Abstract
Schistosomiasis is a neglected tropical disease with a very long endemic history in Asia. Great strides have been made to control the disease in China and the Philippines but the road to elimination is far from over, given the zoonotic nature of the schistosome parasites in both countries.
Keywords: Schistosomiasis, Neglected tropical disease, Asia, Control, Elimination
1. Introduction
Schistosomiasis (bilharzia) is an intravascular disease caused by parasitic trematode worms of the genus Schistosoma. Five species of Schistosoma are known to infect humans: Schistosoma mansoni, Schistosoma japonicum, Schistosoma mekongi, Schistosoma haematobium, and Schistosoma intercalatum. Infections with S. mansoni, S. japonicum, S. mekongi, and S. intercalatum are associated with chronic liver and intestinal fibrosis, whereas chronic S. haematobium infections can lead to fibrosis, stricturing, and calcification of the urinary tract. The burden of disease attributable to the three major human schistosome species (S. mansoni, S. haematobium, and S. japonicum) is estimated to be between 24 and 29 million disability adjusted life years [1]. In Asia, the major endemic foci of human schistosomiasis infection are found in China, The Philippines, and small pockets of Indonesia (S. japonicum); and, to lesser extent, along the Mekong river on the borders of Cambodia and Laos (S. mekongi). In Japan, elimination of the disease was reached through transmission control by environmental management (i.e. land reclamation to enhance agricultural production and cementing ditches used for rice irrigation) and social economic development [2]. The focus of this review is to compare and contrast the schistosomiasis control initiatives leading to potential elimination in the People’s Republic of China and the Republic of the Philippines.
2. Schistosomiasis in China: close to elimination
Archaeological studies have revealed that schistosomiasis has a very long history in China [3]. S. japonicum eggs were identified in a female corpse dating back to the Western Han dynasty some 2100 years ago that was exhumed in 1971 in Hunan Province. Schistosome eggs were also found in the liver of another corpse buried 100 years earlier in Jianglin Hsien, Hubei Province [3]. In old volumes of traditional Chinese medicine, a description of clinical symptoms resembling Katayama syndrome can be traced back to 400 B.C. The first reported clinical diagnosis in modern China was made by an American physician (O. T. Logan) in 1905 in Hunan Province [3]. After the founding of the People’s Republic of China in 1949, large-scale epidemiological surveys were carried out by Chinese scientists to determine the prevalence, incidence, and intensity of S. japonicum infections. The results revealed that schistosomiasis was endemic in 380 counties in 12 provinces, mainly south of the Yangtze River [3]. Approximately 12 million people were infected, with an additional 100 million people at a serious risk. A total of 14,000 km2 of infected Oncomelania flood plains were identified as potential transmission zones [4].
In the early 1990s the World Bank committed a $71 million USD loan (with a complementary $82 million USD from the PRC government) to China over a ten period for schistosomiasis control [3]. The main goal of the World Bank loan was to reduce the prevalence of schistosomiasis in both humans and bovines (cattle and water buffaloes) by approximately 40% [3]. This ambitious task relied primarily on two approaches. The first involves large-scale chemotherapy for humans and bovines with praziquantel, which has been produced in China since 1978, and the other involves the selective treatment of humans (praziquantel) and snail control with molluscicidal programs and/or environmental modification [3]. Mass chemotherapy was used when the prevalence in a community was higher than 15%. Selective treatment was employed when the prevalence ranged between 3 and 15% or when seropositive individuals aged 7–14 years were identified when the prevalence was less than 3% [3]. The World Bank was also involved in a number of research related activities throughout China, including impact and cost analysis of praziquantel, economic assessment of different control strategies, development of new procedures in the diagnosis of schistosomiasis (antigen or antibody detection), cercaria detection, liver examination with the aid of ultrasound, and health education [3].
In China today, schistosomiasis is still considered as a major public health problem and is listed among the top infectious diseases (HIV/AIDS, tuberculosis, and hepatitis B) in the country prioritized for control and elimination [5]. Major endemic foci occur in the marsh and lake (Dongting Lake and Poyang Lake) regions along the Yangtze River basin, where the elimination of transmission has proven difficult (Fig. 1) [6]. Over the past 50 years, the Chinese government has spent close to one billion US dollars on the control of schistosomiasis and this has resulted in a drop in the number of endemic provinces from twelve to seven. Likewise, there has been a substantial reduction in the human prevalence of schistosomiasis. In major endemic foci the current prevalence ranges from 1 to 5%. As of 2012 the estimated number of human cases was reported to be 365,000. It should be pointed out that the schistosomiasis transmission period in China is for only five months per year over two distinct transmission periods [3], whereas, in the Philippines (and Sub-Saharan Africa) it is continuous.
