Abstract
Vaccine efficacy in aquaculture has for a long time depended on evaluating relative percent survival and antibody responses after vaccination. However, current advances in vaccine immunology show that the route in which antigens are delivered into cells is deterministic of the type of adaptive immune response evoked by vaccination. Antigens delivered by the intracellular route induce MHC-I restricted CD8+ responses while antigens presented through the extracellular route activate MHC-II restricted CD4+ responses implying that the route of antigen delivery is a conduit to induction of B- or T-cell immune responses. In finfish, different antigen delivery systems have been explored that include live, DNA, inactivated whole virus, fusion protein, virus-like particles, and subunit vaccines although mechanisms linking these delivery systems to protective immunity have not been studied in detail. Hence, in this review we provide a synopsis of different strategies used to administer viral antigens via the intra- or extracellular compartments. Further, we highlight the differences in immune responses induced by antigens processed by the endogenous route compared to exogenously processed antigens. Overall, we anticipate that the synopsis put together in this review will shed insights into limitations and successes of the current vaccination strategies used in finfish vaccinology.
1. Introduction
The central hallmark of vaccination is to prime the adaptive immune system to develop immune responses that will protect the host organism upon a second encounter with the same pathogen. However, priming the adaptive immune system requires activation of naïve B- and T-lymphocytes into effector cells that translate into protective immunity. While studies on the immunological basis of vaccine protection have for a long time focused on humoral and cellular responses as measures of protective immunity, growing evidence shows that the mode by which antigens are presented to B- or T-lymphocytes has a significant influence on the outcome of adaptive immune responses induced by vaccination which is also influenced by the mode in which antigens are administered to host cells [1, 2]. Put together, these elements drive vaccine development into a cross-talk between vaccinology and immunology in which vaccine design and its delivery (vaccinology) on one hand have to be optimized in order to gain an effective immune response (immunology) on the other. Hence, optimization of antigen design and its delivery into host cells is a prerequisite to inducing an optimal protective immune response.
Unlike B-lymphocytes, which are precursors of antibody secreting cells that can recognize antigens through primed antigen presenting cells (APCs)/activated B-cells [1], T-cell receptors (TCRs) can only “see” antigens that are processed and presented by APCs. TCRs recognize antigen peptides bound on the surface of MHC molecules [2]. Endogenous peptides derived from intracellular sources such as replicating virus are synthesized and processed for presentation to naïve CD8 T-cells by MHC-I molecules while exogenous peptides derived from extracellular sources are processed and presented to naïve CD4 T-cells by MHC-II molecules. An alternative mechanism that permits some extracellular antigens to activate naïve CD8 T-cells called cross presentation exists which occurs via the MHC-I pathway [3, 4]. For antigens delivered via the endosomal route, proteosomes degrade soluble antigens after ubiquitination which have been synthesized in the cytosol or escaped to the endoplasmic reticulum (ER) by cross presentation [5]. Thereafter, the processed antigens are released after proteosomal degradation to generate peptides that are transported into the ER by the transporter-associated antigen processing (TAPs) [5, 6]. Once in the ER, the antigenic peptides are loaded onto MHC-I molecules for presentation on the cell surface where they initiate the activation of naïve CD8 T-cells into effector cytotoxic T-lymphocytes (CTLs) [7–9]. In the case of antigens delivered by the exogenous route, lysosomes degrade endocytosed antigens after endosomal fusion with lysosomes [10]. In general, lysosomes can degrade complex structures such as whole viral particles that are delivered to them via endocytosis by the extracellular route [11]. Presentation of processed peptides by endosomal degradation leads to maturation of APCs into professional APCs which is characterized by expressing MHC-II molecules and antigen specific signaling molecules such CD40L, CD80, and CD86. The resulting professional APCs are the prime initiators of adaptive immune responses that activate naïve T-cells into effector cells through the MHC-peptide complexes and immune modulation molecules. Therefore, it follows that, for a vaccine antigen to turn naïve B- or T-lymphocytes into “protective” cell, there has to be an efficient antigen delivery system that stimulates the activation of cell of the adaptive immune system.
Although studies on antigen presentation in fish immunology have gained prominence in recent years [12–14], there is still limited research on activation of cells of the adaptive immune system by APCs, which precludes our understanding of the role of innate immunity in optimizing vaccine performance. Despite that, several studies have been carried out trying to deliver viral antigens into different compartments of fish cells. Hence, in this review we provide an overview of these delivery systems and based on this approach we highlight the different immune responses induced by antigens delivered by the intracellular route compared to antigens delivered by the extracellular route. In addition, we also highlight the differences in vaccine efficacy from fish immunized using antigens delivered by the exogenous route compared to fish vaccinated using the endogenous route. Overall, we anticipate that the synopsis of different antigen delivery systems put together in this review will shed new insights into limitations and successes of the current vaccination strategies used in fish vaccinology.
2. Fish Antigen Presenting Cells, Adaptive Immune Cells, and Their Receptors
In mammals, antigen presentation is carried out by different cell types that include monocytes, macrophages, and dendritic cells [15]. These cells possess pattern recognition receptors (PRRs) that recognize and bind to pathogen associated molecular patterns (PAMPs) known as “danger signals” on pathogens [16]. Upon binding to PAMPs using PRRs, monocytes mature into macrophages while immature dendritic cells (DCs) also transform into mature dendritic ones to become professional APCs, which induce the expression of proinflammatory cytokines that attract more APCs to the sites of antigen deposition [15, 17]. Upon encounter with the APCs, naïve B- and T-cells undergo maturation to become memory cells capable of recognizing the antigens in subsequent encounters thereby creating the basis acquired immunity.
In teleosts fish, APCs known to possess PRRs having the capacity to bind to different PAMPs on pathogens have been described in different species and these include monocytes, macrophages, and dendritic-like cells [13, 18–26]. In addition fish B-cells have been shown to carry out antigen presentation apart from their role as antibody secreting cells [27]. As a result, in vitro methods for culturing fish monocytes, macrophages, and dendritic-like cells have been developed which makes it easy to study antigen presentation using cell cultures [18, 19, 24, 25]. It is interesting to note that TAP genes comparable to those seen in mammals have been identified and mapped to MHC regions in different cartilaginous and bony fish species suggesting that similar mechanisms of endogenous antigen processing seen in higher vertebrates also exist in teleosts fish [28–31]. Unlike in mammals where APCs carrying processed antigens migrate to the lymph nodes [32], in fish APCs carrying antigens migrate to the head, kidney, and spleen [13], which are the major lymphoid organs [33]. Apart from lymphoid organs, APCs have been detected in other organs such as the gills, skin, and intestines in fish [34, 35]. In addition, different phagocytic cell types have been characterized in fish although their antigen presentation capabilities have not been investigated [36, 37].
Similar to their mammalian counterparts, fish APCs possess a wide range of surface markers that include CD80/CD86, CD83, CD209, MHC-I, and MHC-II proteins [20, 38–41]. In fish, CD83 has been shown to be an activation marker for macrophages [42] and dendritic-like cells [23]. Apart from CD83, other surface markers identified for fish dendritic-like cells include CD208/lysosomal associated membrane protein (LAMP3) [43]. Recently, Zhu et al. [44] showed that fish B-cells act as pivotal APCs in priming the adaptive immune system using CD80/CD86 molecules. In another study, Abós et al. [45] showed upregulation of MHC-II genes that coincided with upregulation of CD80/CD86 genes in a nonlethal infection (no cytopathic effects observed) of viral hemorrhagic septicemia virus (VHSV) in IgM+ cells which consolidates the notion that fish B-cells use CD80/CD86 molecules to activate the adaptive immune system using the MHC-II pathway [44, 46].
As shown in Table 1, all four T-cell receptor chains (α, β, γ, and δ) required for binding to APCs together with the four chains (γ-, δ-, ε-, and ζ-chain) of the CD3 coreceptor complex required for T-cell activation in mammals have been reported in fish. In addition, the T-cell costimulatory marker CD28 and the negative regulatory marker CTLA-4, which bind to CD80 and CD86 receptors on APCs, have also been characterized in fish [47, 48]. As for CD8 T-cells, two subsets have been characterized, namely, CD8α and CD8β, from different fish species of which CD8α has been the most widely used marker for T-cell activation in different studies [49–51]. Moreover, cell mediated cytotoxicity against allogeneic targets and virus infected cells has been reported by different scientists [50, 52–54]. Put together these observations suggest that fish T-cells possess surface receptors essential for the binding to APCs comparable to those found in mammals and that activation of T-cells into effector cytotoxic T-lymphocytes (CTLs) could be based on similar mechanisms to those seen in mammals.
Table 1.
Fish antigen presenting and adaptive immunity cell receptors.
| Protein | Selected examples of fish species | Reference |
|---|---|---|
| (1) Antigen presenting surface markers and MHC molecules | ||
| CD80 (B7.1) | Zebrafish, rainbow trout | [44, 159] |
| CD83 (7.2) | Zebrafish, turbot, Atlantic salmon | [44, 159–161] |
| CD86 | Zebrafish, rainbow trout | [44, 159] |
| CD209 | Zebrafish | [162] |
| MHC-I | Orange spotted grouper, sea bass, grass carp | [49, 163, 164] |
| MHC-II | Zebrafish, lake trout, | [159, 165] |
|
| ||
| (2) T-cell receptors, costimulatory and activator molecules | ||
| CD3γ | Atlantic salmon | [166] |
| CD3ε | Atlantic salmon | [166] |
| CD3ζ | Atlantic salmon | [166, 167] |
| CD3δ | Rainbow trout, Atlantic salmon | [166, 168, 169] |
| TCRα | Rainbow trout and Japanese flounder | [170, 171] |
| TCRβ | Rainbow trout and Japanese flounder | [170, 171] |
| TCRΥ | Japanese flounder | [171] |
| TCRσ | Japanese flounder | [171] |
| CD28 | Rainbow trout | [47, 48] |
| CTLA | Rainbow trout | [47, 48] |
| CD40L | Atlantic salmon and Japanese flounder | [65, 172] |
|
| ||
| (3) T-cells | ||
| CD8α | Rainbow trout, Atlantic salmon | [51, 143, 173] |
| CD8β | Atlantic salmon, Atlantic salmon | [143, 169] |
| CD4 | Atlantic salmon | [169] |
|
| ||
| (4) Immunoglobulins | ||
| IgM | Atlantic salmon, rainbow trout | [56, 174, 175] |
| IgD | Atlantic salmon, rainbow trout | [59, 174] |
| IgT | Rainbow trout, Atlantic salmon | [58, 62] |
| IgZ | Zebrafish | [176] |
There are three immunoglobulin isotypes characterized in fish, this far, and these include IgM [55, 56] and IgD [55, 57] also present in mammals while the recently identified IgT [58] is only found in fish where it exists as membrane bound and secreted form in serum [59, 60]. IgM is the most abundant isotype in serum where it is estimated to be >1000-fold higher than IgT [61, 62]. In addition, IgM has been detected in the mucus of the skin, gills, and intestines although its levels in these organs are far much lower than levels detected in serum [61, 62]. On the contrary, IgT is predominantly found in the mucus of the skin, gut, and intestines [61–63] where it is >100-fold higher than levels detected in sera [61]. It is interesting to note that the costimulatory marker CD40L mostly expressed in activated CD4+ T-cells, which binds to the CD40 receptors on APCs in order to activate B-cell proliferation [64], has been characterized in fish [65]. In addition, transcription factors involved in specification of CD4 T-cells into different T-helper (Th) subtypes have also been characterized, which include T-bet [66, 67], GATA-3 [68–70], and RORγ [71, 72] for the differentiation of naïve CD4 T-cells into Th1, Th2, and Th17 subtypes, respectively. In addition, several cytokines linked to specification of CD4 T-cell into different subtypes have been characterized and these include IL-2, IL-4, IL-6, IL-10, IL-12, IFNγ, IL-15, IL-21, IL-22, and TGFβ [40, 41, 73, 74]. Overall, the characterization of different APCs and adaptive immune cells together with their receptors and regulatory cytokine presented here suggests that teleosts fish antigen presentation mechanisms could be comparable to those used by mammals suggesting that antigen presentation mechanisms have been conserved across the vertebrate taxa.
3. Intracellular Antigen Delivery Systems
Intracellular antigen delivery systems involve immunization strategies that administer the vaccine antigens into the cytoplasm (Figure 1) and these include the following.
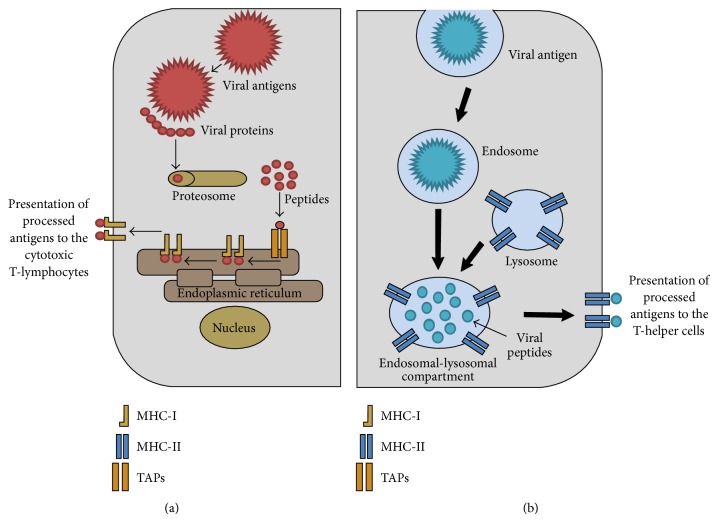
Figure 1.
The endogenous and exogenous pathways of viral antigen entry into host cells. (a) Endogenous pathway shows viral antigens that enter the host cells by the intracellular route. Once internalized, the viral antigens are degraded into peptides by proteasomes. Thereafter, the processed antigenic peptides are transported via the transporter associated with antigen presentation (TAPs) to the endoplasmic reticulum (ER) where they are loaded onto MHC-I molecules for presentation at the cell surface to CD8+ T-cells. (b) Exogenous pathway shows antigens that enter the antigen presenting cells (APCs) via the extracellular route which results in internalization of the antigens in the endosomes. Thereafter, the endosomes fuse with the lysosomes to form the endosomal-lysosomal compartments that have MHC-II complexes. In the endosomal-lysosomal compartments, the antigens are degraded into peptides followed by packaging of the peptides onto MHC-II complexes. Thereafter, the MHC-II complexes carrying the peptides are transported to the cell surface for presentation of the antigenic peptides to the CD4 T-cells.
