Abstract
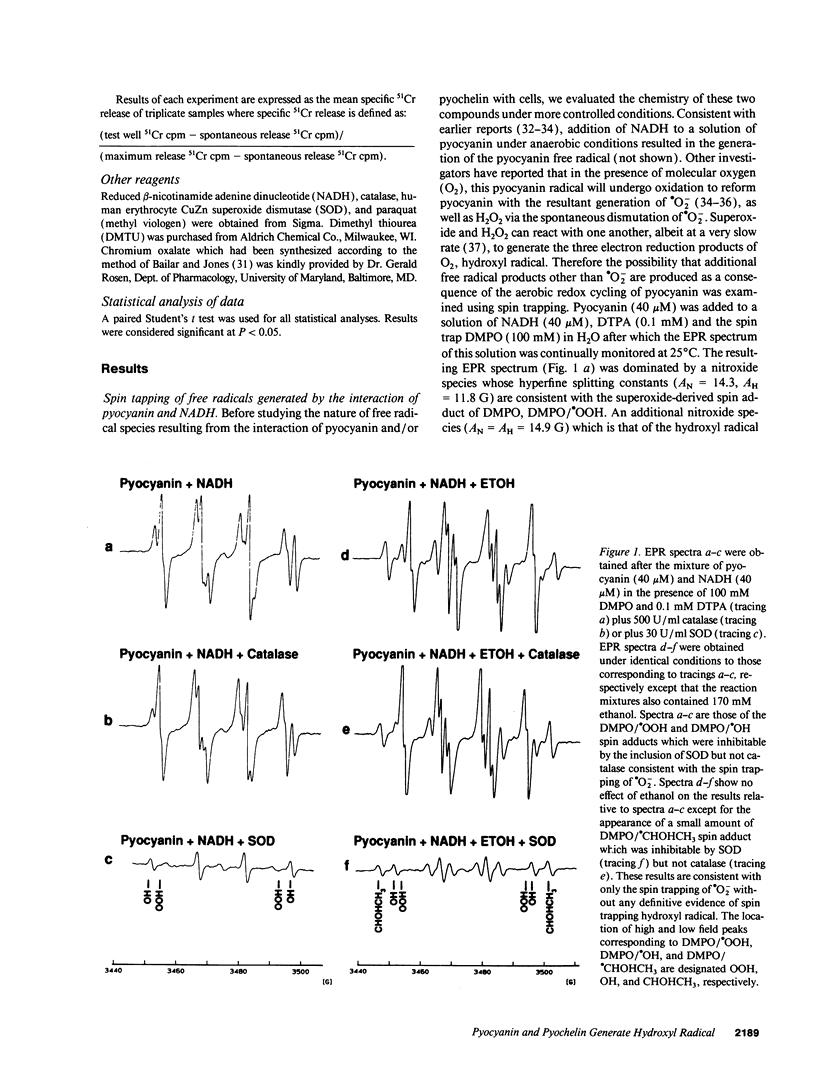
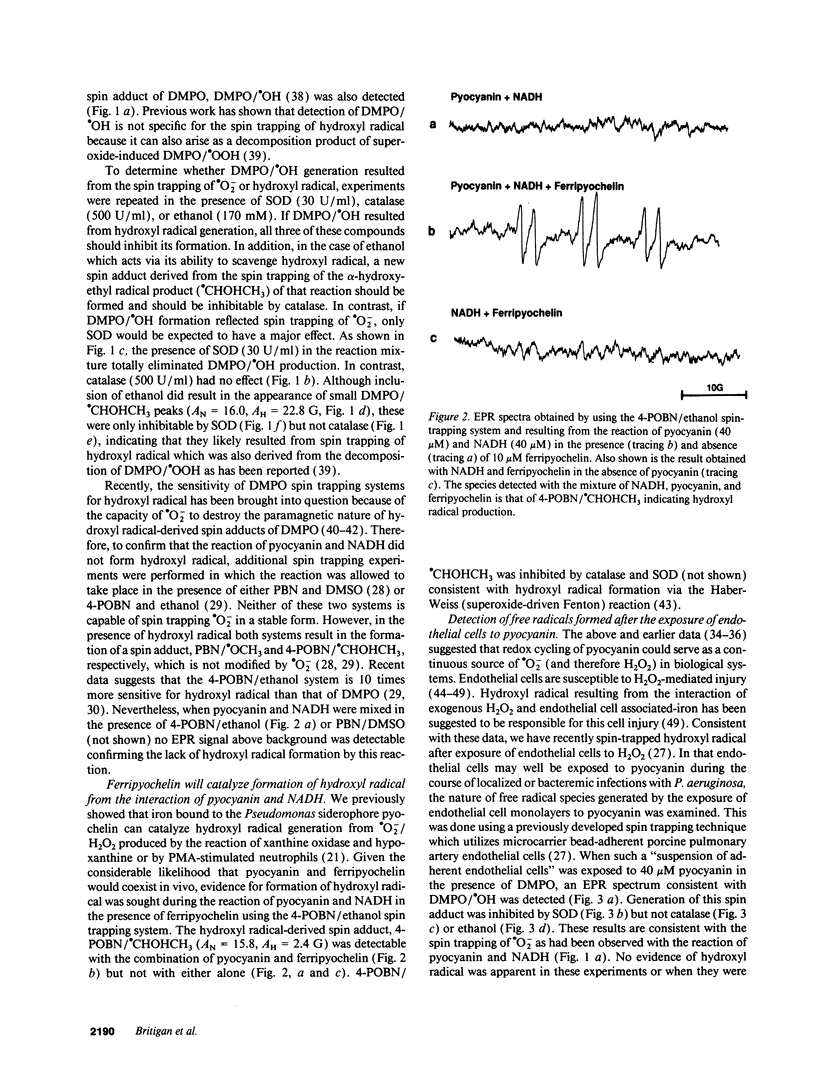
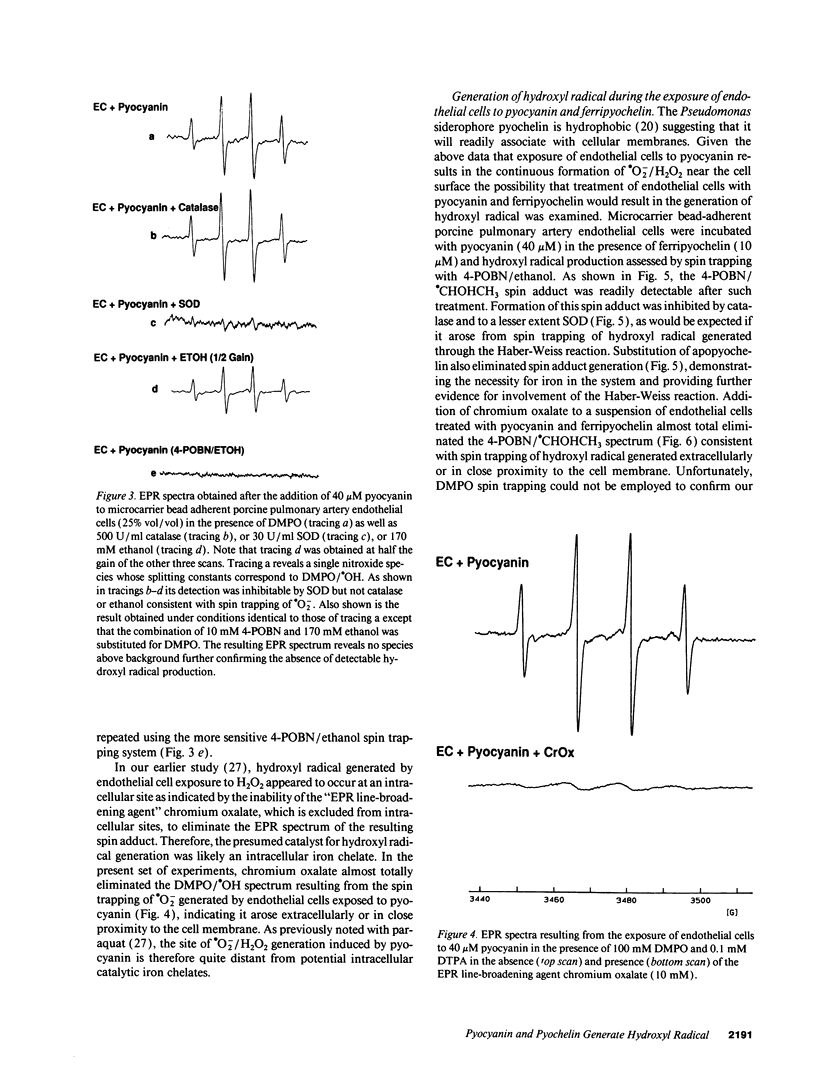
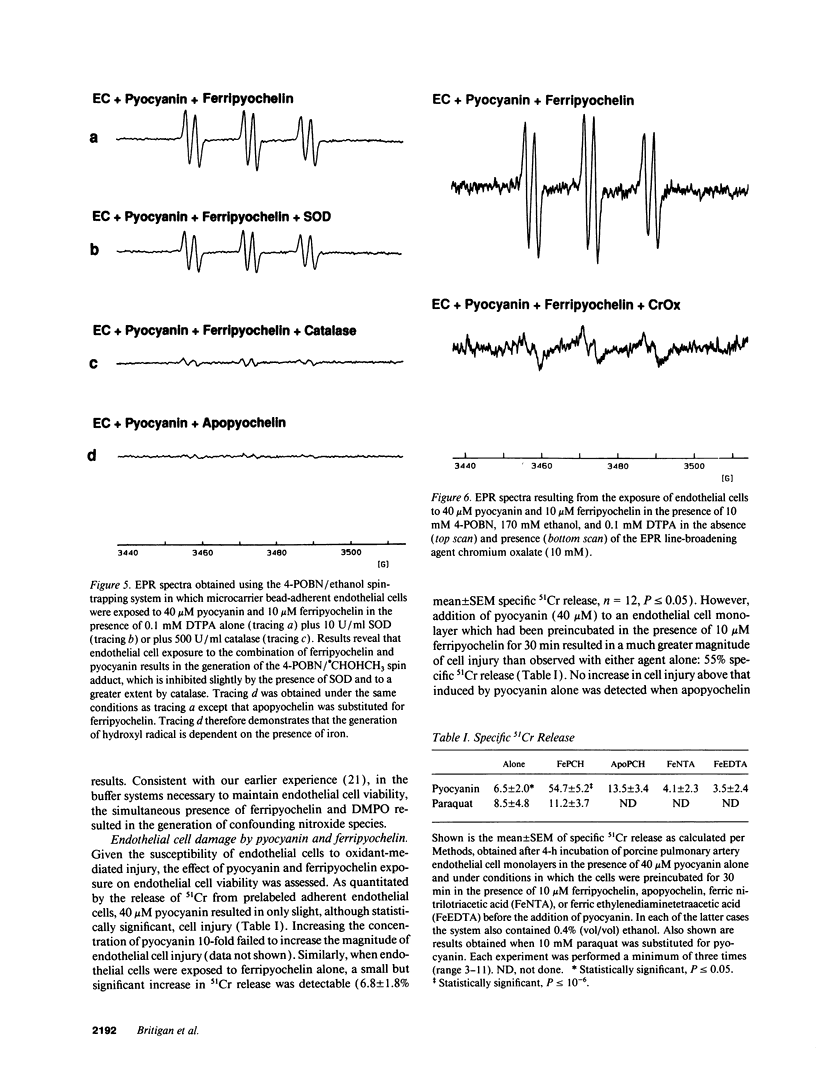
Pyocyanin, a secretory product of Pseudomonas aeruginosa, has the capacity to undergo redox cycling under aerobic conditions with resulting generation of superoxide and hydrogen peroxide. By using spin trapping techniques in conjunction with electron paramagnetic resonance spectrometry (EPR), superoxide was detected during the aerobic reduction of pyocyanin by NADH or porcine endothelial cells. No evidence of hydroxyl radical formation was detected. Chromium oxalate eliminated the EPR spectrum of the superoxide-derived spin adduct resulting from endothelial cell exposure to pyocyanin, suggesting superoxide formation close to the endothelial cell plasma membrane. We have previously reported that iron bound to the P. aeruginosa siderophore pyochelin (ferripyochelin) catalyzes the formation of hydroxyl free radical from superoxide and hydrogen peroxide via the Haber-Weiss reaction. In the present study, spin trap evidence of hydroxyl radical formation was detected when NADH and pyocyanin were allowed to react in the presence of ferripyochelin. Similarly, endothelial cell exposure to pyocyanin and ferripyochelin also resulted in hydroxyl radical production which appeared to occur in close proximity to the cell surface. As assessed by 51Cr release, endothelial cells which were treated with pyocyanin or ferripyochelin alone demonstrated minimal injury. However, endothelial cell exposure to the combination of pyochelin and pyocyanin resulted in 55% specific 51Cr release. Injury was not observed with the substitution of iron-free pyochelin and was diminished by the presence of catalase or dimethyl thiourea. These data suggest the possibility that the P. aeruginosa secretory products pyocyanin and pyochelin may act synergistically via the generation of hydroxyl radical to damage local tissues at sites of pseudomonas infection.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler K. B., Holden-Stauffer W. J., Repine J. E. Oxygen metabolites stimulate release of high-molecular-weight glycoconjugates by cell and organ cultures of rodent respiratory epithelium via an arachidonic acid-dependent mechanism. J Clin Invest. 