Abstract
Glioblastoma (GBM) is the most common malignant adult brain tumor and carries a poor prognosis due to primary and acquired resistance. While many cellular features of GBM have been documented, it is unclear if cells within these tumors extend a primary cilium, an organelle whose associated signaling pathways may regulate proliferation, migration, and survival of neural precursor and tumor cells. Using immunohistochemical and electron microscopy (EM) techniques, we screened human GBM tumor biopsies and primary cell lines for cilia. Immunocytochemical staining of five primary GBM cell lines revealed that between 8 and 25 % of the cells in each line possessed gamma tubulin-positive basal bodies from which extended acetylated, alpha-tubulin-positive axonemes. EM analyses confirmed the presence of cilia at the cell surface and revealed that their axonemes contained organized networks of microtubules, a structural feature consistent with our detection of IFT88 and Arl13b, two trafficked cilia proteins, along the lengths of the axonemes. Notably, cilia were detected in each of 23 tumor biopsies (22 primary and 1 recurrent) examined. These cilia were distributed across the tumor landscape including regions proximal to the vasculature and within necrotic areas. Moreover, ciliated cells within these tumors co-stained with Ki67, a marker for actively dividing cells, and ZEB1, a transcription factor that is upregulated in GBM and linked to tumor initiation, invasion, and chemoresistance. Collectively, our data show that subpopulations of cells within human GBM tumors are ciliated. In view of mounting evidence supporting roles of primary cilia in tumor initiation and propagation, it is likely that further study of the effects of cilia on GBM tumor cell function will improve our understanding of GBM pathogenesis and may provide new directions for GBM treatment strategies.
Keywords: Glioma, Cilium, Intraflagellar transport, Cancer
Introduction
The intrinsic and acquired resistance of glioblastoma (GBM) tumors to aggressive treatments that combine surgery, chemotherapy, and radiation, limits the post-diagnosis median survival of GBM patients to ~12–18 months [1]. Improvements in these patients’ prognoses will require a better understanding of the factors that permit survival, migration, and proliferation of GBM cells within the brain. The GBM microenvironment contains a diverse mixture of cells that includes brain tumor stem cells, microglia, astrocytes, stromal cells, endothelial cells, and pericytes (for reviews see [2–4]). Successful proliferation and survival of GBM cells within this microenvironment have been proposed to be highly dependent on signaling between these cells. Thus, improving our understanding of intra- and inter-cellular communication within the tumor and its microenvironment may provide important new insights that could lead to the development of more effective GBM treatments.
Primary cilia are non-motile, hair-like organelles that play a key role in promoting neural cell proliferation [5, 6], migration [7, 8], and survival [9] in the developing and adult CNS. Their ability to influence these important cellular events is linked to their ability to detect various mitogens [10, 11], neuropeptides [12], and secreted growth factors [13, 14] in the extracellular environment. Previous analyses of five commonly studied astrocytoma and GBM cell lines (U87-MG, T98G, U-373G, U-138MG, U-251 MG) have suggested that these cell lines, which have been in existence for about 30–50 years [15, 16], typically fail to elaborate primary cilia [17]. Given the important roles cilia signaling pathways may play in regulating neural cell behavior in developing and adult CNS, and recent reports showing that basal cell carcinomas and medulloblastoma tumors contain ciliated cells that influence the formation and maintenance of these tumors [18–20], we decided to revisit the question of GBM cell ciliogenesis by examining primary human GBM specimens and derivative primary cell lines using immunostaining and EM to determine whether they contain ciliated cells.
Methods
GBM biopsies and primary GBM cells
GBM biopsies
The brain tumor biopsies analyzed in this study were collected from archived tumor samples housed within the Florida Center for Brain Tumor Research Repository. All tissue samples were obtained with informed consent and were collected using protocols approved by the University of Florida Institutional Review Board approval (to M.R.S, B.A.R, and D.A.S). Biopsies were classified by neuropathologists as either glioblastoma or grade III glioma according to WHO guidelines [21]. For immunohisto-chemistry, we examined a total of 22 biopsies that were fixed overnight at 4 °C in 4 % paraformaldehyde in 0.1 M phosphate buffer (4 % PFA). There were some differences in how the 22 biopsies were handled prior to fixation overnight. Four of the biopsies were immersed in 4 % PFA immediately following collection, which in our hands proved to be optimal for immunohistochemistry. Six biopsies underwent cryopreservation in supplemented NeuroCult™ media (StemCell Technologies, Inc.) containing 10 % DMSO at collection and were then thawed into 4 % PFA for fixation overnight. Finally, 12 biopsies were snap frozen in liquid N2 at collection and then thawed into 4 %PFA for fixation overnight. After fixation, biopsies were rinsed in PBS, placed in 30 % sucrose, frozen in OCT compound over liquid N2, and cryosections (20 μm) were collected onto coated slides. For EM, we fixed one biopsy (~1 h post-surgery) in a mixture of 3 % PFA and 2 % glutaraldehyde in 0.1 M phosphate buffer for 24 h at 4 °C and processed the tissue as described below.
Primary GBM cells and spheres
We examined five GBM primary cell lines in this study (Line 0, Line 1, Line 2, SN179 (S2), and SN186 (S3)) that were isolated from human GBM tumors [22, 23]. Spheres of cells were cultured in Neurobasal media supplemented with 20 ng/ml human epidermal growth factor (hEGF), 10 ng/ml basic fibroblast growth factor (bFGF), and 2 μg/ml heparin. When the spheres reached approximately 150 μm in diameter, they were enzymatically dissociated by digestion with a solution containing trypsin/EDTA (0.05 %) for 3–5 min at 37 °C. Finally, cells were washed, counted using trypan blue to exclude dead cells, and re-plated in fresh media supplemented with hEGF and bFGF. For spheres or cells grown on glass coverslips, Neurobasal media was supplemented with 5 % fetal bovine serum. Spheres or cells after 2 or 24 h of growth, respectively, were fixed for 15 min in 4 % PFA and washed with PBS.
