipocytes and adipocytes. J Biol Chem. 1982 Jun 25;257(12):6691–6696. [PubMed] [Google Scholar]
- Langin D., Portillo M. P., Saulnier-Blache J. S., Lafontan M. Coexistence of three beta-adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharmacol. 1991 Jul 9;199(3):291–301. doi: 10.1016/0014-2999(91)90492-9. [DOI] [PubMed] [Google Scholar]
- Leibel R. L., Berry E. M., Hirsch J. Metabolic and hemodynamic responses to endogenous and exogenous catecholamines in formerly obese subjects. Am J Physiol. 1991 Apr;260(4 Pt 2):R785–R791. doi: 10.1152/ajpregu.1991.260.4.R785. [DOI] [PubMed] [Google Scholar]
- Lemoine H., Ehle B., Kaumann A. J. Direct labelling of beta 2-adrenoceptors. Comparison of binding potency of 3H-ICI 118,551 and blocking potency of ICI 118,551. Naunyn Schmiedebergs Arch Pharmacol. 1985 Oct;331(1):40–51. doi: 10.1007/BF00498850. [DOI] [PubMed] [Google Scholar]
- Lentes K. U., Berrettini W. H., Hoehe M. R., Chung F. Z., Gershon E. S. A biallelic DNA polymorphism of the human beta-2-adrenergic receptor detected by Ban I-Adrbr-2. Nucleic Acids Res. 1988 Mar 25;16(5):2359–2359. doi: 10.1093/nar/16.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde B., Hjemdahl P., Freyschuss U., Juhlin-Dannfelt A. Adipose tissue and skeletal muscle blood flow during mental stress. Am J Physiol. 1989 Jan;256(1 Pt 1):E12–E18. doi: 10.1152/ajpendo.1989.256.1.E12. [DOI] [PubMed] [Google Scholar]
- Lundin A., Arner P., Hellmér J. A new linear plot for standard curves in kinetic substrate assays extended above the Michaelis-Menten constant: application to a luminometric assay of glycerol. Anal Biochem. 1989 Feb 15;177(1):125–131. doi: 10.1016/0003-2697(89)90026-2. [DOI] [PubMed] [Google Scholar]
- Lönnqvist F., Nyberg B., Wahrenberg H., Arner P. Catecholamine-induced lipolysis in adipose tissue of the elderly. J Clin Invest. 1990 May;85(5):1614–1621. doi: 10.1172/JCI114612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnqvist F., Wennlund A., Wahrenberg H., Arner P. Effects of mental stress on lipolysis in humans. Metabolism. 1992 Jun;41(6):622–630. doi: 10.1016/0026-0495(92)90054-e. [DOI] [PubMed] [Google Scholar]
- Marcus C., Karpe B., Bolme P., Sonnenfeld T., Arner P. Changes in catecholamine-induced lipolysis in isolated human fat cells during the first year of life. J Clin Invest. 1987 Jun;79(6):1812–1818. doi: 10.1172/JCI113022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauriège P., De Pergola G., Berlan M., Lafontan M. Human fat cell beta-adrenergic receptors: beta-agonist-dependent lipolytic responses and characterization of beta-adrenergic binding sites on human fat cell membranes with highly selective beta 1-antagonists. J Lipid Res. 1988 May;29(5):587–601. [PubMed] [Google Scholar]
- Mauriège P., Després J. P., Prud'homme D., Pouliot M. C., Marcotte M., Tremblay A., Bouchard C. Regional variation in adipose tissue lipolysis in lean and obese men. J Lipid Res. 1991 Oct;32(10):1625–1633. [PubMed] [Google Scholar]
- Michel M. C., Brodde O. E., Insel P. A. Peripheral adrenergic receptors in hypertension. Hypertension. 1990 Aug;16(2):107–120. doi: 10.1161/01.hyp.16.2.107. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Ostman J., Arner P., Kimura H., Wahrenberg H., Engfeldt P. Influence of fasting on lipolytic response to adrenergic agonists and on adrenergic receptors in subcutaneous adipocytes. Eur J Clin Invest. 1984 Oct;14(5):383–391. doi: 10.1111/j.1365-2362.1984.tb01199.x. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rebuffé-Scrive M., Anderson B., Olbe L., Björntorp P. Metabolism of adipose tissue in intraabdominal depots in severely obese men and women. Metabolism. 1990 Oct;39(10):1021–1025. doi: 10.1016/0026-0495(90)90160-e. [DOI] [PubMed] [Google Scholar]
- Richelsen B., Sørensen N. S. Alpha 2- and beta-adrenergic receptor binding and action in gluteal adipocytes from patients with hypothyroidism and hyperthyroidism. Metabolism. 1987 Nov;36(11):1031–1039. doi: 10.1016/0026-0495(87)90022-9. [DOI] [PubMed] [Google Scholar]
- Valet P., Montastruc J. L., Berlan M., Tran M. A., Lafontan M., Montastruc P. Differential regulation of fat cell beta-2 and beta-1 adrenoceptors by endogenous catecholamines in dog. J Pharmacol Exp Ther. 1989 Apr;249(1):271–277. [PubMed] [Google Scholar]
- Wahrenberg H., Engfeldt P., Arner P., Wennlund A., Ostman J. Adrenergic regulation of lipolysis in human adipocytes: findings in hyper- and hypothyroidism. J Clin Endocrinol Metab. 1986 Sep;63(3):631–638. doi: 10.1210/jcem-63-3-631. [DOI] [PubMed] [Google Scholar]
- Wahrenberg H., Lönnqvist F., Arner P. Mechanisms underlying regional differences in lipolysis in human adipose tissue. J Clin Invest. 1989 Aug;84(2):458–467. doi: 10.1172/JCI114187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahrenberg H., Lönnqvist F., Engfeldt P., Arner P. Abnormal action of catecholamines on lipolysis in adipocytes of type I diabetic patients treated with insulin. Diabetes. 1989 Apr;38(4):524–533. doi: 10.2337/diab.38.4.524. [DOI] [PubMed] [Google Scholar]
- Wang H. Y., Berrios M., Hadcock J. R., Malbon C. C. The biology of beta-adrenergic receptors: analysis in human epidermoid carcinoma A431 cells. Int J Biochem. 1991;23(1):7–20. doi: 10.1016/0020-711x(91)90003-6. [DOI] [PubMed] [Google Scholar]