3.1. Live Vaccines
Live vaccines use attenuated viruses or recombinant antigens encoded by live virus vectors that have the capacity to replicate in host cells with attenuated pathogenicity lacking the ability to cause disease. As analogues of pathogenic viruses, they engage with the cell membrane by binding to surface receptors using epitopes similar to their native virus, thereby gaining entry into endosomal structures and the cytosol where they use the host cell machinery to replicate. Consequently, the processed antigens are presented on the cell surface by MHC-I molecules while soluble antigens expressed by replicating virus are engulfed by APCs to induce humoral immune responses (Figure 1). Hence, live vaccines induce both cellular and humoral immune responses.
In general, different scientists have reported the induction of CTL responses in fish [52, 53, 75]. Utke et al. [76] showed activation of the CTLs by viral hemorrhagic septicemia virus (VHSV) infection in rainbow trout, while we [51] recently showed activation of eomesodermin, a transcription factor involved in activation of CD8α cells in Atlantic salmon exposed to infectious pancreatic necrosis virus (IPNV). Similarly, Chang et al. [49] showed activation of CD8α cells after exposing orange spotted grouper (Epinephelus coioides) to nervous necrosis virus (NNV). Flow cytometry analysis of the spleen cells from fish exposed to NNV showed increased mean fluorescent intensity of the CD8α cells and peripheral blood leukocytes (PBLs) which were linked to increased cytotoxicity and MHC-I restriction of the sorted lymphocytes by recombinant CD8α antibodies. Several fish species have shown upregulation of MHC-I and -II molecules [49, 77–80] as well as expression of high antibody levels after exposure to viral infections [81, 82] suggesting that attenuated viruses administered as live vaccines could evoke both cellular and humoral immunity. Based on these observations, several attempts have been made to develop live viral vaccines for fish (Table 2) and some of the strategies explored this far are outlined below.
Table 2.
Live vaccines.
| Virus | Abbreviation | Fish host | Mode of attenuation | Protection | Reference |
|---|---|---|---|---|---|
| Cyprinid herpesvirus subtype 3 | CyHV-3 | Carp | Natural selection | High | [177] |
|
| |||||
| Viral hemorrhagic septicemia | VHSV | Rainbow trout | Naturally attenuated | High | [178] |
| VHSV | Rainbow trout | Naturally attenuated | High | [84] | |
| VHSV | Olive flounder | Recombinant (RG) modification | High | [179] | |
| VHSV | Rainbow trout | Recombinant (RG) modification | High | [180] | |
| VHSV | Zebra fish | Recombinant (RG) modification | High | [181] | |
|
| |||||
| Infectious hematopoietic necrosis virus | IHNV | Rainbow trout | Multiple serial passage | High | [86] |
| IHNV | Rainbow trout | Naturally attenuated | High | [83] | |
| IHNV | Rainbow trout | Natural selection | High | [182] | |
| IHNV | Rainbow trout | Recombinant (RG) modification | High | [91] | |
| IHNV | Rainbow trout | Recombinant (RG) modification | High | [183] | |
|
| |||||
| Infectious pancreatic necrosis virus | IPNV | Atlantic salmon | Avirulent strain/low dose | High | [82] |
|
| |||||
| Rock bream iridovirus | RSIV | Rock bream | Low temperature | High | [184] |
3.1.1. Natural Selection of Avirulent Strains
Roberti et al. [83] discovered a naturally attenuated mutant of infectious hematopoietic necrosis virus (IHNV) that conferred protection against IHNV in rainbow trout although the vaccine resulted in causing low level mortality after challenge. Adelmann et al. [84] used an oral vaccine against VHSV obtained from a naturally attenuated live virus selected using monoclonal antibodies. In their study, they [84] showed high expression levels of MHC-II and CD4 mRNAs. In addition, they detected antibody responses that were linked to significant protection in rainbow trout after challenge.
3.1.2. Attenuation by Serial Passages
An attenuated IHNV vaccine was developed at Oregon State University by multiple passages using a rainbow trout isolate propagated using the steelhead trout cell culture [85, 86]. The vaccine showed high protection (95%) in vaccinated Chinook salmon while mortality in control fish reached 90%. Although the vaccine was highly protective in Chinook salmon, when used in rainbow trout it showed significant mortality and as such it was stopped [87, 88]. Since the Ab strain of infectious pancreatic necrosis virus (IPNV) was found to be less virulent than the West Buxton, Sp, or Jasper strain, Dorson et al. [89] attempted to develop an attenuated strain of IPNV from the Ab strain after several passages on RTG cells. Neither the serially passaged nor the original Ab strain conferred protection.
3.1.3. Reverse Genetics
Reverse genetics has been used in fish vaccinology to generate avirulent strains for use as live vaccines. For example, recombinant IHNV having a deletion of the NV gene resulted in irreversible attenuation of the wild type virulent strain resulting in the induction of high protection levels in rainbow trout [90]. In another study, recombinant IHNV generated by replacing the NV gene with green fluorescent protein (GFP) or substituting the IHNV G-gene with the G-gene of VHSV induced heterologous protection in rainbow trout [91]. For IPNV, reverse genetics was used to generate an avirulent strain for use as a live vaccine against the wild type strain by substituting amino acids on positions 217 and 221 of the VP2 capsid [92]. These studies showed that the strain encoding the T217A221 motif caused high mortality in Atlantic salmon while the strain encoding the P217T221 motif was avirulent and linked to subclinical infections [93]. Infecting Atlantic salmon with high and low virulent strains at a nonpermissive physiological state (presmoltification stage) did not result in mortality. When the vaccinated fish were challenged at smolt stage (permissive) the avirulent strain was less immunogenic than the virulent vaccine strain [82]. In addition, we observed that the avirulent strain reverted to virulence under stress conditions [37, 94]. In general, the fear of reversion to virulence has been the major hindrance for the licensure of live vaccines in aquaculture.
3.2. DNA Vaccines
The strategy of DNA vaccination is based on the principle that the encoded immunogenic protein is injected into the muscle or other tissues where it enters the host cells and directs the synthesis of its polypeptide antigen from the plasmid vector. Once transfected into host cells, transcribed antigens replicate in the cytosol using the endogenous pathway while soluble or secreted antigens are phagocytized by APC and gain access into the exogenous pathway (Figure 1). In principle, DNA vaccines result in the in vivo synthesis of antigenic proteins using the host cell machinery in a manner identical to natural virus infection in the case of DNA vaccines made for viral diseases. This culminates into antigenic proteins expressed by plasmid DNA gaining access to both the exogenous and endogenous pathways in the activation of both humoral and cellular mediated immune responses.
Boudinot et al. [95] demonstrated the intracellular delivery of the plasmid DNA encoding the recombinant G protein of VHSV inside the muscle cells of vaccinated rainbow trout. Intracellular detection of the G-protein was shown up to 45 days at the injection sites. Transcription of the G-protein was demonstrated by detection of mRNA in muscle tissue extracts, which was linked to expression of high antibody and MHC-II mRNAs levels. In another study, Utke et al. [96] showed activation of the CTLs following immunization using the G-protein of VHSV in rainbow trout. In their study, they [96] used PBLs collected from fish immunized with a DNA vaccine encoding the recombinant G -protein of VHSV and showed that PBLs from vaccinated fish killed the VHSV MHC-I matched RTG-2 cells indicating that the G-proteins had the capacity to induce CTL responses in vaccinated fish. They also showed the homing of leukocytes to the injection site suggesting that cells expressing the recombinant G-protein had a chemoattractant effect. This observation was recently supported by Castro et al. [97] who showed that B-lymphocytes, both IgM+ and IgT+ cells, represent one of the major cell types infiltrating the injection sites expressing the G-protein of VHSV. In their study, they showed upregulation of CXCR3B, a receptor for CXCL11, together with CK5B and CK6 chemokines, which could play chemotactic roles in the early recruitment of B-cells at the injection sites. Put together, these studies show that the intracellular expression of proteins transcribed from DNA vaccines in fish cells leads to homing of leukocytes and B-cells to injection sites with possible involvements of chemoattractant chemokines. Further, these studies suggest that antigens delivered by this endogenous route evoke both the humoral and cellular mediated immune responses in vaccinated fish.
Finally, it is important to point out that immunization using DNA vaccines exhibits many advantages over the live and inactivated vaccines. Intracellular synthesis of the antigenic proteins poses no danger of reversion to virulence and does not require inactivation of viruses using toxic substances. High expression levels of humoral and cellular responses can be achieved at low doses as shown by Corbeil et al. [98] that nanogram quantities of a DNA vaccine protected rainbow trout against IHNV infection after challenge. In addition, intracellular synthesized antigens tend to fold in their native conformation and correctly glycosylated displaying the neutralizing epitopes in a similar pattern to the native virus [99]. In terms of genetic engineering, combinational approaches can easily be adopted. For example, the use of molecularly encoded cytokine adjuvants like IL-2 in DNA engineered vaccines has shown the ability to enhance DNA delivery and increase the duration and magnitude of plasmid DNA expression in vivo [100]. Jimenez et al. [101] coinjected recombinant IL-8 with plasmid DNA encoding the G-protein of VHSV in rainbow trout and showed massive infiltration of neutrophils at the injection site linked to upregulation of proinflammatory cytokines. Table 3 shows the DNA vaccines explored for use in fish, this far, of which only the DNA vaccine for IHNV has been licensed in Canada (Novartis Ltd.).
Table 3.
DNA vaccines explored in fish.
| Classification | Virus family | Pathogen | Abbreviation | Antigen | Protection∗ | Reference |
|---|---|---|---|---|---|---|
| DNA viruses | Iridovirus | Red sea bream iridovirus | RSIV | Major capsid | Moderate | [185] |
| Herpesviridae | Channel catfish virus (CCV) | CCV | ORF 6&59 | Low | [186] | |
|
| ||||||
| RNA virus | Rhabdoviridae | Viral hemorrhagic septicemia virus | VHSV | G | High | [95] |
| Rhabdoviridae | Infectious hematopoietic necrosis virus | IHNV | G | High | [98, 187, 188] | |
| Rhabdoviridae | Spring viremia of carp virus | SVCV | G | High | [189, 190] | |
| Rhabdoviridae | Hirame rhabdovirus | HRV | G | High | [191] | |
| Birnaviridae | Infectious pancreatic necrosis virus | IPNV | SegA/VP2 | Moderate | [103, 140] | |
| Orthomyxoviridae | Infectious salmon anemia virus | ISAV | HE | Moderate | [192] | |
| Togaviridae | Salmon alphavirus subtype 3 | SAV-3 | E2 | Moderate | [157] | |
| Nodaviridae | Atlantic halibut nodavirus | ANHV | Capsid | Low | [193] | |
∗Protection was determined by postchallenge relative percent survival (RPS).
3.3. Fusion Protein Vaccines
This mode of antigen delivery which has been referred to as “the first class ticket to induction of MHC-I responses” relies on receptor mediated internalization of viral antigens to the ER followed by retrograde translocation into the cytosol [102]. Only a few studies have explored this delivery system in fish vaccinology, this far [103, 104]. Li-Li et al. [104] constructed a fusion protein vaccine made by fusing the VP2-VP3 polyprotein of IPNV with the exotoxin of Lactobacillus casei, which resulted in reduced viral loads in vaccinated rainbow trout after challenge while in our group we constructed a fusion protein vaccine made by fusing the VP2 of IPNV with the Pseudomonas aeruginosa exotoxin A (EP) [103]. The exact mechanisms in which viral antigens are translocated into the cytosol are dependent on three bacterial proteins as illustrated from the PE fusion protein in Figure 2. The PE protein has three functional domains, namely, the receptor binding domain-I, transmembrane targeting domain-II, and the toxic moiety domain-III. Domain-I binds to the α2-macroglobulin receptor on the cell surface. After binding to domain-I the ligand-receptor complex is internalized through the receptor mediated endocytosis. After enzymatic cleavage by the protease furin in the endosome, the protein fragment encoding domains-II and -III is delivered into the Golgi by the ER retrograde transport and further into the cytosol using domain-II, which is responsible for transmembrane translocation of the toxin proteins into the cytoplasm. As shown in Figure 2, domain-III, which is toxic to cells, is eliminated and is replaced with the immunogenic protein (VP2) of IPNV bound to the KDEL signaling peptide. The purpose of including the KDEL signaling peptide in the final construct (PE-VP2-KDEL) is that it enables the binding of the whole construct to the Golgi membrane KDEL-receptor. Once bound to the Golgi membrane receptor, the ligand-receptor complex is packaged into vesicles for retrograde transport back to the ER where processed peptides are packaged on MHC-I molecules for presentation on the cell surface. In our studies, we employed the PE-VP2-KDEL fusion protein to deliver the VP2 immunogenic protein of IPNV intracellularly as a vaccine. Although we did not assess the CTL responses induced by this antigen delivery system, our findings show that these vaccines were able to induce a low level antibody response suggesting that antigens delivered using this method could gain access to induction of humoral responses in vaccinated fish. However, there is a need for detailed investigation to determine the role of CTL responses induced by this mode of antigen delivery in vaccinated fish.
Figure 2.
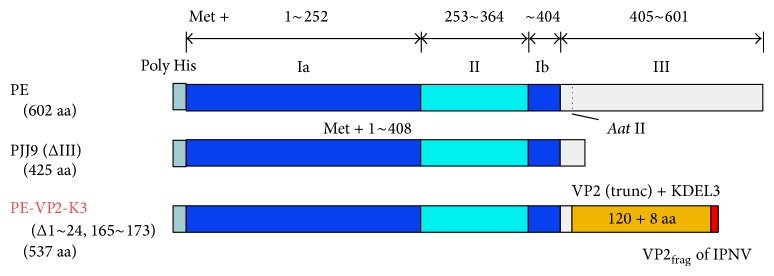
The fusion of the VP2- of IPNV to the Pseudomonas aeruginosa exotoxin A (PE). PE shows a 602 aa exotoxin for Pseudomonas aeruginosa made of three domains. Domain Ia (blue) located on 1–252 aa followed by domain II (green) on the location 253–364 aa, domain Ib (blue) located on 365–404 aa, and finally domain III on the extreme end located on 405–601 aa. PJJ9 (ΔIII) is a 425 aa long intermediate phase in which the toxic moiety of domain III has been cleaved. PE-VP2-KE is the final construct in which the toxic moiety of domain III has been replaced with the truncated VP2 (yellow) immunogenic protein of infectious pancreatic necrosis virus (IPNV). Note that the VP2 is attached to the KDEL3 signaling peptide (red).