Serum starvation
For serum starvation experiments, S2 and S3 cells were seeded onto glass coverslips in 24 well plates in Neuro-basal media containing FBS but no growth factors. Twenty-four hours later, the media in all cultures was changed and in selected wells was replaced with media lacking FBS. After 24 or 72 h, the cultures were fixed in 4 % PFA and the percentage of ciliated cells was determined as described below.
Antibodies
The primary antibodies used for immunocytochemistry (ICC) or immunohistochemistry (IHC) included mouse anti-acetylated alpha-tubulin (1:3000(ICC/IHC); Sigma), rabbit anti-Arl13b (1:1500 (ICC); gift from T. Caspary), mouse anti-gamma tubulin (1:2000 (ICC/IHC); Sigma), rabbit anti-IFT88 (1:200 (ICC); Proteintech), rabbit anti-Ki67 (1:200 (ICC/IHC); Vector), and rabbit anti-ZEB1 (1:1000 (ICC/IHC); Sigma). Sections were blocked with anti-mouse Fab fragments (20 μg/ml: Jackson Immunore-search) when stained sequentially with mouse antibodies against gamma- and acetylated alpha-tubulin. Secondary antibodies were species-specific and were conjugated with fluorescent tags (1:400 (ICC/IHC); Jackson Immunore-search). Slides were coverslipped with Prolong Gold antifade media containing DAPI (Invitrogen).
Immunostaining and quantification of ciliated cells
GBM tumor biopsies and primary cell lines were immunostained with anti-acetylated alpha-tubulin and gamma-tubulin antibodies to label the axoneme and basal bodies, respectively. Primary cilia can be identified by searching for G-tubulin-positive basal bodies proximate to AA-tubulin-positive axonemes [5, 19, 20]. The stained sections were examined using an Olympus IX81-DSU confocal microscope fitted with a 60× water objective and all images were captured as z-stacks (0.5 μm steps). Because we did not observe multi-ciliated cells during our analyses, we quantified the numbers of ciliated cells in dissociated cultures of the primary cell lines by randomly selecting 4–6 microscopic fields per coverslip (3–5 coverslips/cell line) and then counting the number of DAPI-labeled nuclei and cilia in each of the fields/z-stacks. The percentage of ciliated cells was calculated as the number of cilia/number of DAPI-labeled nuclei for each field. To quantify the numbers of ciliated cells in cultured spheres we counted the total number of DAPI-labeled nuclei and cilia within z-stacks of spheres averaging 40–50 cells in size (8–10 spheres/group). The percentage of ciliated cells was calculated as the number of cilia/number of DAPI-labeled nuclei for each sphere. For analyses of GBM biopsies, biopsies were considered to contain ciliated cells if cilia were observed within the first 2–3 z-stacks of each stained section. For analyses of the distribution of ciliated cells within the tumor biopsies, we focused our analyses on four tissue samples that had been immersed in fixative immediately following harvest. The percentage of ciliated cells located within blood vessels, which were identified using rabbit anti-laminin antibody (not shown), or within either a 30 μm or >50 μm radius of a blood vessel was determined by examining z-stack images of randomly selected microscopic fields.
Electron microscopy
Spheres of Line 0 cells were grown in growth-factor supplemented, serum-free Neurobasal media (described above) and were then fixed in a mixture of 3 % PFA and 2 % glutaraldehyde for 1 h. The fixed spheres and biopsy tissues were washed in 0.1 MPB, post-fixed in 1 % osmium tetroxide, dehydrated in ethanol, and embedded in resin. Semithin sections were stained with toluidine blue. Ultrathin sections (70 nm) were collected onto grids, stained with uranyl acetate and lead citrate, and viewed using a Hitachi H-7600 transmission electron microscope at 80 kV. Images were captured with a Hitachi digital camera and proprietary software.
Statistical analyses
Data obtained from the serum starvation experiments were analyzed using two-way ANOVA with treatment and time as main factors. The groups compared (n = 4 coverslips/group) were as follows: 24 h (+FBS), 24 h (–FBS), 72 h (+FBS) and 72 h (–FBS). The significant (p < 0.05) treatment × time interaction identified using two-way ANOVA was followed up with Fisher’s PLSD post hoc comparisons of treatment at each time point. A p-value less than 0.05 was considered significant. All analyses were carried out using StatView 5.0 software (SAS Institute Inc, NC).