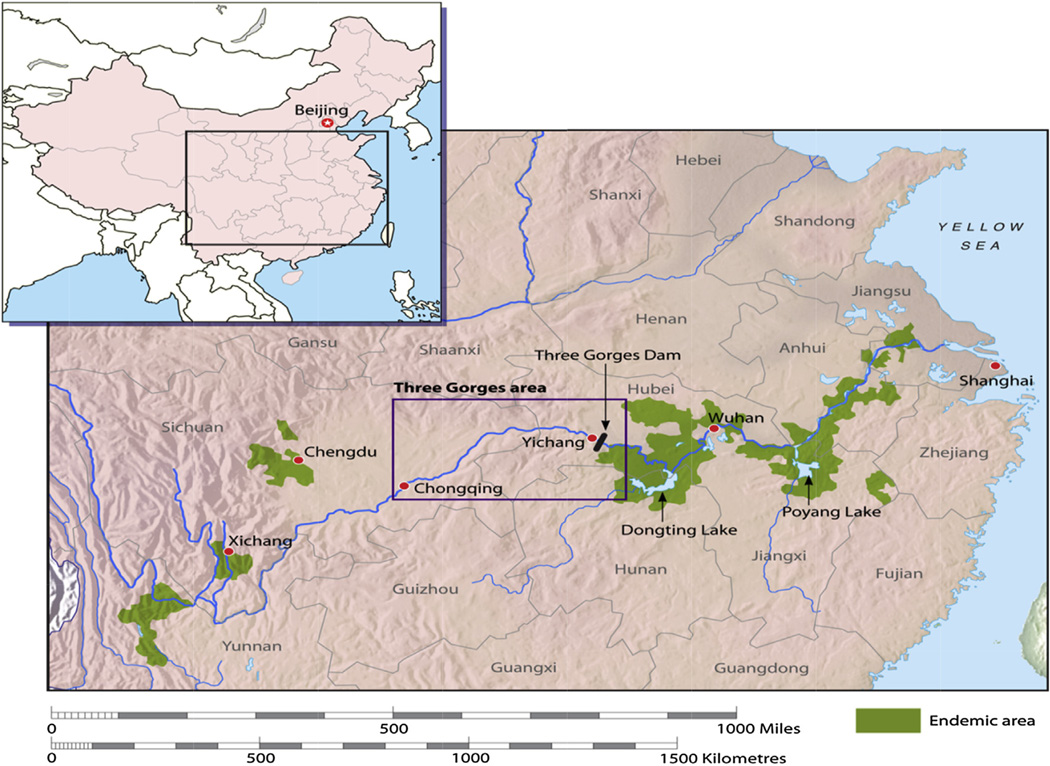
Fig. 1.
The current schistosomiasis-endemic areas (highlighted in green) in the People’s Republic of China. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.) McManus et al., 2010; Ref. [22].
Although it has weaknesses, the schistosomiasis control program for China is recognized as one of the most successful globally, with mass chemotherapy and snail control serving as its cornerstones [3,6] and augmented by various other interventions (as an integrated approach) targeting the S. japonicum transmission pathways. The interventions successfully deployed as part of this integrated control approach include: human and bovine chemotherapy, focal GIS/GPS mollusciciding, sanitation, irrigation, health education (e.g. videos for school children), and, most recently, the replacement of water buffalos with motorized tractors for farming. However, the successful integrated control program in China will be difficult in other countries (e.g. Sub-Saharan Africa) with far weaker economies. China currently employs approximately 20,000 full-time staff working solely on the control of schistosomiasis with an annual operating budget of 120 million US dollars (Table 1). It is noteworthy that since the Chinese economy has prospered over the past decade, many of the young people who resided in the rural endemic villages have now left in search of a better life in the cities. As a direct result, the schistosomiasis-endemic population within China has dropped considerably making disease elimination a possibility in the coming decade.
Table 1.
Summary comparison of the current national control programs for China and the Philippines.
| Index | China | Philippines |
|---|---|---|
| Population | 1344 million | 94 million |
| Endemic provinces | 7 | 24 |
| Estimated human cases | 365,000 | 865,000 |
| Estimated human prevalence | 1–5% | 1–50% |
| Estimated bovine prevalence | <10% | 10–90% |
| Annual budget (@USD) | 120 million | 1.12 million |
| Control staff | 20,000 full-time | 200 part-time |
3. Schistosomiasis in the Philippines: the untold burden
Schistosomiasis was first reported in 1906 in the Philippines and there are approximately 865,000 people infected and another seven million at risk of infection [3]. Major endemic foci are in the poorest regions of the Visayas (Samar and Leyte) and Mindanao (Fig. 2) [7–9]. This includes 24 provinces, 183 municipalities and 1212 barangays (villages) [8]. In 2003 new endemic areas were identified in the northern part of the Philippines. The national schistosomiasis control program was established in 1961 and comprised of passive surveillance and mass praziquantel (PZQ) chemotherapy [8,9]. Unfortunately, control has been sporadic and complicated due to inadequate funding and the zoonotic nature of schistosomiasis japonica. In 1991 the Philippine Health Development Project (PHDP), funded by the World Bank, was initiated for communicable disease control, including schistosomiasis. The PHPD was an intensive case finding and PZQ chemotherapy-based program aimed at treating all infected individuals and was, at first successful in lowering the prevalence of schistosomiasis in the Philippines. However, like similar control programs in Africa and China, following the termination of the PHPD in 1995, schistosome prevalence rebounded due to the inability of PZQ to prevent re-infection and the return to sporadic control [10].