1990 Jan;85(1):75–85. doi: 10.1172/JCI114436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitani R., Wilson R., Rutman A., Read R., Ward C., Burnett D., Stockley R. A., Cole P. J. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol. 1991 Jan;4(1):26–32. doi: 10.1165/ajrcmb/4.1.26. [DOI] [PubMed] [Google Scholar]
- Armstrong A. V., Stewart-Tull D. E., Roberts J. S. Characterisation of the Pseudomonas aeruginosa factor that inhibits mouse-liver mitochondrial respiration. J Med Microbiol. 1971 May;4(2):249–262. doi: 10.1099/00222615-4-2-249. [DOI] [PubMed] [Google Scholar]
- Baldwin D. A., Jenny E. R., Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem. 1984 Nov 10;259(21):13391–13394. [PubMed] [Google Scholar]
- Bodey G. P., Bolivar R., Fainstein V., Jadeja L. Infections caused by Pseudomonas aeruginosa. Rev Infect Dis. 1983 Mar-Apr;5(2):279–313. doi: 10.1093/clinids/5.2.279. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Coffman T. J., Buettner G. R. Spin trapping evidence for the lack of significant hydroxyl radical production during the respiration burst of human phagocytes using a spin adduct resistant to superoxide-mediated destruction. J Biol Chem. 1990 Feb 15;265(5):2650–2656. [PubMed] [Google Scholar]
- Britigan B. E., Edeker B. L. Pseudomonas and neutrophil products modify transferrin and lactoferrin to create conditions that favor hydroxyl radical formation. J Clin Invest. 1991 Oct;88(4):1092–1102. doi: 10.1172/JCI115408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B. E., Roeder T. L., Shasby D. M. Insight into the nature and site of oxygen-centered free radical generation by endothelial cell monolayers using a novel spin trapping technique. Blood. 1992 Feb 1;79(3):699–707. [PubMed] [Google Scholar]
- Buettner G. R. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3(4):259–303. doi: 10.1016/s0891-5849(87)80033-3. [DOI] [PubMed] [Google Scholar]
- Buettner G. R. The reaction of superoxide, formate radical, and hydrated electron with transferrin and its model compound, Fe(III)-ethylenediamine-N,N'-bis[2-(2-hydroxyphenyl)acetic acid] as studied by pulse radiolysis. J Biol Chem. 1987 Sep 5;262(25):11995–11998. [PubMed] [Google Scholar]