Results
GBM primary cell lines contain subpopulations of ciliated cells
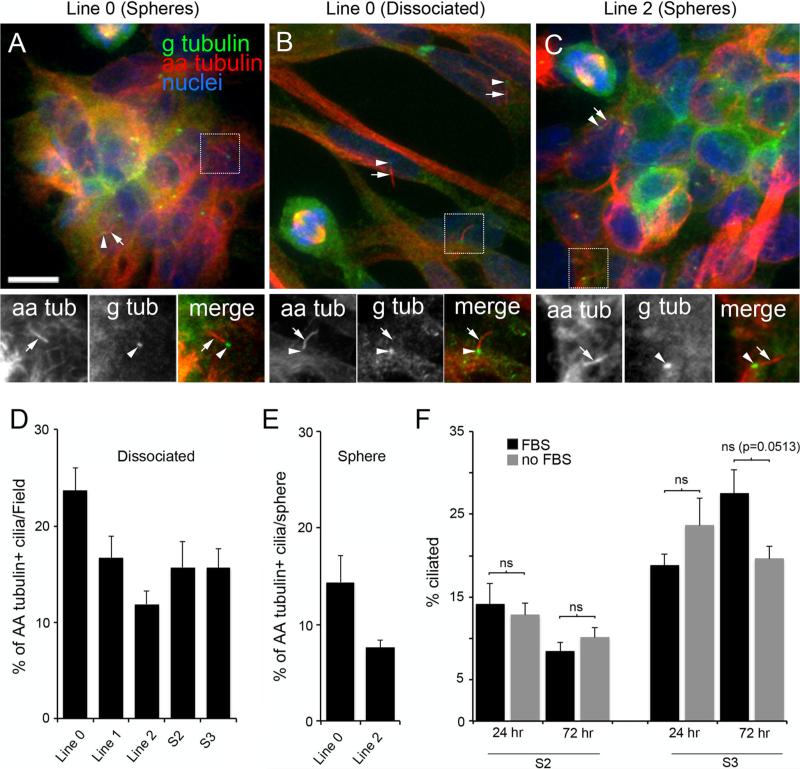
Acetylated alpha-tubulin (AA-tubulin) and gamma-tubulin (G-tubulin) are microtubule proteins that are enriched in the axonemes and anchoring basal bodies of most cilia, respectively, and are critical for maintaining cilia structure [5, 19, 24]. Examination of primary GBM cells and spheres co-stained with antibodies recognizing AA-tubulin and G-tubulin revealed subpopulations of cells that stained positively for both G- and AA-tubulin in a manner consistent with the presence of well-formed primary cilia (Fig. 1a–c). Analyses of the number of ciliated cells in dissociated cell cultures revealed that 23.7 ± 2.4 % of Line 0, 16.7 ± 2.3 % of Line 1, 11.9 ± 1.4 % of Line 2, 15.7 ± 2.6 % of S2, and 15.7 ± 2.0 % of S3 cells were ciliated (mean ± SEM; Fig. 1d). Analyses of the number of ciliated cells in spheres generated from two cell lines revealed that 14.4 ± 2.7 % of Line 0 and 7.6 ± 0.8 % of Line 2 cells in these spheres were ciliated (Fig. 1e). These results show that primary cell lines derived from GBM tumors contain subpopulations of cells that retain their ability to synthesize cilia.
Fig. 1.
Subsets of cultured GBM cell lines derived from human GBM specimens stain positively for primary cilia. (a–c) Sphere aggregates and dissociated Line 0 (a, b) and Line 2 (c) cells were immunostained for gamma-tubulin (G-tubulin or g tub) (green) and acetylated alpha-tubulin (AA-tubulin or aa tub) (red). G-tubulin is enriched in basal bodies and spindle poles, and AA-tubulin is enriched in spindle fibers and the cilia axoneme but can also be detected throughout the cell. The spatial relationship of the staining of these two proteins was used to identify cilia. All images are maximum projections from confocal z-stacks. Scale bar (a) = 10 lm. In a–c, magnified images of the boxed regions showing the staining patterns for aa tub (arrows) and g tub (arrowheads) are displayed below the larger image. The adjacent localization of g tub and aa tub is clearly evident in the merged images. Additional cilia are indicated in the larger images using arrows/arrowheads. d Percentage (±SEM) of AA-tubulin + ciliated cells/field for the indicated cell lines, 24 h after seeding onto glass coverslips. e Percentage (±SEM) of AA-tubulin + ciliated cells/sphere for indicated lines, 2 h after seeding onto glass coverslips. f Percentages (±SEM) of S2 or S3 cells that were ciliated at either 24 or 72 h after serum removal compared to control cells maintained in FBS. Groups were compared using a 2-way ANOVA followed by Fisher's PLSD post hoc analysis. ns not significant
Serum starvation has been used as a strategy to induce ciliogenesis in various cell types [17, 25]. Thus, we asked whether such treatment could increase the number of ciliated cells in dissociated S2 or S3 GBM cells. Our results showed that serum starvation for either 24 or 72 h did not significantly increase the relative numbers of ciliated S2 cells (F(1,12) = 0.014, p = 0.908) or S3 cells (F(1,12) = 0.403, p = 0.537) in our cultures (Fig. 1f). These results suggest that GBM cells may not respond to traditional measures of inducing ciliogenesis.
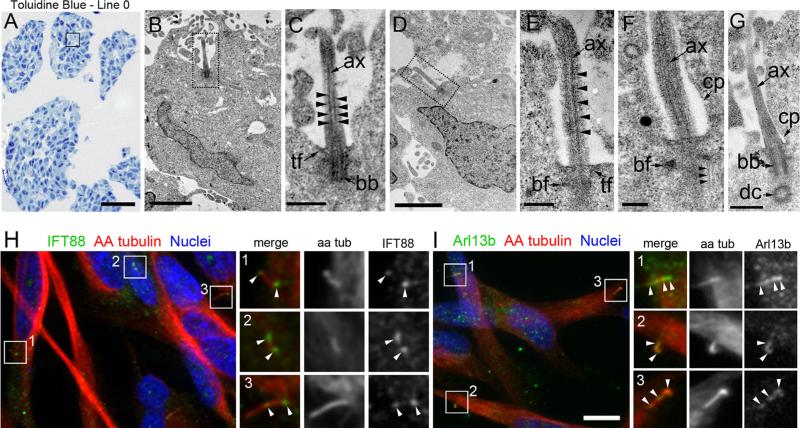
Next, we examined the ultrastructure of the cilia elaborated by cells in Line 0 spheres using EM (Fig. 2a). We detected several cells with docked basal bodies and elon-gated axonemes (Fig. 2b–g). The microtubules within these axonemes were well-organized and extended along the entire length of the visible axoneme (Fig 2c, e). The well-formed, microtubular axoneme backbone observed in these cilia would be expected to support intraflagellar transport (IFT), that is essential for the formation and function of cilia ([26]; for review see [27, 28]). This prediction is supported by our data showing IFT88, a complex B protein that carries cargo to the cilia tip, was localized to the ciliary base, axoneme, and tips of AA-tubulin + cilia (Fig. 2h). In addition, we detected Arl13b, a small GTPase reported to be trafficked into neural cilia [29], localized along the length of the ciliary axonemes of the Line 0 cells, (Fig. 2i). These results suggest that the structure of the cilia elaborated by GBM cells is normal and that they are capable of trafficking cargo along their axonemes.