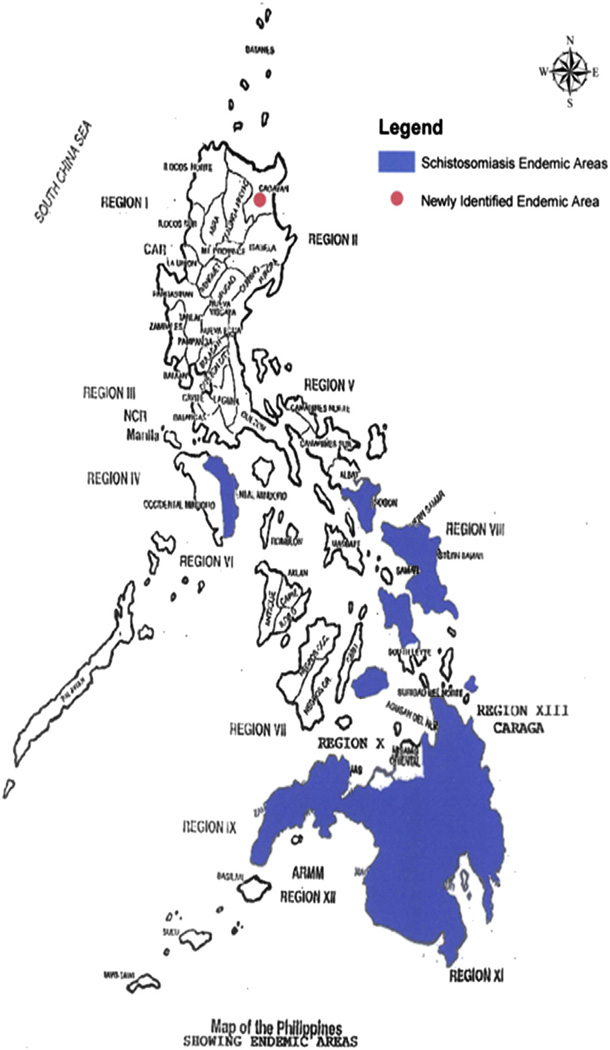
Fig. 2.
Current schistosomiasis endemic areas (highlighted in blue) in the Philippines. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
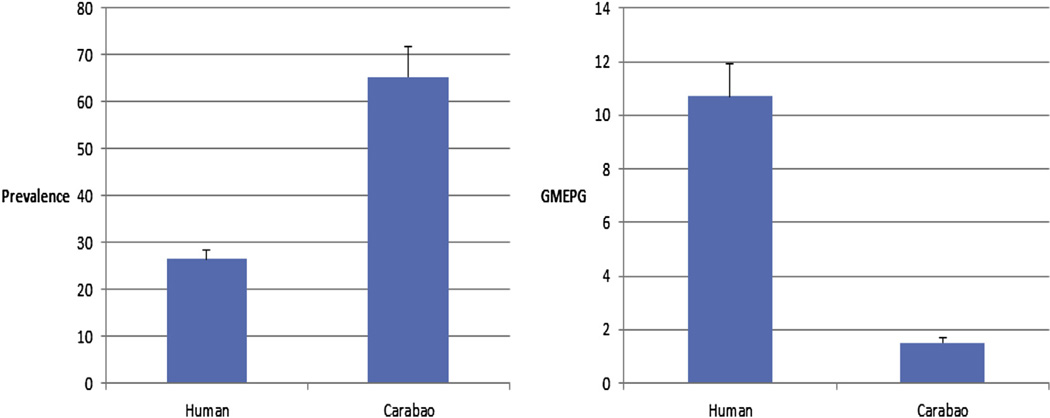
Recent studies by Leonardo and colleagues [11,12], funded by WPRO (World Health Organization Western Pacific Region), reported that the national schistosomiasis prevalence in the Philippines is less than 1% (mean = 0.49%; range = 0.08–3.95). However, these findings grossly understate the current human schistosomias prevalence there. It is notable that in the analysis of their data they used the entire population as the denominator for prevalence calculations rather than the sample size number. We recently (2011) conducted a cross-sectional epidemiological survey (using the Kato-Katz thick smear technique for humans – two stools/three slides per stool; and the FEA-SD technique for carabao) to determine the current schistosomiasis burden across six barangays in Northern Samar, the Philippines. Results are shown in Fig. 3. The overall human prevalence was found to be 26.4% (n = 1955; 95% CI 24.5–28.4%) while in carabao (water buffaloes) it was found to be 65.4%. (n = 211; 95% CI 58.9–71.9%) (Fig. 3, Panel A on left). The corresponding intensities of infection (geometric mean eggs per gram in infected (GMEPG)) were 10.7 (n = 517) and 1.5 (n = 211), respectively, for humans and carabao (Fig. 3, panel B on right). The study was expanded in 2012 to 18 barangays and the human schistosomiasis prevalences in the barangays under study were found to range from 5 to 48%. The results are contrary to those recently reported for Northern Samar where the mean human prevalence was reported to be only 2.45% [11,12]. Moreover, advanced schistosomiasis cases and deaths are now being reported by the media and confirmed by the National Department of Health for Mindanao, Samar, Leyte and Oriental Mindoro (Fig. 4). This latest evidence clearly demonstrates that schistosomiasis has not been eliminated from the Philippines and, indeed, remains a major public health problem.
Fig. 3.
