Abstract
Peripheral Quantitative Computed Tomography (pQCT) can be used for muscle and fat area and density assessments. These may independently influence muscle and fat mass measurements from Dual Energy X-ray Absorptiometry (DXA).
Objective
To determine associations between pQCT-derived soft tissue density and area measures and DXA-derived soft tissue mass.
Methods
Linear regression models were developed based on BMI and calf fat and muscle cross-sectional area (FCSA and MCSA) and density measured by pQCT in healthy women (n=76) and men (n=82) aged 20–59 years. Independent variables for these models were leg and total bone-free lean mass (BFLM) and fat mass (FM) measured by DXA.
Results
Sex differences (p<0.01) were found in both muscle (Mean±SE: Women: 78.6±0.4; Men: 79.9 ± 0.2 mg/cm3) and fat (Women: 0.8±0.4 Men: 9.1±0.6 mg/cm3) density. BMI, fat density, and age (R2=0.86, p<0.01) best accounted for the variability in total FM. FCSA, BMI, and fat density explained the variance in leg FM (R2=0.87, p<0.01). MCSA and muscle density explained the variance in total (R2=0.65, p<0.01) and leg BFLM (R2=0.70, p<0.01).
Conclusion
Calf muscle and fat area and density independently predict lean and fat tissue mass.
Keywords: muscle cross-sectional area, fat cross-sectional area, muscle density, fat density, DXA
Introduction
Peripheral Quantitative Computed Tomography (pQCT) was developed for bone density and bone strength estimation(1–4), but is more commonly being used for soft tissue analysis in training studies or for investigating muscle-bone-fat relationships(5–9). The most common sites of soft-tissue assessment are within the belly of the gastrocnemius muscles (generalized to be at ~66% of tibia length), at the mid-forearm, and, less commonly, at the mid-thigh area7, 8, 10, 11. pQCT-based cross-sectional areas of soft tissue are similar to MRI-based cross-sectional areas and have demonstrated sensitivity in detecting changes in muscle area with training(7, 12). Besides providing similar cross-sectional area results as an MRI scan, which does not emit radiation, a pQCT scan is quicker and emits less radiation than a DXA or CT scan.
An additional capability of pQCT is the ability to generate estimations of muscle and fat density. With regular CT, water has a value of zero, but with pQCT, fat is calibrated to zero mg/cm3 and water has a value close to 55 mg/cm3. A typical muscle density range is ~65–90 mg/cm3. Although fat is calibrated to zero, actual fat densities occur across a range of values, similar to what is observed in CT(13). The variability in this density may relate to alterations in relative fluid content due to differences in adipocyte size and number, extracellular fluid content, or level of blood flow(14, 15). Muscle density values would also differ due to variations in relative hydration, capillarization, and protein, and most drastically, due to variations in lipid content(13). Interest about quantifying muscle quality has increased with the hypothesis that a lower muscle density implies greater fatty infiltration(16). If changes in fatty infiltration can be detected by changes in muscle density with pQCT, then quantifying muscle quality using this approach would provide a non-invasive, low radiation insight into age- and detraining-related losses of muscle function.
Most studies use an additional method (e.g., DXA) for assessing total body composition because equations for body composition from pQCT measures have not been developed in adults7, 8, 17, 18. Muscle and fat mass measurements are not always indicative of the quality of the tissue, and can be influenced by both the size and the density of the tissue. Further, tissue mass and area frequently differs between women and men, but little is known about sex differences in muscle or fat density, which may partly explain size differences. Therefore, the aims of this study were to characterize sex differences in soft tissue quality in healthy adults and characterize the associations between pQCT-derived soft tissue density and area measures and DXA-derived soft tissue mass.
Methods
Participants were healthy adult women (n=76) and men (n=82) aged 20–59 years, were free of any diagnosed chronic disease (e.g., diabetes, cancer, heart disease), and weighed less than 136 kg (DXA weight limit). Both pre and postmenopausal women were enrolled, and premenopausal women were eumenorrheic by self report. Postmenopause was defined as more than one year since their last menstrual period.19 Neither women nor men were taking sex steroids for at least the previous 12 months. Participants were volunteers who were recruited by flyers and mass email in the Greater Oklahoma City Metro Area. All provided written informed consent to participate. This study was approved by the University of Oklahoma Institutional Review Board.
Total Body and Leg Mass
Body height and weight were measured using a wall stadiometer and a Tanita BWB-800 digital scale (Tanita Corporation of America, Inc., Arlington Heights, IL) and body mass index (BMI) was calculated. Total body (including the head) and leg bone free lean mass (BFLM) and fat mass (FM) were measured with Dual Energy X-ray Absorptiometry (DXA; GE Lunar Prodigy, Prodigy enCORE software version 13.31.016, Madison, WI). Scan speeds were determined by the measured thickness of the subject at the naval (Thick = >25 cm and Standard = 13 – 25 cm). Calibrations were performed daily prior to scanning participants using standard methods set by the manufacturer, and the precision (CV%) of the machine is 0.6%. Technician precision was < 2.7% for FM and BFLM. A refractometer (VEE GEE®, Model CLX-1) was used to measure urine specific gravity to ensure normal hydration status prior to being scanned on the DXA. It was calibrated by checking the scale with deionized water. The lower field of the USG should be 1.000. If not, adjustments were made by loosening the set screw and turning the calibration ring until it reads the bottom of the scales which is 1.000. Each sample was measured once unless the specific gravity bordered on abnormal hydration ranges20. All participants were within our predetermined normal hydration range (1.004~1.029).