3.4. Nanoparticle Vaccines
Polymeric nanoparticles formulated from biodegradable polymers have been widely explored as carriers for controlled delivery of vaccine antigens [105, 106]. This system can potentially deliver antigens to the desired location at predetermined rates and durations to generate an optimal immune response [107]. For example, Tian et al. [108–110] and Zheng et al. [111] showed that lymphocytic disease virus (LCDV) encapsulated in particles sustained a much longer release of the DNA antigen than naked DNA injected in Japanese flounder. In addition, carriers protect the antigen from degradation until release as shown by Rajeshkumar et al. [112] that encapsulated DNA antigens were protected from degradation by DNAase for vaccines used in the Asian sea bass (Lates calcarifer).
To deliver the antigens into host cells, nanoparticle materials are internalized by endocytosis [113]. To deliver the antigens into the cytosol, the release of antigens from the acidic endosomes requires membrane disruptive agents, which release the internalized proteins into the cytosol. Therefore, encapsulation carriers should include membrane penetrating peptides and polymers that disrupt the membranes when the pH declines in the endosomes. For example, Standley et al. [114] made acid degradable nanoparticles, which were designed to release encapsulated proteins in a pH-dependent manner. In their study, they made nanoparticles that were stable at pH 7.4 but quickly degradable at pH 5.0 in the acidic endosomal environment enabling the release of antigens into the cytosol, ultimately resulting in upregulation of MHC-I. Another method explored is the use of amphiphilic polymers [115–117], which also have pH-dependent membrane disruptive properties protonated at the endosomal pH range [115, 118]. Upon reduction of the endosomal pH, these particles increase their hydrophobicity to facilitate the disruption and penetration of the endosomal membranes culminating in the release of antigens in the cytosol. These amphiphilic polymers have been shown to increase CD8+ responses and to improve vaccine potency [119]. In summary, these studies show that nanoparticle antigen delivery systems can be designed to deliver antigens through the intra- or extracellular routes to evoke immune responses linked to the MHC-I or -II pathways.
Studies in higher vertebrates have shown that APCs easily carry out phagocytosis of nanoparticles and microparticles between 150 nm and 4.5 μm [120, 121] with the optimal size for phagocytosis being 500 nm [122] while monocytes have been shown to easily phagocytose nanoparticles >100 nm [123]. And, as pointed out by Gutierro et al. [124], nanoparticles that encapsulate antigens resemble pathogens in terms of their uptake into host cells by mirroring the route of pathogen uptake and the immune response triggered after nanoparticle uptake. Fehr et al. [125] and He et al. [120] have also pointed out that nanoparticles can also be used to carry antigens on their surface which would serve as a good stimulant for the induction of B-cell responses. Although antigen delivery using nanoparticle vaccines has been well studied in higher vertebrates, indications are that fish cells use similar mechanisms of antigen uptake from nanoparticle based vaccines. For example, Ruyra et al. [126] showed entry of liposome-based nanoparticles in zebrafish hepatocytes and trout macrophages by endocytosis. Upon entry, the nanoparticle laden cells initiated specific proinflammatory responses while Fredriksen and Grip [127] showed intracellular cytoplasmic localization of polylactic-coglycolic acid (PLGA) nanoparticles in TO-cells [24]. These findings support earlier observations, which showed that because PLGA particles are less hydrophilic than alginates, they are easily incorporated into host cells, which makes them suitable vehicles for delivering antigens into intracellular compartments [128–131]. Recently, we [103] used PLGA nanoparticles to deliver inactivated whole viral particles of IPNV as a vaccine, which expressed low antibody levels comparable to those induced by inactivated whole virus (IWV) vaccines suggesting that delivery of antigens using PLGA nanoparticles has the ability to induce humoral immune responses in vaccinated fish. Although there are limited studies that categorically demonstrate the intracellular delivery of nanoparticle based vaccines in fish cells, Rajeshkumar et al. [112] were able to induce low level cytotoxicity (tested in vitro) using chitosan nanoparticle vaccines in Asian sea bass vaccinated against vibriosis (Listonella anguillarum). Table 4 shows that only a few studies have been carried out using nanoparticle based technologies to administer viral antigens in fish. In general, indications show that nanoparticle based vaccines have the potential to deliver antigens into different host cell compartments and thus that they can induce cellular and humoral immune responses in vaccinated fish. The efficacy of nanoparticle vaccines needs to be improved and explored in more detail.
Table 4.
Nanoparticle vaccines.
| Virus | Virus | Fish host | Admin | Antigen | Type | Protection∗ | Reference |
|---|---|---|---|---|---|---|---|
| Lymphocytic virus | LCDV | Japanese flounder | Oral | Plasmid DNA | PLGA | High | [194] |
| Infectious hematopoietic necrosis virus | IHNV | Rainbow trout | Oral | Plasmid DNA | PLGA | Low | [195] |
| Infectious pancreatic necrosis virus | IPNV | Atlantic salmon | Injection | IWV | PLGA | Low | [127] |
| Infectious pancreatic necrosis virus | IPNV | Atlantic salmon | Injection | IWV | PLGA | Low | [103] |
| White syndromes spot virus | WSSV | Shrimp | Oral | Plasmid DNA | Chitosan | ND | [112] |
| White syndromes spot virus | WSSV | Shrimp | Oral | Plasmid DNA | Chitosan | ND | [196] |
∗Protection was determined by postchallenge relative percent survival (RPS). ND = not done (not tested for protection).
4. Extracellular Antigen Delivery Systems
This approach involves antigen delivery systems that administer viral antigens into the extracellular compartments using the exogenous pathway (Figure 1).
4.1. Inactivated Whole Viral Vaccines
This mode of antigen delivery ensures that the antigenic protein is preserved in its native structure while the virus is rendered nonreplicative using chemical or physical methods. Given that inactivated whole virus (IWV) vaccines are nonreplicative, it follows that their antigens enter the host cells by the exogenous route and their processed peptides are presented to CD4 cells via the MHC-II pathway. And, as such, several studies have shown upregulation of MHC-II genes in response to vaccination using IWV vaccines [77, 132]. In terms of CD4 T-cell differentiation, IWV vaccines have been shown to predominantly activate genes linked to the T-helper 2 (Th2) responses [133]. For example, we showed upregulation of GATA-3, a transcription factor linked to activation of naïve CD4 cells into Th2 responses, when genes linked to activation of Th1 and CD8 T-cell responses were downregulated [51]. In this study, we showed a high correlation between GATA-3 and antibody levels expressed against IPNV [51]. In terms of antibody responses, IWV vaccines [134] have been linked to high expression levels of IgM and IgT in vaccinated fish [135]. In our studies, we showed a high correlation between postchallenge reduction of mortality and systemic IgM levels, suggesting IgM levels could serve as a correlate of protection for IWV vaccines [81]. Overall, these studies strongly suggest that IWV vaccines are to be considered as exogenous antigens, mainly inducing humoral immune responses. Table 5 shows the major IWV vaccines explored in fish vaccinology this far.
Table 5.
Inactivated whole virus vaccines explored in fish.
| Pathogen | Abbreviation | Virus family | Fish species | Protection | Reference |
|---|---|---|---|---|---|
| Viral hemorrhagic septicemia virus | VHSV | Rhabdoviridae | Rainbow trout | High∗ | [197] |
| Infectious hematopoietic necrosis virus | IHNV | Rhabdoviridae | Rainbow trout | High∗ | [198] |
| Spring viremia of carp virus | SVCV | Rhabdoviridae | Carp | High∗ | [199] |
| Infectious pancreatic necrosis virus | IPNV | Birnaviridae | Atlantic salmon | High∗ | [81, 103] |
| Salmon pancreas disease virus | SPDV | Togaviridae | Rainbow trout | High2 | [200] |
| Red seabream iridovirus | RSIV | Iridovirus | Sea bass | High∗ | [201] |
| Singapore grouper iridovirus | SGIV | Iridovirus | Grouper | High∗ | [202] |
| Channel catfish virus | CCV | Herpesviridae | Catfish | Moderate/high∗ | [203] |
| Cyprinid herpesvirus subtype-3 | CyHV-3 | Herpesviridae | Carp | High∗ | [204] |
| Nervous necrosis virus | NNV | Betanodaviridae | Grouper | High1 | [205] |
| Nervous necrosis virus | NNV | Betanodaviridae | Sea bass | High1 | [206] |
| Salmon anemia virus | SAV | Orthomyxoviridae | Atlantic salmon | High∗ | [207] |
Protection measured by ∗relative percent survival (RPS), 1protection against postchallenge virus infection, and 2pathology.
4.2. Subunit Vaccines
The basic principle for this vaccination strategy is that the gene encoding the antigenic proteins is isolated from the native virus and transferred into a heterologous vector that is nonpathogenic for propagation. Table 6 shows the antigenic proteins identified for the major fish viral diseases and the different expression vectors used for propagation. In the case of VHSV and IHNV, production of subunit vaccines has focused on cloning the G-protein into heterologous vectors [136–138] while, for viruses such as IPNV, the strategy has been to clone the entire outer capsid encoding the protective epitopes, instead of protein segments coding the neutralizing epitopes in heterologous vectors [103, 139–141]. Subunit vaccines are nonreplicative and are delivered exogenously to host cells by the extracellular route (Figure 1). Similar to IWV vaccines, subunit vaccines induce humoral immune responses and upregulation of MHC-II genes [139, 142], which is consistent with observations in higher vertebrates in which it has been shown that immune responses induced by subunit vaccines are mainly dependent on the MHC-II pathway and that they elicit antibody responses [143]. Øvergård et al. [144, 145] showed a high correlation between reduction of viral RNA and activation of CD4 markers in Atlantic halibut (Hippoglossus hippoglossus L.) immunized using a subunit vaccine for nodavirus. Although cross presentation of exogenously processed peptides from endosomes into the cytosol has been reported in higher vertebrates [3, 4], there is no study demonstrating cross presentation of peptides processed from endosomes into the cytosol for subunit vaccines in fish.
Table 6.
Subunit vaccines.
| Virus | Abbreviation | Protein | Vector | Efficacy | Fish species | Reference |
|---|---|---|---|---|---|---|
| Infectious pancreatic necrosis virus | IPNV | VP2 | Escherichia coli | Low | Atlantic salmon | [103] |
| IPNV | VP2 | Yeast cells | Low | Rainbow trout | [139] | |
| IPNV | VP2/3 | Lactobacillus casei | Low | Rainbow trout | [208] | |
| IPNV | VP2 | Baculovirus | Low | Atlantic salmon | [141] | |
| IPNV | VP2 | Semliki Forest virus | N/A | CHSE cells | [209] | |
|
| ||||||
| Viral hemorrhagic septicemia virus | VHSV | G | Saccharomyces cerevisiae | — | Rainbow trout | [210] |
| VHSV | G | Escherichia coli | High | Rainbow trout | [197] | |
| VHSV | G | Yersinia ruckeri | Moderate-high | Rainbow trout | [211] | |
| VHSV | G | Baculovirus | high | Rainbow trout | [142] | |
| VHSV | G | Edwardsiella tarda | Moderate | Olive flounder | [212] | |
|
| ||||||
| Infectious hematopoietic necrosis virus | IHNV | G | Escherichia coli | Moderate | Rainbow trout | [138] |
| IHNV | G | Caulobacter crescentus | Low | Rainbow trout | [213] | |
| IHNV | G | Baculovirus | Moderate | Rainbow trout | [214] | |
| IHNV | G | Aeromonas salmonicida | Moderate-high | Rainbow trout | [137] | |
|
| ||||||
| GCRV | VP4 | Escherichia coli | Moderate-high | Grass carp | [215] | |
| GCRV | VP5, VP7 | Escherichia coli | Moderate | Grass carp | [216] | |
|
| ||||||
| VER | Capsid | Escherichia coli | Low | Atlantic halibut | [217] | |
4.3. Virus-Like and Subviral Particle Vaccines
Structural proteins of most viruses self-assemble to forms capsids in different expression systems that resemble the native virus structure in size and morphology and, hence, they are referred to as “virus-like particles” (VLPs). For example, Liu et al. [146] made VLPs of nodavirus expressed in E. coli or Spodoptera frugiperda (Sf21) insect cells that formed small, nonenveloped T = 3 quasi-symmetric particles [147, 148]. They showed that the capsid of malabaricus grouper nervous necrosis virus (MGNNV) spontaneously self-assembled into VLPs when expressed in Sf21 cells infected with a recombinant baculovirus [148]. These VLPs were indistinguishable from the native virus particles by electron microscopy and the 3D structure of the VLPs was resolved at 2.3 nm by cryomicroscopy [147]. Similarly, Fang et al. [149] produced VLPs that were devoid of the nucleoprotein but resembled the outer capsid of the native grass carp reovirus (GCRV). However, in some cases VLPs are formed from replication of surface particulate components that do not form the entire capsid, but they contain elements of the outer capsid that are immunogenic. These protein structures are called “subviral particles” (SVPs). Both VLPs and SVPs do not contain the nucleoprotein and as such they are nonreplicative. Table 7 shows the VLPs, SVPs, and immature virus particles (IVPs) made from different fish viruses. As shown in Table 7, different expression systems were used to make VLPs [139], SVPs [150], and IVPs [151] for IPNV.
Table 7.
Subviral, immature, and virus-like particles used for fish vaccines.
| Virus | Classification | Protein | Cells/vector | Fish host | Protection | Reference |
|---|---|---|---|---|---|---|
| NNV | VLP | Capsid | Escherichia coli | Orange spotted grouper | ND∗ | [153] |
| NNV | VLP | Capsid | Saccharomyces cerevisiae | Red spotted grouper | ND∗ | [218] |
| IPNV | VLP | VP2 | Baculovirus/insect larvae | Rainbow trout | Low | [219] |
| IPNV | IVP | VP2 | CHSE cells | Rainbow trout | ND∗ | [151] |
| IPNV | SVP | VP2 | Yeast cells | Rainbow trout | Low | [150] |
| IPNV | SVP | VP2 | Yeast cells | Rainbow trout | Low | [139] |
| GCRV | SVP | Capsid | Ctenopharyngodon idellus | Grass carp | ND∗ | [149, 220] |
| VNNV | VLP | Capsid | Baculovirus | European sea bass | High | [221] |
| NNV | VLP | Capsid | Baculovirus | Orange spotted grouper | High | [148] |
| NNV | VLP | Capsid | Escherichia coli | Dragon and Malabar grouper | ND∗ | [146] |
| VHSV | Peptide | Nucleoprotein | Rainbow trout | ND∗ | [136] |
ND = Note done (No protection studies carried out).