Fig. 2.
Ultrastructure of Line 0 cilia and detection of IFT88 and Arl13b along their axonemes. a Semithin sections of Line 0 spheres stained with toluidine blue. The box approximates regions where EM analysis was performed. b Low magnification of a cilium (dotted box) extending out of a cell. c Higher magnification of the cilium in (b) reveals a docked basal body anchored by transition fibers and an axoneme comprised of organized, parallel arrangements of microtubules (arrowheads). d Another example of a cilium (dotted box) extending out of the cell body, whose axoneme contained organized, parallel arrangements of microtubules (e, arrowheads). f, g Additional high magnification images of cilia showing axonemes extending out of the ciliary pocket. Typical parallel arrangements of microtubules that impinged on the basal foot (f, arrowheads) and daughter centrioles arranged perpendicularly to basal bodies were observed (g). h Immunostaining for IFT88 (green) revealed localization to the bases, axonemes, and tips of Line 0 cell cilia. Boxes 1–3 highlight IFT88 + cilia that are shown in the magnified images on the right (arrowheads). i Immunostaining for Arl13b (green) revealed localization along the ciliary axonemes and tips. Boxes 1-3 highlight Arl13b + cilia that are shown in the magnified images on the right (arrowheads).ax axoneme, tf transition fibers, bb basal body, bf basal foot, dc daughter centriole, cp ciliary pocket. Scale bars in A = 100 μm, B, D = 2 μm, C = 500 nm, E = 250 nm, F = 200 nm, G = 500 nm, I = 10 μm
Surgically resected primary GBM tumors contain ciliated cells
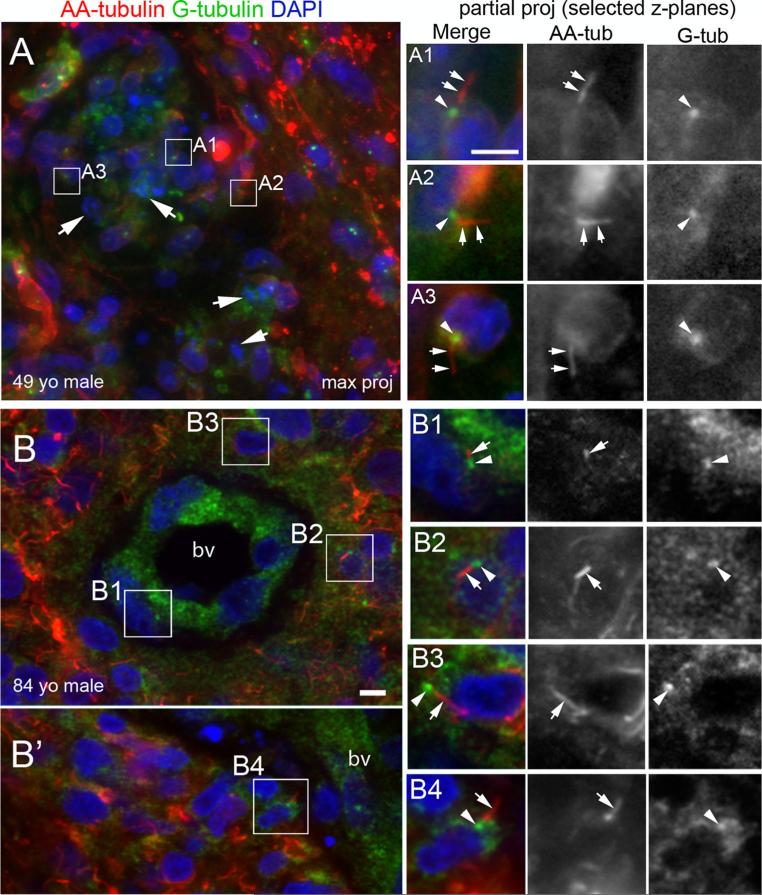
We next asked whether ciliated cells persist in surgically resected specimens. We examined sections of 22 different GBM biopsies (21 primary and 1 recurrent) stained with antibodies to AA-tubulin and G-tubulin to determine if these biopsies contained ciliated cells. We detected G- and AA-tubulin + cilia in all 22 biopsies, including the tumor resected from a patient with recurrent GBM (data not shown). Moreover, cilia appeared to be distributed throughout the GBM microenvironment. Examination of a tumor removed from a 49-year-old male revealed that ciliated cells were present within and surrounding the necrotic zones of the tumor (Fig. 3a). Examination of another tumor from an 84-year-old male revealed subsets of ciliated cells within and immediately surrounding the blood vessels (Fig. 3b and b’, boxed areas show zoomed views of cilia). Ciliated cells were also found adjacent to and within pseudopalisades (data not shown). The presence of cilia around the vasculature prompted an analysis of the spatial distribution of cilia in four GBM biopsies. Overall, we found cilia on 14.1 ± 4.5 % of cells within blood vessels, 24.6 ± 4.2 % of cells within a 30 μm radius of blood vessels, and 18.2 ± 5.2 % of cells 50 μm or further away from blood vessels. Together, these results suggest that ciliated cells can be detected in different regions of the tumor microenvironment.