Panel A on the left illustrates the human and carabao schistosomiasis prevalence in six endemic barangays in Northern Samar, The Philippines. Panel B on the right depicts the geometric mean eggs per gram intensities of infections.
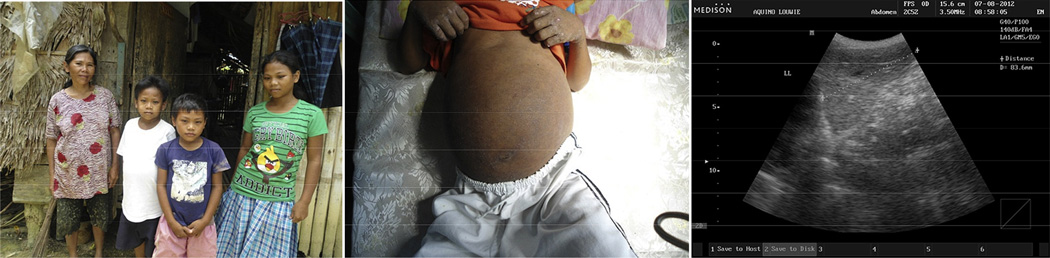
Fig. 4.
Photos depicting a male child age 12 (white shirt, left; white shorts, right) with advanced schistosomiasis and liver fibrosis in Palapag, Northern Samar, The Philippines.
The current (2012) national funding for schistosomiasis control in the Philippines is PHP $47,684,000 ($1,126,477.54 USD). This amount represents only a small fraction (<1%) of what is currently spent on the national schistosomiasis elimination program in China (Table 1). Moreover, the manpower and infrastructure in China far surpasses that of the Philippines. In the Philippines the population in rural endemic areas is growing exponentially; thus more and more individuals are becoming at risk of contracting schistosomiasis. Filipinos residing within schistosomiasis-endemic areas are typically very poor rice farmers with family incomes far below the national average. Most (>95%) are Catholic by faith, usually with 6–10 children and the rates of malnutrition are very high. The Responsible Parenthood and Reproductive Health Act of 2012 (Republic Act No. 10354), informally known as the Reproductive Health Law, is a recent legislation in the Philippines which guarantees universal access to methods of contraception, fertility control, sexual education, and maternal care. What impact this will have on future population growth is yet to be determined. The national population is now quickly approaching 100 million. Over 50% of the endemic population lives in poverty, with rudimentary water, sanitation and hygiene; therefore, the rates of parasitic diseases, acute respiratory infections, diarrhoeal diseases and other communicable diseases are high. In sum, a weak malnourished population will always be susceptible to schistosomiasis infection no matter how many times you treat the individual. The only way forward is to break the life cycle of this persistent disease through multi-component integrated control.
4. Integrated control – the way forward
Schistosomiasis control in China and the Philippines is complicated by the zoonotic nature of the disease, with bovines (water buffalos and cattle) acting as major reservoir hosts. Thus, alternative sustainable control strategies are required to combat schistosomiasis to overcome rapid re-infection, the threat of praziquantel resistance and problems of drug compliance – currently only at 25–40% in China and the Philippines. A multifaceted integrated approach targeting transmission pathways for the disease would comprise: complementing praziquantel treatment with vaccination of bovines and snail control as the key to sustainable control and eventual elimination [10].
In the Philippines, water buffaloes (called carabao) are smaller subspecies (Bubalus bubalis carabanesis) that are found in China, but are common in schistosomiasis-endemic areas interacting with humans, snails and S. japonicum. A study by Wu et al. in Leyte, using a polymerase chain reaction (PCR)-based diagnostic method, showed the S. japonicum prevalence in carabao was 52% [13]. Another recent study of bovines in Samar province in 2010 using real time quantitative PCR (qPCR) showed similar results with greater than 90% of bovines being infected with S. japonicum [14,15]. Thus, there is a little question that bovines play a major role in the transmission of S. japonicum in the Philippines and should thus be targeted for the treatment and vaccination. While dogs, rats and pigs can indeed be infected with S. japonicum in the Philippines and they are considered to be minor contributors to transmission, because rats only produce ~1 g of faeces per day compared with ~250 g produced by humans and dogs and 25 kg produced by carabao. Furthermore, studies have shown that schistosome eggs produced by rats are not viable. Likewise, pigs do not contribute to transmission as they are few in number and are generally penned, limiting the exposure to S. japonicum cercariae. Those pigs that are infected do not release schistosome eggs into snail habitats [16–20].