∗Only immune expression studies were carried out by enzyme linked immunosorbent assay (ELISA) or gene expression.
Given the similarity to their native viral capsids, VLPs provide an excellent platform for displaying viral epitopes [146, 152]. This property was demonstrated by Lai et al. [153] who produced VLPs for NNV expressed in E. coli and showed that NNV failed to infect the Asian sea bass cells that were exposed to the VLPs prior to infection, suggesting that the cell surface receptors were occupied by the VLP-epitopes blocking the wild type virus from entering the cells and thereby protected the cells from developing cytopathic effect (CPE) while control cells not exposed to VLPs developed full CPE. Liu et al. [146] showed that VLPs generated from NNV induced high antibody responses that lasted for more than five months, similar to those produced by the native wild type virus, which were correlated with long-term protection in vaccinated orange spotted grouper. Similarly, Lai et al. [153] produced VLPs in E. coli for NNV that expressed high antibody levels, which were correlated with IgM, MHC-II, and CD4 levels in vaccinated fish. These observations suggest VLPs induce the expression of CD4 responses through the MHC-II pathways in a similar pattern to those induced by subunit vaccines in Mammalia [154].
5. General Discussion and Conclusion
The most explored strategies for the delivery of antigens using the intracellular route in fish vaccinology involve the use of live and DNA vaccines. The use of DNA vaccines in fish has undergone intense investigation in the last decades as a substitute of replicative antigens for live vaccines. Although factors leading to higher performance of DNA vaccines for rhabdoviruses compared to other fish viral families have not been elucidated, similar observations seen in higher vertebrates show that DNA vaccines for rhabdoviruses are more protective [155, 156] than some of the DNA vaccines for other viral families. In general, replicative vaccines delivered via the intracellular route have been linked to activation of cellular and humoral immune responses in vaccinated fish, which makes these vaccines be more protective than nonreplicative vaccines delivered by the extracellular route.
For antigens administered by the extracellular route, several antigen delivery strategies have been explored in fish vaccinology, which include the use of IWV, subunit, SVP, VLP, and IMP vaccines. In general, all exogenous antigens induce humoral immune responses. In terms of cellular immunity, exogenous antigens were linked to expression of MHC-II and CD4 genes. However, new innovations such as the use of fusion protein and nanoparticles vaccines having the potential to deliver nonreplicative antigens into the cytosol are likely to induce CTL responses in vaccinated fish. The use of nanoparticle vaccines has attracted a lot of interest in the delivery of oral vaccines for fish production systems that require a boost vaccination when fish have been transferred in cages to the sea after prime immunization using parenteral vaccines at the freshwater stage. In general, IWV vaccines are superior to subunit vaccines given that they produce high antibody levels, which correlate with protection in vaccinated fish [81, 157]. In addition, these vaccines have been shown to activate the expression of CD4 and Th2 genes that correlate with high antibody levels consolidating the common notion that exogenous antigens stimulate humoral immune responses orchestrated by Th2 cytokines [154].
Although we did not review the role of APC prime/activated B-cells in antigen uptake and presentation to cells of the adaptive immune system in detail given the limited studies carried out on this topic in fish vaccinology, we can conclude that the different antigen delivery systems explored in fish this far deliver their antigens into the intra- and extracellular compartments and that they activate either the cellular or humoral immune response or both depending on the route of antigen delivery. As pointed out by Howarth and Elliot [158], the most protective vaccines are those that stimulate both the CD4+ and CD8+ T-cells responses and, as such, replicative vaccines such as the live and DNA vaccines that stimulate both the MHC-I and -II pathways are likely to produce better protection in fish (Table 8). So far only the IHNV-DNA vaccine for use in Atlantic salmon in Canada is the only one licensed while live viral vaccines are feared to revert to virulence. Hence, the use of IWV vaccines which accounts for the largest proportion of licensed vaccines is likely to continue dominating the vaccine industry in aquaculture [81, 157]. Therefore, the search for better antigen delivery systems that stimulate both CD4+ and CD8+ responses that have the potential to induce long-lasting protective immunity has to continue. Overall, we anticipate that the synopsis of different antigen delivery systems presented here will shed new insights into the limitations and successes of the current immunization strategies used in fish vaccinology.
Table 8.
Comparison of the intra- and extracellular antigen processing parameters.
| Parameters | Intracellular antigen delivery | Extracellular antigen delivery |
|---|---|---|
| Vaccine types | ||
| Viability of antigens | Mostly replicative | Nonreplicative |
| Examples of vaccine types | Live and DNA vaccines | IWV vaccines, subunit vaccines |
| Antigen uptake and presentation | ||
| Pathway of uptake into host cells | Endogenous pathway | Exogenous pathway |
| Antigen uptake and processing | Penetration of host cell membrane | Phagocytosis by APCs |
| Site of antigen deposition | Cytoplasm | Endosome/phagosome |
| Antigen presenting molecules | MHC-I and MHC-II | MHC-II |
| Mode of antigen processing | Proteosomal degradation | Endosomal degradation |
| Adaptive immunity | ||
| Type of immune response induced | Cellular and humoral immune responses | Humoral immune responses |
| Cell types involved | B- and T-lymphocytes | B-lymphocytes |
| T-cell subtypes | CD4 and CD8 T-cells | CD4 T-cells |
| Effector molecules/cells | CTL (cellular) and antibodies (humoral) | Antibodies |
| Effector mechanisms | CTL killing of virus infected cells | Antibody-neutralization of virus |
| Antibody-neutralization of virus |
Acknowledgments
Work leading to preparation of this paper was funded by the TARGETFISH, Targeted Disease Prophylaxis in European Fish Farming, EU Grant 311993. The authors are grateful to Dr. TY Kuo for making the PE-VP2-KDEL fusion protein construct (Figure 2) described in Section 3.3 in the paper and Dr. Inderjit Majara for his part in the PE-VP2-KDEL fusion protein verification studies. The authors are also grateful to Dr. Ida Skaar for the illustrative drawing of Figure 2.
Conflict of Interests
Authors declare no conflict of interests.
Authors' Contribution
Both Hetron Mweemba Munang'andu and Øystein Evensen participated in collecting the information leading to preparation of this paper. Both authors read the paper and approved its publication.
References
- 1.Bishop G. A., Haxhinasto S. A., Stunz L. L., Hostager B. S. Antigen-specific B-lymphocyte activation. Critical Reviews in Immunology. 2003;23(3):149–197. doi: 10.1615/CritRevImmunol.v23.i3.10. [DOI] [PubMed] [Google Scholar]
- 2.Germain R. N. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76(2):287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 3.Schuette V., Burgdorf S. The ins-and-outs of endosomal antigens for cross-presentation. Current Opinion in Immunology. 2014;26(1):63–68. doi: 10.1016/j.coi.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Segura E., Villadangos J. A. A modular and combinatorial view of the antigen cross-presentation pathway in dendritic cells. Traffic. 2011;12(12):1677–1685. doi: 10.1111/j.1600-0854.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 5.Ciechanover A. Intracellular protein degradation: from a vague idea, through the lysosome and the ubiquitin-proteasome system, and onto human diseases and drug targeting (Nobel Lecture) Angewandte Chemie - International Edition. 2005;44(37):5944–5967. doi: 10.1002/anie.200501428. [DOI] [PubMed] [Google Scholar]
- 6.Uebel S., Tampé R. Specificity of the proteasome and the TAP transporter. Current Opinion in Immunology. 1999;11(2):203–208. doi: 10.1016/S0952-7915(99)80034-X. [DOI] [PubMed] [Google Scholar]
- 7.Koopmann J.-O., Post M., Neefjes J. J., Hämmerling G. J., Momburg F. Translocation of long peptides by transporters associated with antigen processing (TAP) European Journal of Immunology. 1996;26(8):1720–1728. doi: 10.1002/eji.1830260809. [DOI] [PubMed] [Google Scholar]
- 8.Koopmann J. O., Hämmerling G. J., Momburg F. Generation, intracellular transport and loading of peptides associated with MHC class I molecules. Current Opinion in Immunology. 1997;9(1):80–88. doi: 10.1016/s0952-7915(97)80163-x. [DOI] [PubMed] [Google Scholar]
- 9.Pamer E., Cresswell P. Mechanisms of MHC class I—restricted antigen processing. Annual Review of Immunology. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 10.Mizushima N., Klionsky D. J. Protein turnover via autophagy: implications for metabolism. Annual Review of Nutrition. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 11.Münz C. Antigen processing via autophagy—not only for MHC class II presentation anymore? Current Opinion in Immunology. 2010;22(1):89–93. doi: 10.1016/j.coi.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haugland G. T., Jordal A. E. O., Wergeland H. I. Characterization of small, mononuclear blood cells from salmon having high phagocytic capacity and ability to differentiate into dendritic like cells. PLoS ONE. 2012;7(11) doi: 10.1371/journal.pone.0049260.e49260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iliev D. B., Thim H., Lagos L., Olsen R., Jørgensen J. B. Homing of antigen-presenting cells in head kidney and spleen—salmon head kidney hosts diverse APC types. Frontiers in Immunology. 2013;4, article 137 doi: 10.3389/fimmu.2013.00137.Article 137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lugo-Villarino G., Balla K. M., Stachura D. L., Bañuelos K., Werneck M. B. F., Traver D. Identification of dendritic antigen-presenting cells in the zebrafish. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(36):15850–15855. doi: 10.1073/pnas.1000494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fearon D. T., Locksley R. M. The instructive role of innate immunity in the acquired immune response. Science. 1996;272(5258):50–54. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 16.Mogensen T. H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical Microbiology Reviews. 2009;22(2):240–273. doi: 10.1128/cmr.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.MacKenzie S., Planas J. V., Goetz F. W. LPS-stimulated expression of a tumor necrosis factor-alpha mRNA in primary trout monocytes and in vitro differentiated macrophages. Developmental and Comparative Immunology. 2003;27(5):393–400. doi: 10.1016/s0145-305x(02)00135-0. [DOI] [PubMed] [Google Scholar]
- 19.Stafford J. L., McLauchlan P. E., Secombes C. J., Ellis A. E., Belosevic M. Generation of primary monocyte-like cultures from rainbow trout head kidney leukocytes. Developmental and Comparative Immunology. 2001;25(5-6):447–459. doi: 10.1016/S0145-305X(01)00015-5. [DOI] [PubMed] [Google Scholar]
- 20.Goetz F. W., Iliev D. B., McCauley L. A. R., et al. Analysis of genes isolated from lipopolysaccharide-stimulated rainbow trout (Oncorhynchus mykiss) macrophages. Molecular Immunology. 2004;41(12):1199–1210. doi: 10.1016/j.molimm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Iliev D. B., Liarte C. Q., MacKenzie S., Goetz F. W. Activation of rainbow trout (Oncorhynchus mykiss) mononuclear phagocytes by different pathogen associated molecular pattern (PAMP) bearing agents. Molecular Immunology. 2005;42(10):1215–1223. doi: 10.1016/j.molimm.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 22.Iliev D. B., Goetz G. W., MacKenzie S., Planas J. V., Goetz F. W. Pathogen-associated gene expression profiles in rainbow trout macrophages. Comparative Biochemistry and Physiology Part D: Genomics & Proteomics. 2006;1(4):416–422. doi: 10.1016/j.cbd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Pettersen E. F., Ingerslev H.-C., Stavang V., Egenberg M., Wergeland H. I. A highly phagocytic cell line TO from Atlantic salmon is CD83 positive and M-CSFR negative, indicating a dendritic-like cell type. Fish & Shellfish Immunology. 2008;25(6):809–819. doi: 10.1016/j.fsi.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Wergeland H. I., Jakobsen R. A. A salmonid cell line (TO) for production of infectious salmon anaemia virus (ISAV) Diseases of Aquatic Organisms. 2001;44(3):183–190. doi: 10.3354/dao044183. [DOI] [PubMed] [Google Scholar]
- 25.Xu C., Evensen Ø., Munang’andu H. M. De novo assembly and transcriptome analysis of Atlantic salmon macrophage/dendritic-like TO cells following type I IFN treatment and Salmonid alphavirus subtype-3 infection. BMC Genomics. 2015;16(1) doi: 10.1186/s12864-015-1302-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bassity E., Clark T. G. Functional identification of dendritic cells in the teleost model, rainbow trout (Oncorhynchus mykiss) PLoS ONE. 2012;7(3) doi: 10.1371/journal.pone.0033196.e33196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunyer J. O. Evolutionary and functional relationships of B cells from fish and mammals: insights into their novel roles in phagocytosis and presentation of particulate antigen. Infectious Disorders—Drug Targets. 2012;12(3):200–212. doi: 10.2174/187152612800564419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohta Y., Haliniewski D. E., Hansen J., Flajnik M. F. Isolation of transporter associated with antigen processing genes, TAP1 and TAP2, from the horned shark Heterodontus francisci . Immunogenetics. 1999;49(11-12):981–986. doi: 10.1007/s002510050582. [DOI] [PubMed] [Google Scholar]