Fig. 3.
Identification of cilia in GBM biopsies. (a, b) Representative examples of G-tubulin/AA-tubulin + cilia in 2 out of 22 stained GBM biopsies. Cilia were detected throughout the tumor microenvironment. a Biopsy from a 49-year-old male immunostained for G-tubulin (blue) and AA-tubulin (red). Nuclei were stained with DAPI (green). This region contained nuclear debris and several apoptotic nuclei (arrows). Ciliated cells are indicated in boxed regions. A1–A3 Magnified images of boxed regions in A (partial projections representing 2–3 collapsed z-stacks within the maximum projected) show the G-tubulin positive basal bodies (arrowheads) and AA-tubulin positive axonemes (arrows) of the cilia. Bars (μm) = 10 (A), 5 (A1). B and B’ Biopsy from an 84 year-old male stained with G-tubulin (blue) and AA-tubulin (red) revealed subsets of ciliated cells within and surrounding blood vessels (bv). B1–B4 Magnified images of boxed regions in B and B’ clearly show adjacent localization of G- and AA-tubulin staining on the cilia. Cilium in B1 is located on a cell within the bv wall. Cilia in B2–B4 are found on cells surrounding the bv. Scale bar B = 5 μm
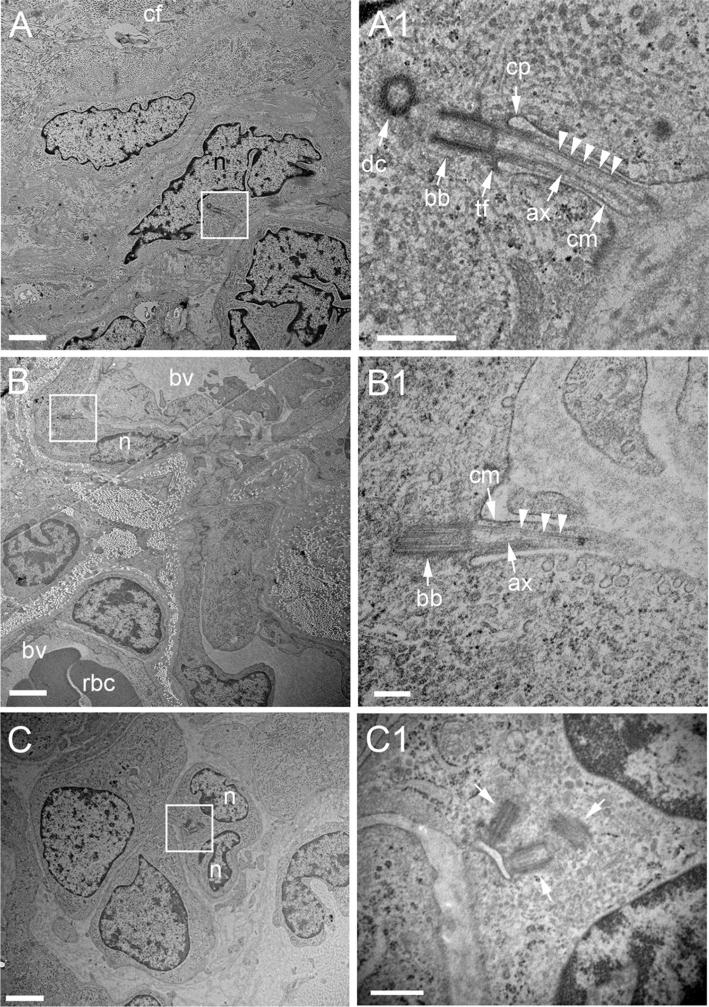
To further confirm our immunohistochemical detection of cilia in GBM, we also performed an EM analysis of a GBM biopsy freshly extracted from the right occipital lobe of a 61-year-old male. Ultrastructurally, this tumor was characterized bydense connective/collagen-like fibers,blood vessels and mitotic cells (Suppl. Fig. 1). Within this environment, we identified cells extending cilia. The cells possessed docked basal bodies and axonemes containing microtubules that appeared well-organized and extended along the length of the visible axoneme (Fig. 4a, b). It is noteworthy that we also observed cells that displayed abnormal basal bodies/centrioles which did not appear to project cilia (Fig. 4c). Together, these results provide ultrastructural confirmation that ciliated cells are present within GBM, and suggest that some GBM cells may be defective in their ability to extend cilia.
Fig. 4.