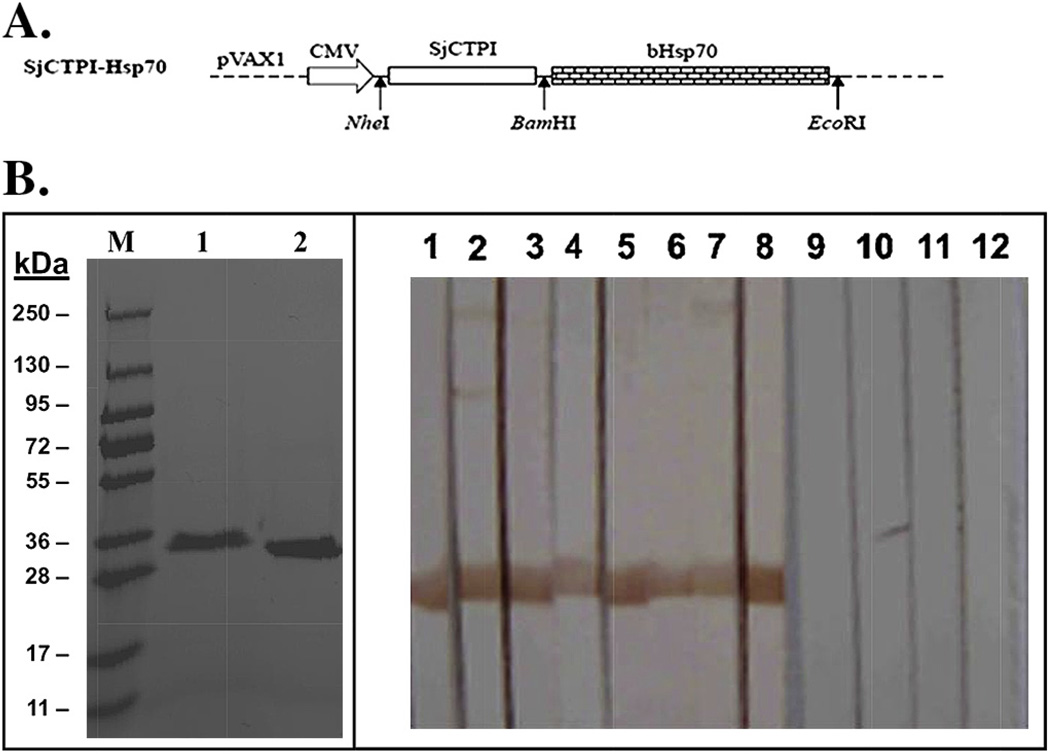
We recently evaluated the efficacy of two schistosome vaccine candidates (SjCTPI and SjC23) as plasmid-DNA vaccines against S. japonicum in water buffaloes in China. Two randomized double-blind control vaccine trials were performed to determine the efficacy levels of the SjCTPI and SjC23 vaccines, both on their own, and where they were fused together with the dendritic cell targeting molecule, heat-shock protein 70 (Hsp70) (SjCTPI-Hsp70 and SjC23-Hsp70) [21]. Both trials used three groups of 15 water buffaloes. Following the prime, all booster vaccines, including controls, were co-administered with an interleukin-12 expressing plasmid as adjuvant. All DNA-vaccine constructs elicited an IgG immune response specific for SjCTPI or SjC23 as determined by Western Blot and ELISA. The DNA vaccine constructs significantly reduced the worm burden (41.5–51.2%) and egg burden (33.2–61.5%) in vaccinated/challenged water buffaloes. Constructs incorporating Hsp70 generated a greater immune response and increased vaccine efficacy. The most successful vaccine was the SjCTPI-Hsp70 construct (Fig. 5), which produced a 51.2% reduction in worm burden, a 61.5% reduction in liver eggs, a 52.1% reduction in faecal eggs and a 52.1% reduction in the hatching of faecal miracidia [21]. Recently we tested a DNA prime protein boost vaccine regimen in water buffalo in China. Using only a prime and boost, with the boost being recombinant proteins in saline with no adjuvant, we achieved an adult worm reduction of 55% and a 57% reduction in the fecal miracidial hatching rate. We developed a new vaccine delivery method that significantly enhances vaccine specific immune responses. We are presently deploying the DNA prime, recombinant protein boost bovine vaccine using the new delivery method as part of an integrated control trial in Northern Samar in the Philippines.
Fig. 5.
A. SjCTPI-Hsp70 construct used for bovine vaccination. B. Expression and Western blotting of SjCTPI-Hsp70.
5. Modelling schistosomiasis elimination
While data are available on the effects of various control measures separately, elimination of schistosomiasis in China and the Philippines will depend on the simultaneous or perhaps, serial integration of a combination of various control options. These would be expected to include chemotherapy (human and/or carabao; mass or targeted), mollusciciding, environmental modification, improved sanitation, and vaccination of definitive bovine hosts. Field trials to estimate the potential for elimination associated with various possible combinations are expensive, time-consuming and problematic to conduct. Mathematical models offer an approach which allows the evaluation of the optimal control strategy to be identified, as well as its associated potential and time-frame for elimination or significantly lowered endemicity. In addition, models can provide information on parameters relating to the configuration of a control program, such as the level of efficacy of individual measures, coverage and the compliance needed to achieve a particular result, as well as the sustainability of the program over time. Estimates of the cost of control measures, and thus cost-effectiveness, can also be derived from a mathematical model and will assist in estimating the effects of these combined strategies and the costs of control. Mathematical models for schistosomiasis were first developed by Hairston [23] and MacDonald [24]. Liang et al. and Spear et al. [24–26] developed mathematical models for schistosomiasis japonica in China, although they did not model transmission in the lake and marshland areas, where the majority of transmission occurs, nor did they model the involvement of bovine hosts in the transmission. Riley et al. [20] developed a S. japonicum transmission dynamics model for the Philippines that tried to account for the additional mammalian hosts and zoonotic transmission. However, there is some conjecture over the parameterization of the model [27] due to the potential underestimation of the bovine S. japonicum prevalence in the Philippines as discussed above.