- 29.Ohta Y., Powis S. J., Coadwell W. J., et al. Identification and genetic mapping of Xenopus TAP2 genes. Immunogenetics. 1999;49(3):171–182. doi: 10.1007/s002510050478. [DOI] [PubMed] [Google Scholar]
- 30.Hansen J. D., Strassburger P., Thorgaard G. H., Young W. P., Du Pasquier L. Expression, linkage, and polymorphism of MHC-related genes in rainbow trout, Oncorhynchus mykiss . Journal of Immunology. 1999;163(2):774–786. [PubMed] [Google Scholar]
- 31.Grimholt U. Transport-associated proteins in Atlantic salmon (Salmo salar) Immunogenetics. 1997;46(3):213–221. doi: 10.1007/s002510050264. [DOI] [PubMed] [Google Scholar]
- 32.von Andrian U. H., Mempel T. R. Homing and cellular traffic in lymph nodes. Nature Reviews Immunology. 2003;3(11):867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 33.Press C. M., Evensen Ø. The morphology of the immune system in teleost fishes. Fish and Shellfish Immunology. 1999;9(4):309–318. doi: 10.1006/fsim.1998.0181. [DOI] [Google Scholar]
- 34.Mulero I., Pilar Sepulcre M., Roca F. J., Meseguer J., García-Ayala A., Mulero V. Characterization of macrophages from the bony fish gilthead seabream using an antibody against the macrophage colony-stimulating factor receptor. Developmental and Comparative Immunology. 2008;32(10):1151–1159. doi: 10.1016/j.dci.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 35.Peleteiro M. C., Richards R. H. Phagocytic-cells in the epidermis of rainbow-trout, Salmo-Gairdneri Richardson. Journal of Fish Diseases. 1990;13(3):225–232. [Google Scholar]
- 36.Fuglem B., Jirillo E., Bjerkås I., et al. Antigen-sampling cells in the salmonid intestinal epithelium. Developmental and Comparative Immunology. 2010;34(7):768–774. doi: 10.1016/j.dci.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Rombout J. H. W. M., Lamers C. H. J., Helfrich M. H., Dekker A., Taverne-Thiele J. J. Uptake and transport of intact macromolecules in the intestinal epithelium of carp (Cyprinus carpio L.) and the possible immunological implications. Cell and Tissue Research. 1985;239(3):519–530. doi: 10.1007/bf00219230. [DOI] [PubMed] [Google Scholar]
- 38.Alejo A., Tafalla C. Chemokines in teleost fish species. Developmental and Comparative Immunology. 2011;35(12):1215–1222. doi: 10.1016/j.dci.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Laing K. J., Secombes C. J. Chemokines. Developmental and Comparative Immunology. 2004;28(5):443–460. doi: 10.1016/j.dci.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Secombes C. J., Wang T., Hong S., et al. Cytokines and innate immunity of fish. Developmental and Comparative Immunology. 2001;25(8-9):713–723. doi: 10.1016/s0145-305x(01)00032-5. [DOI] [PubMed] [Google Scholar]
- 41.Tafalla C., Coll J., Secombes C. J. Expression of genes related to the early immune response in rainbow trout (Oncorhynchus mykiss) after viral haemorrhagic septicemia virus (VHSV) infection. Developmental and Comparative Immunology. 2005;29(7):615–626. doi: 10.1016/j.dci.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Doñate C., Roher N., Balasch J. C., et al. CD83 expression in sea bream macrophages is a marker for the LPS-induced inflammatory response. Fish & Shellfish Immunology. 2007;23(4):877–885. doi: 10.1016/j.fsi.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 43.Johansson P., Corripio-Miyar Y., Wang T., Collet B., Secombes C. J., Zou J. Characterisation and expression analysis of the rainbow trout (Oncorhynchus mykiss) homologue of the human dendritic cell marker CD208/lysosomal associated membrane protein 3. Developmental and Comparative Immunology. 2012;37(3-4):402–413. doi: 10.1016/j.dci.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Zhu L.-Y., Lin A.-F., Shao T., et al. B cells in teleost fish act as pivotal initiating APCs in priming adaptive immunity: an evolutionary perspective on the origin of the B-1 cell subset and B7 molecules. The Journal of Immunology. 2014;192(6):2699–2714. doi: 10.4049/jimmunol.1301312. [DOI] [PubMed] [Google Scholar]
- 45.Abós B., Castro R., Granja G. A., et al. Early activation of teleost B cells in response to rhabdovirus infection. Journal of Virology. 2015;89(3):1768–1780. doi: 10.1128/jvi.03080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pellegrini A., Guiñazú N., Aoki M. P., et al. Spleen B cells from BALB/c are more prone to activation than spleen B cells from C57BL/6 mice during a secondary immune response to cruzipain. International Immunology. 2007;19(12):1395–1402. doi: 10.1093/intimm/dxm107. [DOI] [PubMed] [Google Scholar]
- 47.Bernard D., Riteau B., Hansen J. D., et al. Costimulatory receptors in a teleost fish: typical CD28, elusive CTLA4. The Journal of Immunology. 2006;176(7):4191–4200. doi: 10.4049/jimmunol.176.7.4191. [DOI] [PubMed] [Google Scholar]
- 48.Bernard D., Hansen J. D., Du Pasquier L., Lefranc M.-P., Benmansour A., Boudinot P. Costimulatory receptors in jawed vertebrates: conserved CD28, odd CTLA4 and multiple BTLAs. Developmental and Comparative Immunology. 2007;31(3):255–271. doi: 10.1016/j.dci.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Chang Y.-T., Kai Y.-H., Chi S.-C., Song Y.-L. Cytotoxic CD8α+ leucocytes have heterogeneous features in antigen recognition and class I MHC restriction in grouper. Fish & Shellfish Immunology. 2011;30(6):1283–1293. doi: 10.1016/j.fsi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Fischer U., Utke K., Ototake M., Dijkstra J. M., Köllner B. Adaptive cell-mediated cytotoxicity against allogeneic targets by CD8-positive lymphocytes of rainbow trout (Oncorhynchus mykiss) Developmental and Comparative Immunology. 2003;27(4):323–337. doi: 10.1016/s0145-305x(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 51.Munang'andu H. M., Fredriksen B. N., Mutoloki S., Dalmo R. A., Evensen Ø. The kinetics of CD4+ and CD8+ T-cell gene expression correlate with protection in Atlantic salmon (Salmo salar L) vaccinated against infectious pancreatic necrosis. Vaccine. 2013;31(15):1956–1963. doi: 10.1016/j.vaccine.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 52.Nakanishi T., Fischer U., Dijkstra J. M., et al. Cytotoxic T cell function in fish. Developmental and Comparative Immunology. 2002;26(2):131–139. doi: 10.1016/S0145-305X(01)00055-6. [DOI] [PubMed] [Google Scholar]
- 53.Nakanishi T., Toda H., Shibasaki Y., Somamoto T. Cytotoxic T cells in teleost fish. Developmental and Comparative Immunology. 2011;35(12):1317–1323. doi: 10.1016/j.dci.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 54.Somamoto T., Nakanishi T., Okamoto N. Specific cell-mediated cytotoxicity against a virus-infected syngeneic cell line in isogeneic ginbuna crucian carp. Developmental and Comparative Immunology. 2000;24(6-7):633–640. doi: 10.1016/S0145-305X(00)00018-5. [DOI] [PubMed] [Google Scholar]
- 55.Andersson E., Matsunaga T. Complete cDNA sequence of a rainbow trout IgM gene and evolution of vertebrate IgM constant domains. Immunogenetics. 1993;38(4):243–250. doi: 10.1007/BF00188800. [DOI] [PubMed] [Google Scholar]
- 56.Hordvik I., Voie A. M., Glette J., Male R., Endresen C. Cloning and sequence analysis of two isotypic IgM heavy chain genes from Atlantic salmon, Salmo salar L. European Journal of Immunology. 1992;22(11):2957–2962. doi: 10.1002/eji.1830221130. [DOI] [PubMed] [Google Scholar]
- 57.Hordvik I., Thevarajan J., Samdal I., Bastani N., Krossøy B. Molecular cloning and phylogenetic analysis of the Atlantic salmon immunoglobulin D gene. Scandinavian Journal of Immunology. 1999;50(2):202–210. doi: 10.1046/j.1365-3083.1999.00583.x. [DOI] [PubMed] [Google Scholar]
- 58.Hansen J. D., Landis E. D., Phillips R. B. Discovery of a unique Ig heavy-chain (IgT) in rainbow trout: implications for a distinctive B cell developmental pathway in teleost fish. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(19):6919–6924. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bromage E., Ramirez-Gomez F., Greene W., et al. Secretory IgD has an evolutionarily conserved role in respiratory mucosal defense. The Journal of Immunology. 2012;188 [Google Scholar]
- 60.Ramirez-Gomez F., Greene W., Rego K., et al. Discovery and characterization of secretory IgD in rainbow trout: secretory IgD is produced through a novel splicing mechanism. Journal of Immunology. 2012;188(3):1341–1349. doi: 10.4049/jimmunol.1101938. [DOI] [PubMed] [Google Scholar]
- 61.Parra D., Takizawa F., Sunyer J. O. Evolution of B cell immunity. Annual Review of Animal Biosciences. 2013;1(1):65–97. doi: 10.1146/annurev-animal-031412-103651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang Y.-A., Salinas I., Li J., et al. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nature Immunology. 2010;11(9):827–835. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Z., Gomez D., Parra D., Takizawa F., Sunyer J. O. IgT plays a prominent role in gill immune response of rainbow trout. Fish & Shellfish Immunology. 2013;34(6):1686. doi: 10.1016/j.fsi.2013.03.161. [DOI] [Google Scholar]
- 64.Banchereau J., Bazan F., Blanchard D., et al. The CD40 antigen and its ligand. Annual Review of Immunology. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 65.Lagos L. X., Iliev D. B., Helland R., Rosemblatt M., Jørgensen J. B. CD40L—a costimulatory molecule involved in the maturation of antigen presenting cells in Atlantic salmon (Salmo salar L) Developmental and Comparative Immunology. 2012;38(3):416–430. doi: 10.1016/j.dci.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 66.Takizawa F., Araki K., Ohtani M., et al. Transcription analysis of two Eomesodermin genes in lymphocyte subsets of two teleost species. Fish & Shellfish Immunology. 2014;36(1):215–222. doi: 10.1016/j.fsi.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Wang T., Holland J. W., Martin S. A. M., Secombes C. J. Sequence and expression analysis of two T helper master transcription factors, T-bet and GATA3, in rainbow trout Oncorhynchus mykiss and analysis of their expression during bacterial and parasitic infection. Fish & Shellfish Immunology. 2010;29(5):705–715. doi: 10.1016/j.fsi.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 68.Chi H., Zhang Z., Inami M., Bøgwald J., Zhan W., Dalmo R. A. Molecular characterizations and functional assessments of GATA-3 and its splice variant in Atlantic cod (Gadus morhua L.) Developmental and Comparative Immunology. 2012;36(3):491–501. doi: 10.1016/j.dci.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Kumari J., Bogwald J., Dalmo R. A. Transcription factor GATA-3 in Atlantic salmon (Salmo salar): molecular characterization, promoter activity and expression analysis. Molecular Immunology. 2009;46(15):3099–3107. doi: 10.1016/j.molimm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 70.Takizawa F., Koppang E. O., Ohtani M., et al. Constitutive high expression of interleukin-4/13A and GATA-3 in gill and skin of salmonid fishes suggests that these tissues form Th2-skewed immune environments. Molecular Immunology. 2011;48(12-13):1360–1368. doi: 10.1016/j.molimm.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 71.Du L., Yang X., Yang L., Wang X., Zhang A., Zhou H. Molecular evidence for the involvement of RORα and RORγ in immune response in teleost. Fish & Shellfish Immunology. 2012;33(2):418–426. doi: 10.1016/j.fsi.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 72.Monte M. M., Wang T. H., Costa M. M., Harun N. O., Secombes C. J. Cloning and expression analysis of two ROR-γ homologues (ROR-γa1 and ROR-γa2) in rainbow trout Oncorhynchus mykiss . Fish & Shellfish Immunology. 2012;33(2):365–374. doi: 10.1016/j.fsi.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 73.Rivas-Aravena A., Guajardo S., Valenzuela B., et al. Ribavirin stimulates the immune response of Atlantic salmon. Veterinary Immunology and Immunopathology. 2015;164(1-2):93–100. doi: 10.1016/j.vetimm.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 74.Wang T., Diaz-Rosales P., Costa M. M., et al. Functional characterization of a nonmammalian IL-21: rainbow trout Oncorhynchus mykiss IL-21 upregulates the expression of the Th cell signature cytokines IFN-γ, IL-10, and IL-22. The Journal of Immunology. 2011;186(2):708–721. doi: 10.4049/jimmunol.1001203. [DOI] [PubMed] [Google Scholar]
- 75.Zhou H., Stuge T. B., Miller N. W., et al. Heterogeneity of channel catfish CTL with respect to target recognition and cytotoxic mechanisms employed. The Journal of Immunology. 2001;167(3):1325–1332. doi: 10.4049/jimmunol.167.3.1325. [DOI] [PubMed] [Google Scholar]
- 76.Utke K., Bergmann S., Lorenzen N., Köllner B., Ototake M., Fischer U. Cell-mediated cytotoxicity in rainbow trout, Oncorhynchus mykiss, infected with viral haemorrhagic septicaemia virus. Fish & Shellfish Immunology. 2007;22(3):182–196. doi: 10.1016/j.fsi.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 77.Kai Y. H., Wu Y. C., Chi S. C. Immune gene expressions in grouper larvae (Epinephelus coioides) induced by bath and oral vaccinations with inactivated betanodavirus. Fish and Shellfish Immunology. 2014;40(2):563–569. doi: 10.1016/j.fsi.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 78.Kai Y.-H., Chi S.-C. Efficacies of inactivated vaccines against betanodavirus in grouper larvae (Epinephelus coioides) by bath immunization. Vaccine. 2008;26(11):1450–1457. doi: 10.1016/j.vaccine.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 79.Ingerslev H.-C., Rønneseth A., Pettersen E. F., Wergeland H. I. Differential expression of immune genes in Atlantic salmon (Salmo salar L.) challenged intraperitoneally or by cohabitation with IPNV. Scandinavian Journal of Immunology. 2009;69(2):90–98. doi: 10.1111/j.1365-3083.2008.02201.x. [DOI] [PubMed] [Google Scholar]