- Busch C., Cancilla P. A., DeBault L. E., Goldsmith J. C., Owen W. G. Use of endothelium cultured on microcarriers as a model for the microcirculation. Lab Invest. 1982 Nov;47(5):498–504. [PubMed] [Google Scholar]
- Bynoe L. A., Pou S., Gottsch J. D., Rosen G. M. Light-dependent spin trapping of hydroxyl radical from human erythrocytes. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1305–1310. doi: 10.1016/0006-291x(91)91715-o. [DOI] [PubMed] [Google Scholar]
- Coffman T. J., Cox C. D., Edeker B. L., Britigan B. E. Possible role of bacterial siderophores in inflammation. Iron bound to the Pseudomonas siderophore pyochelin can function as a hydroxyl radical catalyst. J Clin Invest. 1990 Oct;86(4):1030–1037. doi: 10.1172/JCI114805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Britigan B. E., Hassett D. J., Rosen G. M. Do humans neutrophils form hydroxyl radical? Evaluation of an unresolved controversy. Free Radic Biol Med. 1988;5(2):81–88. doi: 10.1016/0891-5849(88)90033-0. [DOI] [PubMed] [Google Scholar]
- Cox C. D., Adams P. Siderophore activity of pyoverdin for Pseudomonas aeruginosa. Infect Immun. 1985 Apr;48(1):130–138. doi: 10.1128/iai.48.1.130-138.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D., Rinehart K. L., Jr, Moore M. L., Cook J. C., Jr Pyochelin: novel structure of an iron-chelating growth promoter for Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4256–4260. doi: 10.1073/pnas.78.7.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox C. D. Role of pyocyanin in the acquisition of iron from transferrin. Infect Immun. 1986 Apr;52(1):263–270. doi: 10.1128/iai.52.1.263-270.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crim C., Simon R. H. Effects of oxygen metabolites on rat alveolar type II cell viability and surfactant metabolism. Lab Invest. 1988 Apr;58(4):428–437. [PubMed] [Google Scholar]
- Davis G., Thornalley P. J. Free radical production from the aerobic oxidation of reduced pyridine nucleotides catalysed by phenazine derivatives. Biochim Biophys Acta. 1983 Sep 30;724(3):456–464. doi: 10.1016/0005-2728(83)90106-8. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr Pathogenesis of the pseudomonas lung lesion in cystic fibrosis. Chest. 