Williams et al. [28] developed a multi-host model to simulate transmission dynamics of schistosomiasis japonica in the lake and marshland areas of China and to predict the effects of different control strategies. It extended the two-host model of Barbour [29], using human and bovine (water buffalo (carabao) or cattle) definitive hosts to allow for heterogeneity within human and carabao definitive hosts. It consists of a set of simultaneous equations which model the rate of change in prevalence over time:
Where: P = prevalence in the definitive host population; y = prevalence in the intermediate host population (i.e. Oncomelania snails); i = 1, 2, 3,…n hosts; D = definitive host; I = intermediate host; g = cure rate in definitive host; μ = cure rate in intermediate host; tIDi and tDIi = composite transmission parameters for intermediate host → ith definitive host and ith definitive → intermediate host transmission respectively, defined as:
and, where: a = infectious contact rate: intermediate to definitive host; b = infectious contact rate: definitive to intermediate host; Δ = inverse density of intermediate host; and Φ = density of definitive host. The model is parameterized using data of S. japonicum epidemiology including: the distribution of endemic prevalences within host classes, and known features of schistosomiasis japonica such as infection duration. Interventions can then be imposed on the system, and the equations solved numerically to predict the consequences for prevalence and incidence.
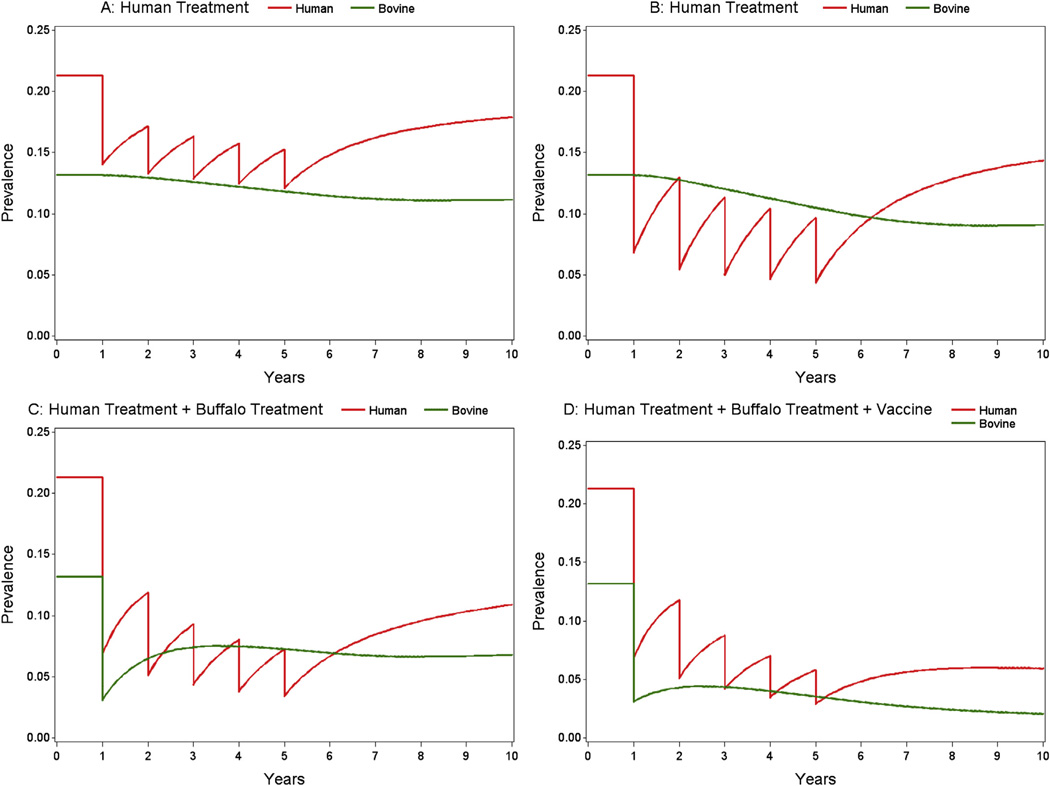
Simulation of transmission and control using this model has predicted that bovines are major reservoirs for human infection in the lake and marshland areas of China [27,29], that human mass chemotherapy alone is not sufficient for long-term sustainable control [29,30], and that an integrated approach, such as a combination of human chemotherapy and bovine vaccination, is required for the long-term, sustainable control of schistosomiasis in China [21,27,28]. With regard to the last scenario, the model predicts that a hypothetical S. japonicum vaccine capable of reducing the fecal egg output of water buffaloes by 45% alone or in conjunction with PZQ treatment will lead to a significant reduction in transmission [28]. In fact, the two DNA plasmid vaccines, SjCTPI-Hsp70 and SjC23-Hsp70, developed and tested experimentally by Da’Dara et al. [21] both exceeded this hypothetical level. Indeed, mathematical modelling of SjCTPIHsp70 and SjC23-Hsp70 alone and in conjunction with human chemotherapy showed a significant reduction in transmission, almost to the point of elimination (Fig. 6).
Fig. 6.