- 80.Hetland D. L., Jørgensen S. M., Skjødt K., et al. In situ localisation of major histocompatibility complex class I and class II and CD8 positive cells in infectious salmon anaemia virus (ISAV)-infected Atlantic salmon. Fish & Shellfish Immunology. 2010;28(1):30–39. doi: 10.1016/j.fsi.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 81.Munang'andu H. M., Fredriksen B. N., Mutoloki S., Dalmo R. A., Evensen Ø. Antigen dose and humoral immune response correspond with protection for inactivated infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L) Veterinary Research. 2013;44(1, article 7) doi: 10.1186/1297-9716-44-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Munang'andu H. M., Sandtrø A., Mutoloki S., Brudeseth B. E., Santi N., Evensen Ø. Immunogenicity and cross protective ability of the central VP2 amino acids of infectious pancreatic necrosis virus in Atlantic salmon (Salmo salar L.) PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0054263.e54263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roberti K. A., Rohovec J. S., Winton J. R. Vaccination of rainbow trout against infectious hematopoietic necrosis (IHN) by using attenuated mutants selected by neutralizing monoclonal antibodies. Journal of Aquatic Animal Health. 1998;10(4):328–337. doi: 10.1577/1548-8667(1998)010<0328:VORTAI>2.0.CO;2. [DOI] [Google Scholar]
- 84.Adelmann M., Köllner B., Bergmann S. M., et al. Development of an oral vaccine for immunisation of rainbow trout (Oncorhynchus mykiss) against viral haemorrhagic septicaemia. Vaccine. 2008;26(6):837–844. doi: 10.1016/j.vaccine.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 85.Fryer J. L., Rohovec J. S., Tebbit G. L., McMichael J. S., Pilcher K. S. Vaccination for the control of infectious diseases in Pacific salmon. Fish Pathology. 1976;10(2):155–164. [Google Scholar]
- 86.Tebbit G. L. Viruses infecting salmonid fishes from Oregon. A. The occurence and distribution of infectious pancreatic necrosis virus; B. The development of an attenuated strain of infectious hematopoietic necrosis virus (IHNV) for the immunization of salmonids [Ph.D. thesis] Corvallis, Ore, USA: Oregon State University; 1976. [Google Scholar]
- 87.Leong J. C., Fryer J. L. Viral vaccines for aquaculture. Annual Review of Fish Diseases. 1993;3:225–240. doi: 10.1016/0959-8030(93)90036-b. [DOI] [Google Scholar]
- 88.Rohovec J. S., Winton J. R., Fryer J. L. Bacterins and vaccines for the control of infectious diseases of fish. Proceedings of the Republic of China-United State Cooperative Science Seminar on Fish diseases; 1981; Beijing, China. National Science Council; [Google Scholar]
- 89.Dorson M., Castric J., Torchy C. Infectious pancreatic necrosis virus of salmonids: biological and antigenic features of a pathogenic strain and of a non-pathogenic variant selected in RTG-2 cells. Journal of Fish Diseases. 1978;1(4):309–320. doi: 10.1111/j.1365-2761.1978.tb00035.x. [DOI] [Google Scholar]
- 90.Thoulouze M. I., Bouguyon E., Carpentier C., Brémont M. ssential role of the NV protein of Novirhabdovirus for pathogenicity in rainbow trout. Journal of Virology. 2004;78(8):4098–4107. doi: 10.1128/jvi.78.8.4098-4107.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Romero A., Figueras A., Thoulouze M.-I., Bremont M., Novoa B. Recombinant infectious hematopoietic necrosis viruses induce protection for rainbow trout Oncorhynchus mykiss . Diseases of Aquatic Organisms. 2008;80(2):123–135. doi: 10.3354/dao01932. [DOI] [PubMed] [Google Scholar]
- 92.Santi N., Vakharia V. N., Evensen Ø. Identification of putative motifs involved in the virulence of infectious pancreatic necrosis virus. Virology. 2004;322(1):31–40. doi: 10.1016/j.virol.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 93.Mutoloki S., Munang'andu H., Evensen Ø. Clinical and subclinical forms of infectious pancreatic necrosis virus infections show specific viral genetic fingerprints that link differences in virulence to immunogenicity. Fish & Shellfish Immunology. 2013;34(6):p. 1667. doi: 10.1016/j.fsi.2013.03.103. [DOI] [Google Scholar]
- 94.Gadan K., Sandtrø A., Marjara I. S., Santi N., Munang'andu H. M., Evensen Ø. Stress-induced reversion to virulence of infectious pancreatic necrosis virus in naive fry of Atlantic salmon (Salmo salar L.) PLoS ONE. 2013;8(2) doi: 10.1371/journal.pone.0054656.e54656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boudinot P., Blanco M., De Kinkelin P., Benmansour A. Combined DNA immunization with the glycoprotein gene of viral hemorrhagic septicemia virus and infectious hematopoietic necrosis virus induces double-specific protective immunity and nonspecific response in rainbow trout. Virology. 1998;249(2):297–306. doi: 10.1006/viro.1998.9322. [DOI] [PubMed] [Google Scholar]
- 96.Utke K., Kock H., Schuetze H., et al. Cell-mediated immune responses in rainbow trout after DNA immunization against the viral hemorrhagic septicemia virus. Developmental and Comparative Immunology. 2008;32(3):239–252. doi: 10.1016/j.dci.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 97.Castro R., Martínez-Alonso S., Fischer U., et al. DNA vaccination against a fish rhabdovirus promotes an early chemokine-related recruitment of B cells to the muscle. Vaccine. 2014;32(10):1160–1168. doi: 10.1016/j.vaccine.2013.11.062. [DOI] [PubMed] [Google Scholar]
- 98.Corbeil S., Lapatra S. E., Anderson E. D., Kurath G. Nanogram quantities of a DNA vaccine protect rainbow trout fry against heterologous strains of infectious hematopoietic necrosis virus. Vaccine. 2000;18(25):2817–2824. doi: 10.1016/s0264-410x(00)00078-5. [DOI] [PubMed] [Google Scholar]
- 99.Shedlock D. J., Weiner D. B. DNA vaccination: antigen presentation and the induction of immunity. Journal of Leukocyte Biology. 2000;68(6):793–806. [PubMed] [Google Scholar]
- 100.Fu F., Lang Y., Li X., et al. Evaluation of the enhancing ability of three adjuvants for DNA vaccination using the porcine circovirus type 2 ORF2 (capsid) gene in mice. Virus Research. 2013;171(1):247–251. doi: 10.1016/j.virusres.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 101.Jimenez N., Coll J., Salguero F. J., Tafalla C. Co-injection of interleukin 8 with the glycoprotein gene from viral haemorrhagic septicemia virus (VHSV) modulates the cytokine response in rainbow trout (Oncorhynchus mykiss) Vaccine. 2006;24(27-28):5615–5626. doi: 10.1016/j.vaccine.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 102.Smith D. C., Spooner R. A., Watson P. D., et al. Internalized pseudomonas exotoxin A can exploit multiple pathways to reach the endoplasmic reticulum. Traffic. 2006;7(4):379–393. doi: 10.1111/j.1600-0854.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 103.Munang'andu H. M., Fredriksen B. N., Mutoloki S., et al. Comparison of vaccine efficacy for different antigen delivery systems for infectious pancreatic necrosis virus vaccines in Atlantic salmon (Salmo salar L.) in a cohabitation challenge model. Vaccine. 2012;30(27):4007–4016. doi: 10.1016/j.vaccine.2012.04.039. [DOI] [PubMed] [Google Scholar]
- 104.Li-Li Z., Min L., Jun-Wei G., Xin-Yuan Q., Yi-Jing L., Di-Qiu L. Expression of infectious pancreatic necrosis virus (IPNV) VP2-VP3 fusion protein in Lactobacillus casei and immunogenicity in rainbow trouts. Vaccine. 2012;30(10):1823–1829. doi: 10.1016/j.vaccine.2011.12.132. [DOI] [PubMed] [Google Scholar]
- 105.Akagi T., Baba M., Akashi M. Polymers in Nanomedicine. Vol. 247. Berlin, Germany: Springer; 2012. Biodegradable nanoparticles as vaccine adjuvants and delivery systems: regulation of immune responses by nanoparticle-based vaccine; pp. 31–64. (Advances in Polymer Science). [DOI] [Google Scholar]
- 106.Hans M. L., Maxwell C., Ehrlichman R. S., et al. Evaluation of in vitro release and in vivo efficacy of mPEG-PLA-haloperidol conjugate micelle-like structures. Journal of Biomedical Materials Research B: Applied Biomaterials. 2007;83(2):422–430. doi: 10.1002/jbm.b.30812. [DOI] [PubMed] [Google Scholar]
- 107.Schlosser E., Mueller M., Fischer S., et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine. 2008;26(13):1626–1637. doi: 10.1016/j.vaccine.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 108.Tian J. Y., Sun X. Q., Chen X. G., Yuc J., Qu L. Y., Wang L. C. The formulation and immunisation of oral poly(DL-lactide-co-glycolide) microcapsules containing a plasmid vaccine against lymphocystis disease virus in Japanese flounder (Paralichthys olivaceus) International Immunopharmacology. 2008;8(6):900–908. doi: 10.1016/j.intimp.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 109.Tian J. Y., Sun X. Q., Chen X. G. Formation and oral administration of alginate microspheres loaded with pDNA coding for lymphocystis disease virus (LCDV) to Japanese flounder. Fish and Shellfish Immunology. 2008;24(5):592–599. doi: 10.1016/j.fsi.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 110.Tian J., Yu J., Sun X. Q. Chitosan microspheres as candidate plasmid vaccine carrier for oral immunisation of Japanese flounder (Paralichthys olivaceus) Veterinary Immunology and Immunopathology. 2008;126(3-4):220–229. doi: 10.1016/j.vetimm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 111.Zheng F.-R., Sun X.-Q., Liu H.-Z., Zhang J.-X. Study on the distribution and expression of a DNA vaccine against lymphocystis disease virus in Japanese flounder (Paralichthys olivaceus) Aquaculture. 2006;261(4):1128–1134. doi: 10.1016/j.aquaculture.2006.05.020. [DOI] [Google Scholar]
- 112.Rajeshkumar S., Venkatesan C., Sarathi M., et al. Oral delivery of DNA construct using chitosan nanoparticles to protect the shrimp from white spot syndrome virus (WSSV) Fish and Shellfish Immunology. 2009;26(3):429–437. doi: 10.1016/j.fsi.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 113.Sahay G., Alakhova D. Y., Kabanov A. V. Endocytosis of nanomedicines. Journal of Controlled Release. 2010;145(3):182–195. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Standley S. M., Kwon Y. J., Murthy N., et al. Acid-degradable particles for protein-based vaccines: Enhanced survival rate for tumor-challenged mice using ovalbumin model. Bioconjugate Chemistry. 2004;15(6):1281–1288. doi: 10.1021/bc049956f. [DOI] [PubMed] [Google Scholar]
- 115.Jones R. A., Cheung C. Y., Black F. E., et al. Poly(2-alkylacrylic acid) polymers deliver molecules to the cytosol by pH-sensitive disruption of endosomal vesicles. Biochemical Journal. 2003;372(1):65–75. doi: 10.1042/bj20021945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murthy N., Robichaud J. R., Tirrell D. A., Stayton P. S., Hoffman A. S. The design and synthesis of polymers for eukaryotic membrane disruption. Journal of Controlled Release. 1999;61(1-2):137–143. doi: 10.1016/s0168-3659(99)00114-5. [DOI] [PubMed] [Google Scholar]
- 117.Yoshikawa T., Okada N., Oda A., et al. Development of amphiphilic γ-PGA-nanoparticle based tumor vaccine: potential of the nanoparticulate cytosolic protein delivery carrier. Biochemical and Biophysical Research Communications. 2008;366(2):408–413. doi: 10.1016/j.bbrc.2007.11.153. [DOI] [PubMed] [Google Scholar]
- 118.Kusonwiriyawong C., van de Wetering P., Hubbell J. A., Merkle H. P., Walter E. Evaluation of pH-dependent membrane-disruptive properties of poly(acrylic acid) derived polymers. European Journal of Pharmaceutics and Biopharmaceutics. 2003;56(2):237–246. doi: 10.1016/s0939-6411(03)00093-6. [DOI] [PubMed] [Google Scholar]
- 119.Foster S., Duvall C. L., Crownover E. F., Hoffman A. S., Stayton P. S. Intracellular delivery of a protein antigen with an endosomal-releasing polymer enhances CD8 T-cell production and prophylactic vaccine efficacy. Bioconjugate Chemistry. 2010;21(12):2205–2212. doi: 10.1021/bc100204m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.He C. B., Hu Y. P., Yin L. C., Tang C., Yin C. H. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657–3666. doi: 10.1016/j.biomaterials.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 121.Thiele L., Rothen-Rutishauser B., Jilek S., Wunderli-Allenspach H., Merkle H. P., Walter E. Evaluation of particle uptake in human blood monocyte-derived cells in vitro. Does phagocytosis activity of dendritic cells measure up with macrophages? Journal of Controlled Release. 2001;76(1-2):59–71. doi: 10.1016/S0168-3659(01)00412-6. [DOI] [PubMed] [Google Scholar]
- 122.Burgdorf S., Kurts C. Endocytosis mechanisms and the cell biology of antigen presentation. Current Opinion in Immunology. 2008;20(1):89–95. doi: 10.1016/j.coi.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 123.Agnihotri S. A., Mallikarjuna N. N., Aminabhavi T. M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. Journal of Controlled Release. 2004;100(1):5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 124.Gutierro I., Hernández R. M., Igartua M., Gascón A. R., Pedraz J. L. Size dependent immune response after subcutaneous, oral and intranasal administration of BSA loaded nanospheres. Vaccine. 2002;21(1-2):67–77. doi: 10.1016/s0264-410x(02)00435-8. [DOI] [PubMed] [Google Scholar]