1989 Jul;96(1):158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- Friedheim E. A. The effect of pyocyanine on the respiration of some normal tissues and tumours. Biochem J. 1934;28(1):173–179. doi: 10.1042/bj0280173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon D. E., Varani J., Phan S. H., Ward J. H., Kaplan J., Till G. O., Simon R. H., Ryan U. S., Ward P. A. Source of iron in neutrophil-mediated killing of endothelial cells. Lab Invest. 1987 Jul;57(1):37–44. [PubMed] [Google Scholar]
- Haas B., Kraut J., Marks J., Zanker S. C., Castignetti D. Siderophore presence in sputa of cystic fibrosis patients. Infect Immun. 1991 Nov;59(11):3997–4000. doi: 10.1128/iai.59.11.3997-4000.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Harlan J. M., Callahan K. S. Role of hydrogen peroxide in the neutrophil-mediated release of prostacyclin from cultured endothelial cells. J Clin Invest. 1984 Aug;74(2):442–448. doi: 10.1172/JCI111440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Mechanism of the antibiotic action pyocyanine. J Bacteriol. 1980 Jan;141(1):156–163. doi: 10.1128/jb.141.1.156-163.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett D. J., Charniga L., Bean K., Ohman D. E., Cohen M. S. Response of Pseudomonas aeruginosa to pyocyanin: mechanisms of resistance, antioxidant defenses, and demonstration of a manganese-cofactored superoxide dismutase. Infect Immun. 1992 Feb;60(2):328–336. doi: 10.1128/iai.60.2.328-336.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner J. E., Repine J. E. Pulmonary strategies of antioxidant defense. Am Rev Respir Dis. 1989 Aug;140(2):531–554. doi: 10.1164/ajrccm/140.2.531. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Ota S., Mutoh H., Sugimoto T., Razandi M., Ivey K. J. Oxygen metabolites stimulate mucous glycoprotein secretion from cultured rat gastric mucous cells. Am J Physiol. 1991 Oct;261(4 Pt 1):G662–G668. doi: 10.1152/ajpgi.1991.261.4.G662. [DOI] [PubMed] [Google Scholar]
- Ishizu K., Dearman H. H., Huang M. T., White J. R. Electron paramagnetic resonance observations on biogenic semiquinone and 5-methyl phenazinium radicals. Biochim Biophys Acta. 1968 Sep 3;165(2):283–285. doi: 10.1016/0304-4165(68)90056-1. [DOI] [PubMed] [Google Scholar]