Ultrastructural detection of cilia in a GBM biopsy from a 61 year-old male. a Example of a cell close to densely packed collagen-like fibers (also see Suppl. Fig. 1) extending a cilium (boxed region). a1 Higher magnification of the cilium in (a) reveals a docked basal body anchored by transition fibers and an axoneme comprised of organized, parallel arrangements of microtubules (arrowheads). b Example a cell adjacent to blood vessels (bv) projecting a cilium (boxed region, magnified in B1). c Example of cell that appears to contain multiple nuclei and abnormal numbers of basal bodies/centrioles (arrows). ax axoneme, tf transition fibers, bb basal body, dc daughter centriole, cf collagen-like fibers, cp ciliary pocket, cm ciliary membrane, n nucleus. Scale bars in A, B, C = 2 μm, A1, C1 = 500 nm, B1 = 200 nm
Ciliated cells within GBM tumors also express Ki67 and ZEB1
It has been suggested that cilia are required for normal and tumorigenic cell proliferation [5, 6, 18, 19]. Thus, we sought to determine whether actively dividing GBM tumor cells are ciliated by co-staining GBM Line 0 cells with antibodies against the proliferating antigen, Ki67, [30] and AA-tubulin. We found that Ki67+ Line 0 cells possessed AA- tubulin + cilia (Fig. 5a). We then co-immunostained sections of all of our GBM biopsies (n = 22) that contained G-tubulin/AA-tubulin + cilia (Fig. 3) with the Ki67 antibody and found that 17/22 biopsies contained Ki67+ cells that were also ciliated (Fig. 5b, e). This finding prompted us to determine whether ciliated cells in the GBM tumors were expressing other markers of tumorigenesis. We recently reported that levels of the transcription factor, ZEB1, in GBM are significantly elevated and that increased expression of ZEB1 is associated with cells that are invasive and chemo-resistant [31]. We found that ZEB1+ S2 cells possessed G- and AA-tubulin + cilia (Fig. 5c). Staining of GBM sections with a ZEB1 antibody revealed that 19/22 of the biopsies contained subsets of cells bearing G- and AA-tubulin + cilia that also stained for ZEB1 (Fig. 5d, e). Together, these results suggest that the cilia present in GBM tumors are associated with cells displaying proliferative and potentially invasive and chemoresistant capabilities.
Fig. 5.
Detection of primary cilia on Ki67+ and ZEB1+ GBM cells. a Line 0 cells were immunostained for Ki67 (green) and AA-tubulin (red) and nuclei were labeled with DAPI (blue). AA-tubulin + cilia (arrow, enlarged in inset) could be readily identified on Ki67+ cells. b Immunostaining of a biopsy (same as Fig. 3b) shows a G-tubulin+/AA-tubulin + cilium (arrow, enlarged in inset) associated with a Ki67+ cell in the tumor. c S2 cells were immunostained for ZEB1 (green), AA-tubulin (red), G-tubulin (blue in inset) and nuclei were labeled with DAPI (blue). AA-tubulin+/G-tubulin + cilia (arrow, enlarged in inset) could be identified on ZEB1 + cells. d Immuno-staining of a different biopsy shows an example of a ZEB1 + nucleus associated with a G-tubulin+/AA-tubulin + cilium (arrow, enlarged in inset). e Percentage of GBM biopsies that contained cells positive for cilia, both cilia and Ki67, or both cilia and ZEB1. Scale bars in A, B = 10 μm
Discussion
Our immunohistological and EM analyses of GBM tumors and primary cell lines consistently show that subsets of cells within these tumors possessed well-formed primary cilia. Moreover, these cilia were associated with cells expressing protein markers indicative of proliferative and invasive/chemoresistant capabilities. While the numbers of ciliated cells associated with various cancers, including GBM, are low [17, 32–36], we suggest that the presence of subpopulations of ciliated cells in GBM may be clinically relevant given that primary cilia have been shown to play roles in the proliferation, migration, and survival of some cell types.
We consistently identified ciliated cells in GBM tumors and primary GBM cell lines, a result that differs from that reported by Moser et al. (2009), who reported that fully formed primary cilia were either rare, absent, or abnormal in five highly propagated GBM cell lines. Differences in cell lines and their growth conditions may contribute to the disparities between these results. The cell lines examined by Moser et al. (2009) were well-established, older GBM cell lines, whereas we examined recently derived primary tumor cell lines and tumor biopsies. Unlike the culture media used by Moser et al. (2009), we used media supplemented with bFGF and EGF in an attempt to mirror conditions resembling those found in primary glioblastomas [37]. Alternatively, since we observed that only 8–25 % of the cells examined in vitro and in the biopsies were ciliated, an estimate that may be low because not all primary cilia are positive for gamma- or acetylated alpha-tubulin [38–40], it is possible that the majority of GBM cells are unable to grow cilia, a state that would be expected to lead to dysregulation of the cell cycle [41]. Our observations that serum starvation did not increase the numbers of ciliated GBM cells in our cultures and that nonciliated cells were identified by ultrastructural analysis of a GBM biopsy are consistent with this possibility, and are findings consistent with those reported by Moser et al. (2009). It is noteworthy that a recent study of U-251 GBM cells, which typically lack cilia [17], reported that expression of cell cycle-related kinase (CCRK), a protein often upregulated in GBM, is abnormally high in these cells [42]. These authors found that knockdown of CCRK levels increased ciliogenesis and inhibited U-251 proliferation, results suggesting GBM cells that fail to elaborate cilia may contribute to GBM tumor growth. Future studies will be needed to determine what factors determine whether GBM cells generate cilia and whether failure of GBM cells to generate cilia contributes to tumor cell propagation.