Prevalence of schistosomiasis infection in humans and bovines for four different control strategies over a ten year period. A: Annual mass human treatment for five years, with 85% efficacy and 40% coverage; B: annual mass human treatment, for five years, with 85% efficacy and 80% coverage; C: annual mass human treatment, for five years, with 85% efficacy and 80% coverage, plus one mass bovine treatment with 85% efficacy and 90% coverage; D: annual mass human treatment, for five years, with 85% efficacy and 80% coverage, plus one mass bovine treatment, plus bovine vaccine with 52% efficacy and 90% coverage.
6. Challenges ahead
The public health problem of schistosomiasis in China and the Philippines is a formidable one. Differences in the habitat and ecology of the intermediate snail host found in the Philippines have made it difficult to duplicate the successful snail control programs in China. Climatic conditions and rice farming methods have also made snail control difficult. During the pre-praziquantel era, approaches used were aimed at decreasing transmission by reducing the numbers of the snail intermediate host and by limiting human exposure to the infective form of the parasite. There were significant achievements, but not impressive enough to remarkably reduce the national prevalence of S. japonicum infection in the Philippines. Improved sanitation was an essential component of the control program but was difficult to sustain in areas where no more than one third of the population have satisfactory latrines. So, what parts of an integrated control package will work and be sustainable in the long run remains a question. We are presently conducting a clinical trial in Northern Samar to answer that question. Whatever the package will ultimately be, we are confident that a transmission blocking vaccine targeting bovines will serve as a vital component in the Philippines.
Acknowledgements
The authors would like to thank the UBS-Optimus Foundation, the National Health and Medical Research Council, Australia for providing financial support for the schistosomiasis research in the Philippines, and the National Institutes of Health Award (A1068109) to DAH (USA) for support of production of the schistosome vaccines. YSL is an Australian Research Council Future fellow; DJG is an Australian Research Council fellow (DECRA); DPM is an NHMRC Senior Principal Research Fellow.
References
- 1.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Utzinger J, Zhou XN, Chen MG, Bergquist R. Conquering schistosomiasis in China: the long march. Acta Trop. 2005;96:69–96. doi: 10.1016/j.actatropica.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Ross AG, Sleigh AC, Li YS, Davis GM, Williams GM, Jiang Z, Feng Z, McManus DP. Schistosomiasis in the People’s Republic of China: prospects and challenges for the 21st century. Clin. Microbiol. Rev. 2001;14:270–295. doi: 10.1128/CMR.14.2.270-295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou XN, Guo J, Wu XH, Jiang QW, Zheng J, Dang H, Wang XH, Xu J, Zhu HQ, Wu GL, Li YS, Xu XJ, Chen HG, Wang TP, Zhu YC, Qiu DC, Dong XQ, Zhao GM, Zhang SJ, Zhao NQ, Xia G, Wang LY, Zhang SQ, Lin DD, Chen MG, Hao Y. Epidemiology of schistosomiasis in the Peoples’ Republic of China. Emerging Infect. Dis. 2007;13:1470–1476. doi: 10.3201/eid1310.061423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Utzinger J, Zhou XN. Schistosomiasis control: experiences and lessons from China. Lancet. 2008;372:1793–1795. doi: 10.1016/S0140-6736(08)61358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou XN, Wang LY, Chen MG, Wu XH, Jiang QW, Chen XY, Zheng J, Utzinger J. The public health significance and control of schistosomiasis in China—then and now. Acta Trop. 2005;96:97–105. doi: 10.1016/j.actatropica.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Blas BL, et al. The schistosomiasis problem in the Philippines: a review. Parasitol. Int. 2004;53:127–134. doi: 10.1016/j.parint.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Leonardo LR, et al. Difficulties and strategies in the control of schistosomiasis in the Philippines. Acta Trop. 2002;82:295–299. doi: 10.1016/s0001-706x(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 9.Tarafder MR, et al. Across-sectional study of the prevalence of intensity of infection with Schistosoma japonicum in 50 irrigated and rain-fed villages in Samar Province, the Philippines. BMC Public Health. 2006;6:1–10. doi: 10.1186/1471-2458-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray DJ, McManus DP, Li YS, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide at future. Lancet Infect. Dis. 2010;10:733–736. doi: 10.1016/S1473-3099(10)70099-2. [DOI] [PubMed] [Google Scholar]