- Jackowski J. T., Szepfalusi Z., Wanner D. A., Seybold Z., Sielczak M. W., Lauredo I. T., Adams T., Abraham W. M., Wanner A. Effects of P. aeruginosa-derived bacterial products on tracheal ciliary function: role of O2 radicals. Am J Physiol. 1991 Feb;260(2 Pt 1):L61–L67. doi: 10.1152/ajplung.1991.260.2.L61. [DOI] [PubMed] [Google Scholar]
- King R. J., Coalson J. J., Seidenfeld J. J., Anzueto A. R., Smith D. B., Peters J. I. O2- and pneumonia-induced lung injury. II. Properties of pulmonary surfactant. J Appl Physiol (1985) 1989 Jul;67(1):357–365. doi: 10.1152/jappl.1989.67.1.357. [DOI] [PubMed] [Google Scholar]
- Lewis M. S., Whatley R. E., Cain P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Hydrogen peroxide stimulates the synthesis of platelet-activating factor by endothelium and induces endothelial cell-dependent neutrophil adhesion. J Clin Invest. 1988 Dec;82(6):2045–2055. doi: 10.1172/JCI113825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., 2nd, Gadek J. E., Hunninghake G. W., Crystal R. G. Oxidant injury of lung parenchymal cells. J Clin Invest. 1981 Nov;68(5):1277–1288. doi: 10.1172/JCI110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. M., Dearborn D. G., Sorensen R. U. In vitro effect of synthetic pyocyanine on neutrophil superoxide production. Infect Immun. 1987 Mar;55(3):559–563. doi: 10.1128/iai.55.3.559-563.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M., Sorrell T. C. Effect of pyocyanin and 1-hydroxyphenazine on the assay of lactate dehydrogenase activity. J Infect Dis. 1991 Sep;164(3):610–612. doi: 10.1093/infdis/164.3.610a. [DOI] [PubMed] [Google Scholar]
- Muller M., Sorrell T. C. Production of leukotriene B4 and 5-hydroxyeicosatetraenoic acid by human neutrophils is inhibited by Pseudomonas aeruginosa phenazine derivatives. Infect Immun. 1991 Sep;59(9):3316–3318. doi: 10.1128/iai.59.9.3316-3318.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro N. C., Barker A., Rutman A., Taylor G., Watson D., McDonald-Gibson W. J., Towart R., Taylor W. A., Wilson R., Cole P. J. Effect of pyocyanin and 1-hydroxyphenazine on in vivo tracheal mucus velocity. J Appl Physiol (1985) 1989 Jul;67(1):316–323. doi: 10.1152/jappl.1989.67.1.316. [DOI] [PubMed] [Google Scholar]
- Mühlradt P. F., Tsai H., Conradt P. Effects of pyocyanine, a blue pigment from Pseudomonas aeruginosa, on separate steps of T cell activation: interleukin 2 (IL 2) production, IL 2 receptor formation, proliferation and induction of cytolytic activity. Eur J Immunol. 1986 Apr;16(4):434–440. doi: 10.1002/eji.1830160421. [DOI] [PubMed] [Google Scholar]