Could subpopulations of ciliated GBM cells trigger and/or maintain tumor growth+ There is growing evidence that cilia support cell tumorigenesis. For example, knockout of Kif3a, a protein required for cilia formation and function, in cerebellar granule neuron precursors [18, 19] and in human basal cell carcinoma [20] has been reported to inhibit the formation and growth of medulloblastoma and basal cell carcinoma, respectively. Our observations that KIF3A (data not shown) and ciliated cells are present in GBM tumors suggest the possibility that cilia may also play a role in regulating growth of these tumors. We also identified ciliated cells that were actively dividing (Ki67+) and expressing a transcription factor (ZEB1+) reported to mediate GBM initiation, invasion and chemoresistance [31], throughout the tumor biopsies. The close proximity of Ki67+ and ZEB1+ ciliated cells to blood vessels could facilitate their responses to proliferation, migratory or survival cues originating from the bloodstream, cues that could promote growth of the tumor. Clearly, the ability of cilia to transduce changes in the extracellular environment into adaptive cellular responses would be advantageous to tumors. Finally, GBM tumors are notoriously invasive with cells infiltrating both adjacent and remote brain regions. It has recently been shown that migrating cells in the developing brain use cilia to navigate across the forebrain, and that Arl13b is involved in this process [7, 8]. This is noteworthy since we detected Arl13b in the cilia of Line 0 cells, cells that form lethal and invasive tumors when xenografted into mice [22, 31, 43]. Thus, it is conceivable that cilia could also play a role in GBM invasion by guiding tumor cell migration. Taken together, our results suggest several potential lines of investigation that may lead to improved understanding of how ciliated GBM cells might contribute to the characteristic aggressiveness and treatment resistance of this cancer.
Supplementary Material
Acknowledgments
We would like to thank the UF COM Core EM Facility and R. Martuscello for technical assistance, and T. Caspary for the rabbit Arl13b antibody. We would also like to thank B. Frentzen and R. McTiernan of the Florida Center for Brain Tumor Research for providing us with tumor samples. This work was supported, in part, by funds from the McKnight Brain Research Foundation and the Evelyn F. and William L. McKnight Brain Institute at the University of Florida (to M.R.S., B.A.S and D.A.S.), an American Cancer Society Chris DiMarco Institutional Research Grant Junior Investigator Award (to M.R.S), and an American Cancer Society Research Scholar Grant (#RSG-13-031-01-DDC) (to M.R.S.). M.R.S. would like to dedicate this study to the memory of his younger brother, Andrew T. Sarkisian, who died in 2007 at the age of 28 from blood cancer.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11060-013-1340-y) contains supplementary material, which is available to authorized users.
Conflict of Interest The authors declare that they have no conflict of interest.
Contributor Information
Matthew R. Sarkisian, Department of Neuroscience, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA
Dorit Siebzehnrubl, Department of Neuroscience, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA.
Lan Hoang-Minh, Department of Neuroscience, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA.
Loic Deleyrolle, Department of Neurosurgery, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA.
Daniel J. Silver, Department of Neurosurgery, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA
Florian A. Siebzehnrubl, Department of Neurosurgery, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA
Sarah M. Guadiana, Department of Neuroscience, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA
Gayathri Srivinasan, Department of Neuroscience, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA.
Susan Semple-Rowland, Department of Neuroscience, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA.
Jeffrey K. Harrison, Department of Pharmacology and Therapeutics, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA
Dennis A. Steindler, Department of Neurosurgery, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA
Brent A. Reynolds, Department of Neurosurgery, McKnight Brain Institute, University of Florida College of Medicine, Gainesville, FL 32610, USA
References
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. The brain tumor microenvironment. Glia. 2012;60:502–514. doi: 10.1002/glia.21264. [DOI] [PubMed] [Google Scholar]
- 3.Hjelmeland AB, Lathia JD, Sathornsumetee S, Rich JN. Twisted tango: brain tumor neurovascular interactions. Nat Neurosci. 2011;14:1375–1381. doi: 10.1038/nn.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siebzehnrubl FA, Reynolds BA, Vescovi A, Steindler DA, Deleyrolle LP. The origins of glioma: E Pluribus Unum? Glia. 2011;59:1135–1147. doi: 10.1002/glia.21143. [DOI] [PubMed] [Google Scholar]
- 5.Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, Wang B, Flavell RA, Rakic P, Town T. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci USA. 2008;105:13127–13132. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 7.Baudoin JP, Viou L, Launay PS, Luccardini C, Espeso Gil S, Kiyasova V, Irinopoulou T, Alvarez C, Rio JP, Boudier T, Lechaire JP, Kessaris N, Spassky N, Metin C. Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron. 2012;76:1108–1122. doi: 10.1016/j.neuron.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 8.Higginbotham H, Eom TY, Mariani LE, Bachleda A, Hirt J, Gukassyan V, Cusack CL, Lai C, Caspary T, Anton ES. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Dev Cell. 2012;23:925–938. doi: 10.1016/j.devcel.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshimura K, Kawate T, Takeda S. Signaling through the primary cilium affects glial cell survival under a stressed environment. Glia. 2011;59:333–344. doi: 10.1002/glia.21105. [DOI] [PubMed] [Google Scholar]
- 10.Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 2003;426:83–87. doi: 10.1038/nature02061. [DOI] [PubMed] [Google Scholar]