- 11.Leonardo L, Rivera P, Saniel O, Villacorte E, Lebanan MA, Crisostomo B, Hernandez L, Baquilod M, Erce E, Martinez R, Velayudhan R. A national baseline prevalence survey of schistosomiasis in the Philippines using stratified two-step systematic cluster sampling design. J. Trop. Med. 2012;2012:936128. doi: 10.1155/2012/936128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonardo LR, Rivera P, Saniel O, Villacorte E, Crisostomo B, Hernandez L, Baquilod M, Erce E, Martinez R, Velayudhan R. Prevalence survey of schistosomiasis in Mindanao and the Visayas, The Philippines. Parasitol. Int. 2008;57:246–251. doi: 10.1016/j.parint.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Qin Y, Chu K, Meng R, Liu Y, McGarvey S, Olveda RM, Acosta LP, Ji MJ, Fernandez T, Friedman J, Kurtis J. High prevalence of Schistosoma japonicum infection in water buffaloes in the Philippines assessed by real time polymerase chain reaction. Am. J. Trop. Med. Hyg. 2010;82:646–652. doi: 10.4269/ajtmh.2010.09-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu B, Gordon CA, Hu W, McManus DP, Chen HG, Gray DJ, Ju C, Zeng XJ, Gobert GN, Ge J, Lan WM, Xie SY, Jiang WS, Ross AG, Acosta LP, Olveda R, Feng Z. A novel procedure for precise quantification of Schistosoma japonicum eggs in bovine faeces. PLoS Neglected Trop. Dis. 2012;6:e1885. doi: 10.1371/journal.pntd.0001885. http://dx.doi.org/10.1371/journal.pntd.0001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon CA, Acosta LP, Gray DJ, Olveda RM, Jarilla B, Gobert GN, Ross AG, McManus DP. High prevalence of Schistosoma japonicum infection in carabao from Samar Province, the Philippines: implications for transmission and control. PLoS Neglected Trop. Dis. 2012;6:e1778. doi: 10.1371/journal.pntd.0001778. http://dx.doi.org/10.1371/journal.pntd.0001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumag PU, et al. Epidemiology of animal schistosomiasis in the Philippines. Philipp. J. Anim. Ind. 1981;36:1–23. [Google Scholar]
- 17.Fernandez TJ, et al. An epidemiological study on Schistosoma japonicum in domestic animals in Leyte, Philippines. Southeast Asian J. Trop. Med. Public Health. 1982;13:575–579. [PubMed] [Google Scholar]
- 18.McGarvey ST, et al. Cross-sectional associations between intensity of animal and human infection with Schistosoma japonicum in Western Samar Province, Philippines. Bull. World Health Organ. 2006;84:446–452. doi: 10.2471/blt.05.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez TJ, et al. Prevalence of Schistosoma japonicum infection among animals in fifty villages of Samar Province, the Philippines. Vector Borne Zoonotic Dis. 2007;7:147–155. doi: 10.1089/vbz.2006.0565. [DOI] [PubMed] [Google Scholar]
- 20.Riley S, Carabin H, Belisle P, Joseph L, Tallo V, Balabong E, Willingham AL, Fernandez TJ, Gonzales RO, Olveda RM, McGarvey ST. Multi-host transmission dynamics of Schistosoma japonicum in Samar Province, the Philippines. PLoS Med. 2008;5:e18. doi: 10.1371/journal.pmed.0050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Da’Dara AA, Li YS, Xiong T, Zhou J, Williams GM, McManus DP, Feng Z, Yu XL, Gray DJ, Harn DA. DNA-based vaccine protects against zoonotic schistosomiasis in water buffalo. Vaccine. 2008;26:3617–3625. doi: 10.1016/j.vaccine.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McManus DP, Gray DJ, Li YS, Feng Z, Williams GM, Stewart D, Rey-Ladino J, Ross AG. Schistosomiasis in the Peoples’ Republic of China: the era of the Three Gorges Dam. Clin. Microbiol. Rev. 2010;23:442–466. doi: 10.1128/CMR.00044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hairston NG. On the mathematical analysis of schistosome populations. Bull. World Health Organ. 1965;33:45–62. [PMC free article] [PubMed] [Google Scholar]
- 24.Macdonald G. The dynamics of helminth infections, with special reference to schistosomes. Trans. R. Soc. Trop. Med. Hyg. 1965;59:489–506. doi: 10.1016/0035-9203(65)90152-5. [DOI] [PubMed] [Google Scholar]
- 25.Liang S, Seto E, Remais JV, Zhong B, Yang C, Hubbard A, Davis GM, Gu X, Qiu D, Spear RC. Environmental effects on parasitic disease transmission exemplified by schistosomiasis in western China. Proc. Natl. Acad. Sci. 2007;104:7110–7115. doi: 10.1073/pnas.0701878104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang S, Spear RC, Seto E, Hubbard A, Qiu D. A multi-group model of Schistosoma japonicum transmission dynamics and control: model calibration and control prediction. Trop. Med. Int. Health. 2005;10:263–278. doi: 10.1111/j.1365-3156.2005.01386.x. [DOI] [PubMed] [Google Scholar]
- 27.Spear RC, Hubbard A, Liang S, Seto E. Disease transmission models for public health decision making: toward an approach for designing intervention strategies for schistosomiasis japonica. Environ. Health Perspect. 2002;110:907–915. doi: 10.1289/ehp.02110907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray DJ, Williams GM, Li YS, McManus DP. Transmission dynamics of Schistosoma japonicum in the lake and marshlands region of China. PLoS One. 2008;3:e4058. doi: 10.1371/journal.pone.0004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams GM, Sleigh AC, Li YS, Feng Z, Davis GM, Chen H, Ross AG, Bergquist R, McManus DP. Mathematical modelling of schistosomiasis japonica: comparison of control strategies in the Peoples’ Republic of China. Acta Trop. 2002;82:253–262. doi: 10.1016/s0001-706x(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 30.Guo J, Li YS, Gray DJ, Ning A, Hu G, Chen H, Davis GM, Sleigh AC, Zheng F, McManus DP, Williams GM. A drug-based intervention study on the importance of buffaloes for human Schistosoma japonicum infection around Poyang Lake, People’s Republic of China. Am. J. Trop. Med. Hyg. 2006;74:335–341. [PubMed] [Google Scholar]