- Müller P. K., Krohn K., Mühlradt P. F. Effects of pyocyanine, a phenazine dye from Pseudomonas aeruginosa, on oxidative burst and bacterial killing in human neutrophils. Infect Immun. 1989 Sep;57(9):2591–2596. doi: 10.1128/iai.57.9.2591-2596.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutman J., Berger M., Chase P. A., Dearborn D. G., Miller K. M., Waller R. L., Sorensen R. U. Studies on the mechanism of T cell inhibition by the Pseudomonas aeruginosa phenazine pigment pyocyanine. J Immunol. 1987 May 15;138(10):3481–3487. [PubMed] [Google Scholar]
- O'Connell M., Halliwell B., Moorhouse C. P., Aruoma O. I., Baum H., Peters T. J. Formation of hydroxyl radicals in the presence of ferritin and haemosiderin. Is haemosiderin formation a biological protective mechanism? Biochem J. 1986 Mar 15;234(3):727–731. doi: 10.1042/bj2340727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki M., Kawabata T., Awai M. Iron release from haemosiderin and production of iron-catalysed hydroxyl radicals in vitro. Biochem J. 1988 Mar 1;250(2):589–595. doi: 10.1042/bj2500589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker N. B., Berger E. M., Curtis W. E., Muldrow M. E., Linas S. L., Repine J. E. Hydrogen peroxide causes dimethylthiourea consumption while hydroxyl radical causes dimethyl sulfoxide consumption in vitro. J Free Radic Biol Med. 1985;1(5-6):415–419. doi: 10.1016/0748-5514(85)90155-2. [DOI] [PubMed] [Google Scholar]
- Patel K. D., Zimmerman G. A., Prescott S. M., McEver R. P., McIntyre T. M. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol. 1991 Feb;112(4):749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pou S., Cohen M. S., Britigan B. E., Rosen G. M. Spin-trapping and human neutrophils. Limits of detection of hydroxyl radical. J Biol Chem. 1989 Jul 25;264(21):12299–12302. [PubMed] [Google Scholar]
- Ramos C. L., Pou S., Britigan B. E., Cohen M. S., Rosen G. M. Spin trapping evidence for myeloperoxidase-dependent hydroxyl radical formation by human neutrophils and monocytes. J Biol Chem. 1992 Apr 25;267(12):8307–8312. [PubMed] [Google Scholar]