- 125.Fehr T., Skrastina D., Pumpens P., Zinkernagel R. M. T cell-independent type I antibody response against B cell epitopes expressed repetitively on recombinant virus particles. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(16):9477–9481. doi: 10.1073/pnas.95.16.9477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ruyra A., Cano-Sarabia M., MacKenzie S. A., Maspoch D., Roher N. A novel liposome based nanocarrier loaded with an LPS-dsRNA cocktail for fish innate immune system stimulation. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0076338.e76338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fredriksen B. N., Grip J. PLGA/PLA micro- and nanoparticle formulations serve as antigen depots and induce elevated humoral responses after immunization of Atlantic salmon (Salmo salar L.) Vaccine. 2012;30(3):656–667. doi: 10.1016/j.vaccine.2011.10.105. [DOI] [PubMed] [Google Scholar]
- 128.Broos S., Lundberg K., Akagi T., et al. Immunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: implications for specific immunotherapy. Vaccine. 2010;28(31):5075–5085. doi: 10.1016/j.vaccine.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 129.Diwan M., Elamanchili P., Lane H., Gainer A., Samuel J. Biodegradable nanoparticle mediated antigen delivery to human cord blood derived dendritic cells for induction of primary T cell responses. Journal of Drug Targeting. 2003;11(8–10):495–507. doi: 10.1080/10611860410001670026. [DOI] [PubMed] [Google Scholar]
- 130.Katare Y. K., Muthukumaran T., Panda A. K. Influence of particle size, antigen load, dose and additional adjuvant on the immune response from antigen loaded PLA microparticles. International Journal of Pharmaceutics. 2005;301(1-2):149–160. doi: 10.1016/j.ijpharm.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 131.Samuel J., Elamanchili P., Chong C., et al. Biodegradable nanoparticles for targeted delivery of therapeutic vaccines to dendritic cells. The FASEB Journal. 2003;17(7):p. C332. [Google Scholar]
- 132.Haugland Ø., Torgersen J., Syed M., Evensen Ø. Expression profiles of inflammatory and immune-related genes in Atlantic salmon (Salmo salar L.) at early time post vaccination. Vaccine. 2005;23(48-49):5488–5499. doi: 10.1016/j.vaccine.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 133.Munang'andu H. M., Mutoloki S., Evensen Ø. Acquired immunity and vaccination against infectious pancreatic necrosis virus of salmon. Developmental & Comparative Immunology. 2014;43(2):184–196. doi: 10.1016/j.dci.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 134.Munang'andu H. M., Mutoloki S., Evensen O. Fish Vaccination. chapter 3. Wiley; 2014. Non-replicating vaccines; p. p. 22. [DOI] [Google Scholar]
- 135.Salinas I., Zhang Y. A., Sunyer J. O. Mucosal immunoglobulins and B cells of teleost fish. Developmental and Comparative Immunology. 2011;35(12):1346–1365. doi: 10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Estepa A., Coll J. M. Enhancement of fish mortality by rhabdovirus infection after immunization with a viral nucleoprotein peptide. Viral Immunology. 1993;6(4):237–243. doi: 10.1089/vim.1993.6.237. [DOI] [PubMed] [Google Scholar]
- 137.Noonan B., Enzmann P. J., Trust T. J. Recombinant infectious hematopoietic necrosis virus and viral hemorrhagic septicemia virus glycoprotein epitopes expressed in Aeromonas salmonicida induce protective immunity in rainbow trout (Oncorhynchus mykiss) Applied and Environmental Microbiology. 1995;61(10):3586–3591. doi: 10.1128/aem.61.10.3586-3591.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Oberg L. A., Wirkkula J., Mourich D., Leong J. C. Bacterially expressed nucleoprotein of infectious hematopoietic necrosis virus augments protective immunity induced by the glycoprotein vaccine in fish. Journal of Virology. 1991;65(8):4486–4489. doi: 10.1128/jvi.65.8.4486-4489.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allnutt F. C. T., Bowers R. M., Rowe C. G., Vakharia V. N., LaPatra S. E., Dhar A. K. Antigenicity of infectious pancreatic necrosis virus VP2 subviral particles expressed in yeast. Vaccine. 2007;25(26):4880–4888. doi: 10.1016/j.vaccine.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 140.Mikalsen A. B., Torgersen J., Aleström P., Hellemann A.-L., Koppang E.-O., Rimstad E. Protection of Atlantic salmon, Salmo salar L, against infectious pancreatic necrosis after DNA vaccination. Diseases of Aquatic Organisms. 2004;60(1):11–20. doi: 10.3354/dao060011. [DOI] [PubMed] [Google Scholar]
- 141.Shivappa R. B., McAllister P. E., Edwards G. H., Santi N., Evensen Ø., Vakharia V. N. Development of a subunit vaccine for infectious pancreatic necrosis virus using a baculovirus insect/larvae system. Developments in Biologicals. 2005;121:165–174. [PubMed] [Google Scholar]
- 142.Lecocq-Xhonneux F., Thiry M., Dheur I., et al. A recombinant viral haemorrhagic septicaemia virus glycoprotein expressed in insect cells induces protective immunity in rainbow trout. Journal of General Virology. 1994;75(7):1579–1587. doi: 10.1099/0022-1317-75-7-1579. [DOI] [PubMed] [Google Scholar]
- 143.Moore A. C., Hutchings C. L. Combination vaccines: synergistic simultaneous induction of antibody and T-cell immunity. Expert Review of Vaccines. 2007;6(1):111–121. doi: 10.1586/14760584.6.1.111. [DOI] [PubMed] [Google Scholar]
- 144.Øvergård A.-C., Patel S., Nøstbakken O. J., Nerland A. H. Atlantic halibut (Hippoglossus hippoglossus L.) T-cell and cytokine response after vaccination and challenge with nodavirus. Vaccine. 2013;31(19):2395–2402. doi: 10.1016/j.vaccine.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 145.Øvergård A.-C., Nerland A. H., Fiksdal I. U., Patel S. Atlantic halibut experimentally infected with nodavirus shows increased levels of T-cell marker and IFNγ transcripts. Developmental & Comparative Immunology. 2012;37(1):139–150. doi: 10.1016/j.dci.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 146.Liu W., Hsu C.-H., Chang C.-Y., Chen H.-H., Lin C.-S. Immune response against grouper nervous necrosis virus by vaccination of virus-like particles. Vaccine. 2006;24(37–39):6282–6287. doi: 10.1016/j.vaccine.2006.05.073. [DOI] [PubMed] [Google Scholar]
- 147.Tang L., Lin C.-S., Krishna N. K., Yeager M., Schneemann A., Johnson J. E. Virus-like particles of a fish nodavirus display a capsid subunit domain organization different from that of insect nodaviruses. Journal of Virology. 2002;76(12):6370–6375. doi: 10.1128/JVI.76.12.6370-6375.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lin C.-S., Lu M.-W., Tang L., et al. Characterization of virus-like particles assembled in a recombinant baculovirus system expressing the capsid protein of a fish nodavirus. Virology. 2001;290(1):50–58. doi: 10.1006/viro.2001.1157. [DOI] [PubMed] [Google Scholar]
- 149.Fang Q., Seng E. K., Ding Q. Q., Zhang L. L. Characterization of infectious particles of grass carp reovirus by treatment with proteases. Archives of Virology. 2008;153(4):675–682. doi: 10.1007/s00705-008-0048-3. [DOI] [PubMed] [Google Scholar]
- 150.Dhar A. K., Bowers R. M., Rowe C. G., Allnutt F. C. T. Expression of a foreign epitope on infectious pancreatic necrosis virus VP2 capsid protein subviral particle (SVP) and immunogenicity in rainbow trout. Antiviral Research. 2010;85(3):525–531. doi: 10.1016/j.antiviral.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 151.Rivas-Aravena A., Martin M. C.-S., Galaz J., et al. Evaluation of the immune response against immature viral particles of infectious pancreatic necrosis virus (IPNV): a new model to develop an attenuated vaccine. Vaccine. 2012;30(34):5110–5117. doi: 10.1016/j.vaccine.2012.05.062. [DOI] [PubMed] [Google Scholar]
- 152.Lu M. W., Lin C.-S. Involvement of the terminus of grouper betanodavirus capsid protein in virus-like particle assembly. Archives of Virology. 2003;148(2):345–355. doi: 10.1007/s00705-002-0901-8. [DOI] [PubMed] [Google Scholar]
- 153.Lai Y.-X., Jin B.-L., Xu Y., et al. Immune responses of orange-spotted grouper, Epinephelus coioides, against virus-like particles of betanodavirus produced in Escherichia coli . Veterinary Immunology and Immunopathology. 2014;157(1-2):87–96. doi: 10.1016/j.vetimm.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 154.Goldsby R. A., Kindt T., Kiby J., Osborne B. Immunology. 5th. W.H. Freeman; 2003. [Google Scholar]
- 155.Osinubi M. O. V., Wu X., Franka R., et al. Enhancing comparative rabies DNA vaccine effectiveness through glycoprotein gene modifications. Vaccine. 2009;27(51):7214–7218. doi: 10.1016/j.vaccine.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 156.Ray N. B., Ewalt L. C., Lodmell D. L. Nanogram quantities of plasmid DNA encoding the rabies virus glycoprotein protect mice against lethal rabies virus infection. Vaccine. 1997;15(8):892–895. doi: 10.1016/S0264-410X(96)00281-2. [DOI] [PubMed] [Google Scholar]
- 157.Xu C., Mutoloki S., Evensen Ø. Superior protection conferred by inactivated whole virus vaccine over subunit and DNA vaccines against salmonid alphavirus infection in Atlantic salmon (Salmo salar L.) Vaccine. 2012;30(26):3918–3928. doi: 10.1016/j.vaccine.2012.03.081. [DOI] [PubMed] [Google Scholar]
- 158.Howarth M., Elliott T. The processing of antigens delivered as DNA vaccines. Immunological Reviews. 2004;199:27–39. doi: 10.1111/j.0105-2896.2004.00141.x. [DOI] [PubMed] [Google Scholar]
- 159.Shao T., Zhu L.-Y., Nie L., et al. Characterization of surface phenotypic molecules of teleost dendritic cells. Developmental & Comparative Immunology. Mar 2015;49(1):38–43. doi: 10.1016/j.dci.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 160.Li M.-F., Li Y.-X., Sun L. CD83 is required for the induction of protective immunity by a DNA vaccine in a teleost model. Developmental & Comparative Immunology. 2015;51(1):141–147. doi: 10.1016/j.dci.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 161.Wiik-Nielsen J., Løvoll M., Fritsvold C., et al. Characterization of myocardial lesions associated with cardiomyopathy syndrome in atlantic salmon, Salmo salar L., using laser capture microdissection. Journal of Fish Diseases. 2012;35(12):907–916. doi: 10.1111/j.1365-2761.2012.01431.x. [DOI] [PubMed] [Google Scholar]
- 162.Lin A.-F., Xiang L.-X., Wang Q.-L., Dong W.-R., Gong Y.-F., Shao J.-Z. The DC-SIGN of zebrafish: insights into the existence of a CD209 homologue in a lower vertebrate and its involvement in adaptive immunity. The Journal of Immunology. 2009;183(11):7398–7410. doi: 10.4049/jimmunol.0803955. [DOI] [PubMed] [Google Scholar]
- 163.Pinto R. D., Randelli E., Buonocore F., Pereira P. J. B., dos Santos N. M. S. Molecular cloning and characterization of sea bass (Dicentrarchus labrax, L.) MHC class I heavy chain and β2-microglobulin. Developmental and Comparative Immunology. 2013;39(3):234–254. doi: 10.1016/j.dci.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 164.Xu Z. Y., Nie P., Chang M. X., Sun B. J. Cloning, characterization and expression analysis of SIMP (source of immunodominant MHC-associated peptides) in grass carp Ctenopharyngodon idella. Fish & Shellfish Immunology. 2008;24(6):701–714. doi: 10.1016/j.fsi.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 165.Noakes M. A., Reimer T., Phillips R. B. Genotypic characterization of an MHC class II locus in lake trout (Salvelinus namaycush) from Lake Superior by single-stranded conformational polymorphism analysis and reference strand-mediated conformational analysis. Marine Biotechnology. 2003;5(3):270–278. doi: 10.1007/s10126-002-0079-9. [DOI] [PubMed] [Google Scholar]
- 166.Liu Y., Moore L., Koppang E. O., Hordvik I. Characterization of the CD3ζ, CD3γδ and CD3ε subunits of the T cell receptor complex in Atlantic salmon. Developmental & Comparative Immunology. 2008;32(1):26–35. doi: 10.1016/j.dci.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 167.Laing K. J., Hansen J. D. Fish T cells: recent advances through genomics. Developmental and Comparative Immunology. 2011;35(12):1282–1295. doi: 10.1016/j.dci.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 168.Ronza P., Bermúdez R., Losada A. P., Robles A., Quiroga M. I. Mucosal CD3ε+ cell proliferation and gut epithelial apoptosis: implications in rainbow trout gastroenteritis (RTGE) Journal of Fish Diseases. 2011;34(6):433–443. doi: 10.1111/j.1365-2761.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- 169.Maisey K., Toro-Ascuy D., Montero R., Reyes-López F. E., Imarai M. Identification of CD3ε, CD4, CD8β splice variants of Atlantic salmon. Fish & Shellfish Immunology. 2011;31(6):815–822. doi: 10.1016/j.fsi.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 170.Partula S., De Guerra A., Fellah J. S., Charlemagne J. Structure and diversity of the TCR α-chain in a teleost fish. The Journal of Immunology. 1996;157(1):207–212. [PubMed] [Google Scholar]
- 171.Nam B. H., Hirono I., Aoki T. The four TCR genes of teleost fish: The cDNA and genomic DNA analysis of Japanese flounder (Paralichthys olivaceus) TCR alpha-, beta-, gamma-, and delta-chains. Journal of Immunology. 2003;170(6):3081–3090. doi: 10.4049/jimmunol.170.6.3081. [DOI] [PubMed] [Google Scholar]
- 172.Park C.-I., Hwang J. Y., Hirono I., Aoki T. Characterization and expression of a CD40 homolog gene in Japanese flounder Paralichthys olivaceus . Immunogenetics. 2005;57(9):682–689. doi: 10.1007/s00251-005-0032-y. [DOI] [PubMed] [Google Scholar]
- 173.Takizawa F., Dijkstra J. M., Kotterba P., et al. The expression of CD8α discriminates distinct T cell subsets in teleost fish. Developmental and Comparative Immunology. 2011;35(7):752–763. doi: 10.1016/j.dci.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 174.Hordvik I. Immunoglobulin isotypes in Atlantic salmon, Salmo Salar . Biomolecules. 2015;5(1):166–177. doi: 10.3390/biom5010166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bromage E., Kataria P., Ramirez-Gomez F. The structure of rainbow trout IgM influences antibody-mediated phagocytosis of bacteria. The Journal of Immunology. 2012;188 [Google Scholar]