- 11.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 12.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet–Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA. 2008;105:4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakravarthy B, Gaudet C, Menard M, Atkinson T, Chiarini A, Dal Pra I, Whitfield J. The p75 neurotrophin receptor is localized to primary cilia in adult murine hippocampal dentate gyrus granule cells. Biochem Biophys Res Commun. 2010;401:458–462. doi: 10.1016/j.bbrc.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 14.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr Biol. 2005;15:1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Ponten J, Macintyre EH. Long term culture of normal and neoplastic human glia. Acta Pathol Microbiol Scand. 1968;74:465–486. doi: 10.1111/j.1699-0463.1968.tb03502.x. [DOI] [PubMed] [Google Scholar]
- 16.Stein GH. T98G: an anchorage-independent human tumor cell line that exhibits stationary phase G1 arrest in vitro. J Cell Physiol. 1979;99:43–54. doi: 10.1002/jcp.1040990107. [DOI] [PubMed] [Google Scholar]
- 17.Moser JJ, Fritzler MJ, Rattner JB. Primary ciliogenesis defects are associated with human astrocytoma/glioblastoma cells. BMC Cancer. 2009;9:448. doi: 10.1186/1471-2407-9-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barakat MT, Humke EW, Scott MP. Kif3a is necessary for initiation and maintenance of medulloblastoma. Carcinogenesis. 2013;34:1382–1392. doi: 10.1093/carcin/bgt041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han YG, Kim HJ, Dlugosz AA, Ellison DW, Gilbertson RJ, Alvarez-Buylla A. Dual and opposing roles of primary cilia in medulloblastoma development. Nat Med. 2009;15:1062–1065. doi: 10.1038/nm.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Jr, Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med. 2009;15:1055–1061. doi: 10.1038/nm.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deleyrolle LP, Harding A, Cato K, Siebzehnrubl FA, Rahman M, Azari H, Olson S, Gabrielli B, Osborne G, Vescovi A, Reynolds BA. Evidence for label-retaining tumour-initiating cells in human glioblastoma. Brain. 2011;134:1331–1343. doi: 10.1093/brain/awr081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hothi P, Martins TJ, Chen L, Deleyrolle L, Yoon JG, Reynolds B, Foltz G. High-throughput chemical screens identify disulfiram as an inhibitor of human glioblastoma stem cells. Oncotarget. 2012;3:1124–1136. doi: 10.18632/oncotarget.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldaz H, Rice LM, Stearns T, Agard DA. Insights into microtubule nucleation from the crystal structure of human gamma-tubulin. Nature. 2005;435:523–527. doi: 10.1038/nature03586. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464:1048–1051. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa H, Marshall WF. Ciliogenesis: building the cell's antenna. Nature Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 28.Santos N, Reiter JF. Building it up and taking it down: the regulation of vertebrate ciliogenesis. Dev Dyn. 2008;237:1972–1981. doi: 10.1002/dvdy.21540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 31.Siebzehnrubl FA, Silver DJ, Tugertimur B, Deleyrolle LP, Siebzehnrubl D, Sarkisian MR, Devers KG, Yachnis AT, Kupper MD, Neal D, Nabilsi NH, Kladde MP, Suslov O, Brabletz S, Brabletz T, Reynolds BA, Steindler DA. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol Med. 2013;5:1196–1212. doi: 10.1002/emmm.201302827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Basten SG, Willekers S, Vermaat JS, Slaats GG, Voest EE, van Diest PJ, Giles RH. Reduced cilia frequencies in human renal cell carcinomas versus neighboring parenchymal tissue. Cilia. 2013;2:2. doi: 10.1186/2046-2530-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J, Dabiri S, Seeley ES. Primary cilium depletion typifies cutaneous melanoma in situ and malignant melanoma. PLoS One. 2011;6:e27410. doi: 10.1371/journal.pone.0027410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seeley ES, Carriere C, Goetze T, Longnecker DS, Korc M. Pancreatic cancer and precursor pancreatic intraepithelial neoplasia lesions are devoid of primary cilia. Cancer Res. 2009;69:422–430. doi: 10.1158/0008-5472.CAN-08-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Asselt SJ, de Vries EG, van Dullemen HM, Brouwers AH, Walenkamp AM, Giles RH, Links TP. Pancreatic cyst development: insights from von Hippel-Lindau disease. Cilia. 2013;2:3. doi: 10.1186/2046-2530-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan K, Frolova N, Xie Y, Wang D, Cook L, Kwon YJ, Steg AD, Serra R, Frost AR. Primary cilia are decreased in breast cancer: analysis of a collection of human breast cancer cell lines and tissues. J Histochem Cytochem. 2010;58:857–870. doi: 10.1369/jhc.2010.955856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Past-orino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 38.Leask A, Obrietan K, Stearns T. Synaptically coupled central nervous system neurons lack centrosomal gamma-tubulin. Neurosci Lett. 1997;229:17–20. doi: 10.1016/s0304-3940(97)00412-6. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor AK, Malarkey EB, Berbari NF, Croyle MJ, Haycraft CJ, Bell PD, Hohenstein P, Kesterson RA, Yoder BK. An inducible CiliaGFP mouse model for in vivo visualization and analysis of cilia in live tissue. Cilia. 2013;2:8. doi: 10.1186/2046-2530-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukushima N, Furuta D, Hidaka Y, Moriyama R, Tsujiuchi T. Post-translational modifications of tubulin in the nervous system. J Neurochem. 2009;109:683–693. doi: 10.1111/j.1471-4159.2009.06013.x. [DOI] [PubMed] [Google Scholar]
- 41.Plotnikova OV, Golemis EA, Pugacheva EN. Cell cycle-dependent ciliogenesis and cancer. Cancer Res. 2008;68:2058–2061. doi: 10.1158/0008-5472.CAN-07-5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang Y, Roine N, Makela TP. CCRK depletion inhibits glioblastoma cell proliferation in a cilium-dependent manner. EMBO Rep. 2013;14:741–747. doi: 10.1038/embor.2013.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silver DJ, Siebzehnrubl FA, Schildts MJ, Yachnis AT, Smith GM, Smith AA, Scheffler B, Reynolds BA, Silver J, Steindler DA. Chondroitin sulfate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor micro-environment. J Neurosci. 2013;33:15603–15617. doi: 10.1523/JNEUROSCI.3004-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.