- Ras G. J., Anderson R., Taylor G. W., Savage J. E., Van Niekerk E., Wilson R., Cole P. J. Proinflammatory interactions of pyocyanin and 1-hydroxyphenazine with human neutrophils in vitro. J Infect Dis. 1990 Jul;162(1):178–185. doi: 10.1093/infdis/162.1.178. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Britigan B. E., Cohen M. S., Ellington S. P., Barber M. J. Detection of phagocyte-derived free radicals with spin trapping techniques: effect of temperature and cellular metabolism. Biochim Biophys Acta. 1988 May 13;969(3):236–241. doi: 10.1016/0167-4889(88)90057-2. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G. M., Hassett D. J., Yankaskas J. R., Cohen M. S. Detection of free radicals as a consequence of dog tracheal epithelial cellular xenobiotic metabolism. Xenobiotica. 1989 Jun;19(6):635–643. doi: 10.3109/00498258909042300. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A., Black C. D., Krishna C. M., Malech H. L., Bernstein E. F., Russo A. Hydroxyl radical production by stimulated neutrophils reappraised. J Biol Chem. 1988 Sep 25;263(27):13797–13801. [PubMed] [Google Scholar]
- Schellhorn H. E., Pou S., Moody C., Hassan H. M. An electron spin resonance study of oxyradical generation in superoxide dismutase- and catalase-deficient mutants of Escherichia coli K-12. Arch Biochem Biophys. 1989 Jun;271(2):323–331. doi: 10.1016/0003-9861(89)90282-8. [DOI] [PubMed] [Google Scholar]
- Shasby D. M., Lind S. E., Shasby S. S., Goldsmith J. C., Hunninghake G. W. Reversible oxidant-induced increases in albumin transfer across cultured endothelium: alterations in cell shape and calcium homeostasis. Blood. 1985 Mar;65(3):605–614. [PubMed] [Google Scholar]
- Shasby D. M., Shasby S. S., Peach M. J. Granulocytes and phorbol myristate acetate increase permeability to albumin of cultured endothelial monolayers and isolated perfused lungs. Role of oxygen radicals and granulocyte adherence. Am Rev Respir Dis. 1983 Jan;127(1):72–76. doi: 10.1164/arrd.1983.127.1.72. [DOI] [PubMed] [Google Scholar]
- Sorensen R. U., Klinger J. D., Cash H. A., Chase P. A., Dearborn D. G. In vitro inhibition of lymphocyte proliferation by Pseudomonas aeruginosa phenazine pigments. Infect Immun. 1983 Jul;41(1):321–330. doi: 10.1128/iai.41.1.321-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. C., Wood R. E., Boat T. F., Matthews L. W., Tucker A. S., Doershuk C. F. Treatment and prognosis of massive hemoptysis in cystic fibrosis. Am Rev Respir Dis. 1978 May;117(5):825–828. doi: 10.1164/arrd.1978.117.5.825. [DOI] [PubMed] [Google Scholar]