- 176.Hu Y.-L., Xiang L.-X., Shao J.-Z. Identification and characterization of a novel immunoglobulin Z isotype in zebrafish: implications for a distinct B cell receptor in lower vertebrates. Molecular Immunology. 2010;47(4):738–746. doi: 10.1016/j.molimm.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 177.Perelberg A., Ronen A., Hutoran M., Smith Y., Kotler M. Protection of cultured Cyprinus carpio against a lethal viral disease by an attenuated virus vaccine. Vaccine. 2005;23(26):3396–3403. doi: 10.1016/j.vaccine.2005.01.096. [DOI] [PubMed] [Google Scholar]
- 178.de Kinkelin P., Bearzotti-Le Berre M., Bernard J. Viral hemorrhagic septicemia of rainbow trout: selection of a thermoresistant virus variant and comparison of polypeptide synthesis with the wild-type virus strain. Journal of Virology. 1980;36(3):652–658. doi: 10.1128/jvi.36.3.652-658.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim W. S., Kim C. S., Cho J. K., Myung J. I., Oh M. J. Disease control efficacy of synthetic double-stranded RNA Poly(I:C) administration for viral nervous necrosis (VNN) in sevenband grouper (Epinephelus septemfasciatus) Aquaculture. 2012;364-365:259–262. doi: 10.1016/j.aquaculture.2012.08.014. [DOI] [Google Scholar]
- 180.Enzmann P.-J., Fichtner D., Schütze H., Walliser G. Development of vaccines against VHS and IHN: oral application, molecular marker and discrimination of vaccinated fish from infected populations. Journal of Applied Ichthyology. 1998;14(3-4):179–183. doi: 10.1111/j.1439-0426.1998.tb00639.x. [DOI] [Google Scholar]
- 181.Novoa B., Romero A., Mulero V., Rodríguez I., Fernández I., Figueras A. Zebrafish (Danio rerio) as a model for the study of vaccination against viral haemorrhagic septicemia virus (VHSV) Vaccine. 2006;24(31-32):5806–5816. doi: 10.1016/j.vaccine.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 182.Kim C. H., Winton J. R., Leong J. C. Neutralization-resistant variants of infectious hematopoietic necrosis virus have altered virulence and tissue tropism. Journal of Virology. 1994;68(12):8447–8453. doi: 10.1128/jvi.68.12.8447-8453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ristow S. S., LaPatra S. E., Dixon R., et al. Responses of cloned rainbow trout Oncorhynchus mykiss to an attenuated strain of infectious hematopoietic necrosis virus. Diseases of Aquatic Organisms. 2000;42(3):163–172. doi: 10.3354/dao042163. [DOI] [PubMed] [Google Scholar]
- 184.Oh S.-Y., Nishizawa T. Optimizing the quantitative detection of red seabream iridovirus (RSIV) genome from splenic tissues of rock bream Oplegnathus fasciatus using a qPCR Assay. Fish Pathology. 2013;48(1):21–24. doi: 10.3147/jsfp.48.21. [DOI] [Google Scholar]
- 185.Caipang C. M. A., Takano T., Hirono I., Aoki T. Genetic vaccines protect red seabream, Pagrus major, upon challenge with red seabream iridovirus (RSIV) Fish & Shellfish Immunology. 2006;21(2):130–138. doi: 10.1016/j.fsi.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 186.Nusbaum K. E., Smith B. F., DeInnocentes P., Bird R. C. Protective immunity induced by DNA vaccination of channel catfish with early and late transcripts of the channel catfish herpesvirus (IHV-1) Veterinary Immunology and Immunopathology. 2002;84(3-4):151–168. doi: 10.1016/S0165-2427(01)00399-3. [DOI] [PubMed] [Google Scholar]
- 187.Leong J. C., Anderson E., Bootland L. M., et al. Fish vaccine antigens produced or delivered by recombinant DNA technologies. Fish Vaccinology. 1997;90:267–277. [PubMed] [Google Scholar]
- 188.Winton J. R. Immunization with viral antigens: infectious haematopoietic necrosis. Fish Vaccinology. 1997;90:211–220. [PubMed] [Google Scholar]
- 189.Emmenegger E. J., Kurath G. DNA vaccine protects ornamental koi (Cyprinus carpio koi) against North American spring viremia of carp virus. Vaccine. 2008;26(50):6415–6421. doi: 10.1016/j.vaccine.2008.08.071. [DOI] [PubMed] [Google Scholar]
- 190.Kanellos T., Sylvester I. D., D'Mello F., et al. DNA vaccination can protect Cyprinus Carpio against spring viraemia of carp virus. Vaccine. 2006;24(23):4927–4933. doi: 10.1016/j.vaccine.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 191.Takano T., Iwahori A., Hirono I., Aoki T. Development of a DNA vaccine against hirame rhabdovirus and analysis of the expression of immune-related genes after vaccination. Fish & Shellfish Immunology. 2004;17(4):367–374. doi: 10.1016/j.fsi.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 192.Mikalsen A. B., Sindre H., Torgersen J., Rimstad E. Protective effects of a DNA vaccine expressing the infectious salmon anemia virus hemagglutinin-esterase in Atlantic salmon. Vaccine. 2005;23(41):4895–4905. doi: 10.1016/j.vaccine.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 193.Sommerset I., Lorenzen E., Lorenzen N., Bleie H., Nerland A. H. A DNA vaccine directed against a rainbow trout rhabdovirus induces early protection against a nodavirus challenge in turbot. Vaccine. 2003;21(32):4661–4667. doi: 10.1016/S0264-410X(03)00526-7. [DOI] [PubMed] [Google Scholar]
- 194.Tian J. Y., Yu J. Corrigendum to ‘poly (lactic-co-glycolic acid) nanoparticles as candidate DNA vaccine carrier for oral immunization of Japanese flounder (Paralichthys olivaceus) against lymphocystis disease virus’ Fish Shellfish Immun. 30 (2011) 109–117. Fish & Shellfish Immunology. 2011;31(2):p. 364. doi: 10.1016/j.fsi.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 195.Adomako M., St-Hilaire S., Zheng Y., et al. Oral DNA vaccination of rainbow trout, Oncorhynchus mykiss (Walbaum), against infectious haematopoietic necrosis virus using PLGA [Poly(D,L-Lactic-Co-Glycolic Acid)] nanoparticles. Journal of Fish Diseases. 2012;35(3):203–214. doi: 10.1111/j.1365-2761.2011.01338.x. [DOI] [PubMed] [Google Scholar]
- 196.Vimal S., Abdul Majeed S., Taju G., et al. Chitosan tripolyphosphate (CS/TPP) nanoparticles: preparation, characterization and application for gene delivery in shrimp. Acta Tropica. 2013;128(3):486–493. doi: 10.1016/j.actatropica.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 197.de Kinkelin P., Béarzotti M., Castric J., Nougayrède P., Lecocq-Xhonneux F., Thiry M. Eighteen years of vaccination against viral haemorrhagic septicaemia in France. Veterinary Research. 1995;26(5-6):379–387. [PubMed] [Google Scholar]
- 198.Anderson E., Clouthier S., Shewmaker W., Weighall A., LaPatra S. Inactivated infectious haematopoietic necrosis virus (IHNV) vaccines. Journal of Fish Diseases. 2008;31(10):729–745. doi: 10.1111/j.1365-2761.2008.00960.x. [DOI] [PubMed] [Google Scholar]
- 199.Tesarcík J., Macura B., Dedek L., Valicek L., Smíd B. Isolation and electron microscopy of a rhabdovirus from the acute form of infectious dropsy of carp (spring viraemia of carp) Zentralblatt für Veterinärmedizin Reihe B. 1977;24(4):340–343. doi: 10.1111/j.1439-0450.1977.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 200.López-Dóriga M. V., Smail D. A., Smith R. J., et al. Isolation of salmon pancreas disease virus (SPDV) in cell culture and its ability to protect against infection by the ‘wild-type’ agent. Fish & Shellfish Immunology. 2001;11(6):505–522. doi: 10.1006/fsim.2000.0330. [DOI] [PubMed] [Google Scholar]
- 201.Caipang C. M. A., Hirono I., Aoki T. Immunogenicity, retention and protective effects of the protein derivatives of formalin-inactivated red seabream iridovirus (RSIV) vaccine in red seabream, Pagrus major. Fish & Shellfish Immunology. 2006;20(4):597–609. doi: 10.1016/j.fsi.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 202.Ou-Yang Z. L., Wang P. R., Huang X. H., et al. Immunogenicity and protective effects of inactivated Singapore grouper iridovirus (SGIV) vaccines in orange-spotted grouper, Epinephelus coioides . Developmental and Comparative Immunology. 2012;38(2):254–261. doi: 10.1016/j.dci.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 203.Dixon P. Immunization with viral antigens: viral diseases of carp and catfish. Fish Vaccinology. 1997;90:221–232. [PubMed] [Google Scholar]
- 204.Yasumoto S., Kuzuya Y., Yasuda M., Yoshimura T., Miyazaki T. Oral immunization of common carp with a liposome vaccine fusing koi herpesvirus antigen. Fish Pathology. 2006;41(4):141–145. doi: 10.3147/jsfp.41.141. [DOI] [Google Scholar]
- 205.Pakingking R., Jr., Bautista N. B., de Jesus-Ayson E. G., Reyes O. Protective immunity against viral nervous necrosis (VNN) in brown-marbled grouper (Epinephelus fuscogutattus) following vaccination with inactivated betanodavirus. Fish & Shellfish Immunology. 2010;28(4):525–533. doi: 10.1016/j.fsi.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 206.Pakingking R., Jr., Seron R., Dela Peña L., Mori K., Yamashita H., Nakai T. Immune responses of Asian sea bass, Lates calcarifer Bloch, against an inactivated betanodavirus vaccine. Journal of Fish Diseases. 2009;32(5):457–463. doi: 10.1111/j.1365-2761.2009.01040.x. [DOI] [PubMed] [Google Scholar]
- 207.Lauscher A., Krossøy B., Frost P., et al. Immune responses in Atlantic salmon (Salmo salar) following protective vaccination against Infectious salmon anemia (ISA) and subsequent ISA virus infection. Vaccine. 2011;29(37):6392–6401. doi: 10.1016/j.vaccine.2011.04.074. [DOI] [PubMed] [Google Scholar]
- 208.Min L., Li-Li Z., Jun-Wei G., Xin-Yuan Q., Yi-Jing L., Di-Qiu L. Immunogenicity of Lactobacillus-expressing VP2 and VP3 of the infectious pancreatic necrosis virus (IPNV) in rainbow trout. Fish and Shellfish Immunology. 2012;32(1):196–203. doi: 10.1016/j.fsi.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 209.McKenna B. M., Fitzpatrick R. M., Phenix K. V., Todd D., Vaughan L. M., Atkins G. J. Formation of infectious pancreatic necrosis virus-like particles following expression of segment a by recombinant semliki forest virus. Marine Biotechnology. 2001;3(2):103–110. doi: 10.1007/s101260000049. [DOI] [PubMed] [Google Scholar]
- 210.de Kinkelin P. Vaccination in fish. Veterinary Research. 1995;26(3):205–206. [PubMed] [Google Scholar]
- 211.Estepa A., Thiry M., Coll J. M. Recombinant protein fragments from haemorrhagic septicaemia rhabdovirus stimulate trout leukocyte anamnestic responses in vitro . Journal of General Virology. 1994;75(6):1329–1338. doi: 10.1099/0022-1317-75-6-1329. [DOI] [PubMed] [Google Scholar]
- 212.Choi S. H., Kim M. S., Kim K. H. Immunization of olive flounder (Paralichthys olivaceus) with an auxotrophic Edwardsiella tarda mutant harboring the VHSV DNA vaccine. Fish & Shellfish Immunology. 2012;33(3):569–574. doi: 10.1016/j.fsi.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 213.Simon B., Nomellini J., Chiou P., et al. Recombinant vaccines against infectious hematopoietic necrosis virus: production by the Caulobacter crescentus S-layer protein secretion system and evaluation in laboratory trials. Diseases of Aquatic Organisms. 2001;44(1):17–27. doi: 10.3354/dao044017. [DOI] [PubMed] [Google Scholar]
- 214.Cain K. D., LaPatra S. E., Shewmaker B., Jones J., Byrne K. M., Ristow S. S. Immunogenicity of a recombinant infectious hematopoietic necrosis virus glycoprotein produced in insect cells. Diseases of Aquatic Organisms. 1999;36(1):67–72. doi: 10.3354/dao036067. [DOI] [PubMed] [Google Scholar]
- 215.Tian Y. Y., Ye X., Zhang L. L., Deng G. C., Bai Y. Q. Development of a novel candidate subunit vaccine against Grass carp reovirus Guangdong strain (GCRV-GD108) Fish & Shellfish Immunology. 2013;35(2):351–356. doi: 10.1016/j.fsi.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 216.Shao L., Sun X., Fang Q. Antibodies against outer-capsid proteins of grass carp reovirus expressed in E. coli are capable of neutralizing viral infectivity. Virology Journal. 2011;8, article 347 doi: 10.1186/1743-422x-8-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sommerset I., Skern R., Biering E., et al. Protection against Atlantic halibut nodavirus in turbot is induced by recombinant capsid protein vaccination but not following DNA vaccination. Fish and Shellfish Immunology. 2005;18(1):13–29. doi: 10.1016/j.fsi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 218.Choi Y. R., Kim H. J., Lee J. Y., Kang H. A., Kim H.-J. Chromatographically-purified capsid proteins of red-spotted grouper nervous necrosis virus expressed in Saccharomyces cerevisiae form virus-like particles. Protein Expression and Purification. 2013;89(2):162–168. doi: 10.1016/j.pep.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 219.Martinez-Alonso S., Vakharia V. N., Saint-Jean S. R., Pérez-Prieto S., Tafalla C. Immune responses elicited in rainbow trout through the administration of infectious pancreatic necrosis virus-like particles. Developmental and Comparative Immunology. 2012;36(2):378–384. doi: 10.1016/j.dci.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 220.Fang Q., Shah S., Liang Y., Zhou Z. H. 3D reconstruction and capsid protein characterization of grass carp reovirus. Science in China, Series C: Life Sciences. 2005;48(6):593–600. doi: 10.1360/062004-105. [DOI] [PubMed] [Google Scholar]
- 221.Thiéry R., Cozien J., Cabon J., Lamour F., Baud M., Schneemann A. Induction of a protective immune response against viral nervous necrosis in the European sea bass Dicentrarchus labrax by using betanodavirus virus-like particles. Journal of Virology. 2006;80(20):10201–10207. doi: 10.1128/jvi.01098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]