- Tate R. M., Morris H. G., Schroeder W. R., Repine J. E. Oxygen metabolites stimulate thromboxane production and vasoconstriction in isolated saline-perfused rabbit lungs. J Clin Invest. 1984 Aug;74(2):608–613. doi: 10.1172/JCI111458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer A. J., Pryjma J., Tarnok Z., Ernst M., Flad H. D. Inhibitory and stimulatory effects of Pseudomonas aeruginosa pyocyanine on human T and B lymphocytes and human monocytes. Infect Immun. 1990 Mar;58(3):808–815. doi: 10.1128/iai.58.3.808-815.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercellotti G. M., Severson S. P., Duane P., Moldow C. F. Hydrogen peroxide alters signal transduction in human endothelial cells. J Lab Clin Med. 1991 Jan;117(1):15–24. [PubMed] [Google Scholar]
- Weiss S. J. Oxygen, ischemia and inflammation. Acta Physiol Scand Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M. J., Shasby D. M., Husted R. M. Oxidants increase paracellular permeability in a cultured epithelial cell line. J Clin Invest. 1985 Sep;76(3):1155–1168. doi: 10.1172/JCI112071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Pitt T., Taylor G., Watson D., MacDermot J., Sykes D., Roberts D., Cole P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest. 1987 Jan;79(1):221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R., Sykes D. A., Watson D., Rutman A., Taylor G. W., Cole P. J. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 1988 Sep;56(9):2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter M., Wilson J. S., Bedell K., Shasby D. M. The conductance of cultured epithelial cell monolayers: oxidants, adenosine triphosphate, and phorbol dibutyrate. Am J Respir Cell Mol Biol. 1990 Apr;2(4):355–363. doi: 10.1165/ajrcmb/2.4.355. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Lactoferrin-catalysed hydroxyl radical production. Additional requirement for a chelating agent. Biochem J. 1983 Jan 15;210(1):15–19. doi: 10.1042/bj2100015. [DOI] [PMC free article] [PubMed] [Google Scholar]