Calf Soft Tissue Area and Density
Muscle and fat cross-sectional areas (MCSA, FCSA)) and density of the calf (65% of tibia length) were measured using pQCT (XCT 3000 with software version 6.00; Stratec Medizintechnik GmbH, Pforzheim, Germany). The percentage of the cross section that was fat (FCSA %) was calculated as (FCSA/Total CSA) * 100. Tibia length was measured from the medial tibia plateau to the medial malleolus. Quality assurance scans were performed daily prior to scanning participants using standard methods set by the manufacturer and must be within 99% accuracy to pass. The voxel size for scanning was 0.4 mm, and the scan speed was 20 mm/sec. Images were segmented into muscle, fat and bone with the integrated software using a median filter mode for noise suppression (F03F05). The ‘F03F05’ filter combines a 3×3 median filter with a threshold range of −500 to 500 mg/cm3 with a 5×5 median filter with a threshold range of −500 to 300 mg/cm3. The segmentation threshold value range used to separate fat + marrow from muscle + bone was −100 to 40 mg/cm3, and the threshold value range used to separate bone from muscle and marrow from fat was 710 and 40 mg/cm3. Precision (CV%) for the 3 technicians was <2% for soft tissue area measurements.
Statistical Analyses
Data were analyzed using SAS 9.3 (SAS Institute Inc., Cary NC) and are presented at mean ± SE unless otherwise stated. Paired t-tests were used to determine sex differences in outcome variables. Linear stepwise (p entry = 0.05, p exit = 0.10) regression was used to determine predictors of total body and leg BFLM and FM. Independent variables were sex, BMI, age, [muscle density and MCSA for BFLM estimates], and [fat density and FCSA for FM estimates]. Regression analyses were also performed without including sex. We ensured that n≥20 per variable entered into each regression. Coefficients of determination are also reported between DXA mass variables and pQCT CSA and density variables and between CSA and density from pQCT. Significance was set at p<0.05.
Results
Of the women, 34.2% (n = 26) were postmenopausal, and the time since menopause ranged from 1 to 20 years. Postmenopausal women were older (p<0.001), and had greater total FM (p<0.02) and percent body fat (p<0.001) than premenopausal women. Differences in weight (p=0.086) and muscle density (p=0.094) neared significance. Other soft tissue comparisons between pre and postmenopausal women were not significant. As expected, men were taller and had more DXA-derived total and leg BFLM, resulting in a greater weight than women (all p<0.01; Table 1). Total (p<0.05) and leg (p<0.01) fat mass (DXA) were higher in women. These sex differences were consistent with the sex differences seen in pQCT-derived MCSA and FCSA (p<0.01). Muscle and fat density (pQCT) were both higher (p<0.01) in men.
Table 1.
Body composition in men and women (mean ± SE)
| Men | Women | |||
|---|---|---|---|---|
|
| ||||
| Mean ± SE | Min, Max | Mean ± SE | Min, Max | |
| Age (y) | 38.6 ± 1.4 | 20.1, 59.9 | 41.8 ± 1.5 | 20.0, 59 |
| Height (cm) | 178.3 ± 0.7** | 164, 194.5 | 164.0 ± 0.8 | 147.5, 187.5 |
| Weight (kg) | 86.8 ± 1.6** | 62.9, 131.8 | 71.3 ± 1.9 | 46.8, 123.2 |
| BMI (kg/m2) | 27.2 ± 0.4 | 21.4, 38.4 | 26.6 ± 0.7 | 18.4, 45.5 |
| DXA Parameters | ||||
| Total BFLM (kg) | 60.0 ± 0.8** | 45.4, 86.2 | 40.9 ± 0.7 | 29.1, 61.3 |
| Total FM (kg) | 23.1 ± 1.2* | 4.4, 58.1 | 27.3 ± 1.3 | 9.4, 58.0 |
| Leg BFLM (kg) | 20.6 ± 0.3** | 14.2, 31.7 | 13.8 ± 0.3 | 9.3, 20.1 |
| Leg FM (kg) | 7.0 ± 0.4** | 1.5, 15.3 | 10.4 ± 0.5 | 3.1, 23.9 |
| Body fat % | 25.8 ± 1.0** | 6.2, 44.6 | 37.1 ± 1.0 | 15.3, 51.5 |
| pQCT Parameters | ||||
| MCSA (cm2) | 89.0 ± 1.5** | 61.2, 134.8 | 68.7 ± 1.3 | 45.0, 97.2 |
| FCSA (cm2) | 18.2 ± 1.0** | 3.6, 43.2 | 32.4 ± 1.7 | 9.3, 96.1 |
| Muscle Den. (mg/cm3) | 79.9 ± 0.2** | 75.8, 82.9 | 78.6 ± 0.4 | 59.2, 84.1 |
| Fat Den. (mg/cm3) | 9.1 ± 0.6** | −1.3, 25.3 | 0.8 ± 0.4 | −9.8, 15.3 |
| FCSA (%)** | 15.1 ± 0.7 | 3.3, 30.1 | 28.6 ± 1.0 | 11.8, 52.5 |
p<0.01,
p<0.05
Sex difference. BFLM: Bone-Free Lean Mass; FM: Fat Mass; MCSA: Muscle Cross-Sectional Area; FCSA: Fat Cross-Sectional Area; Den: Density
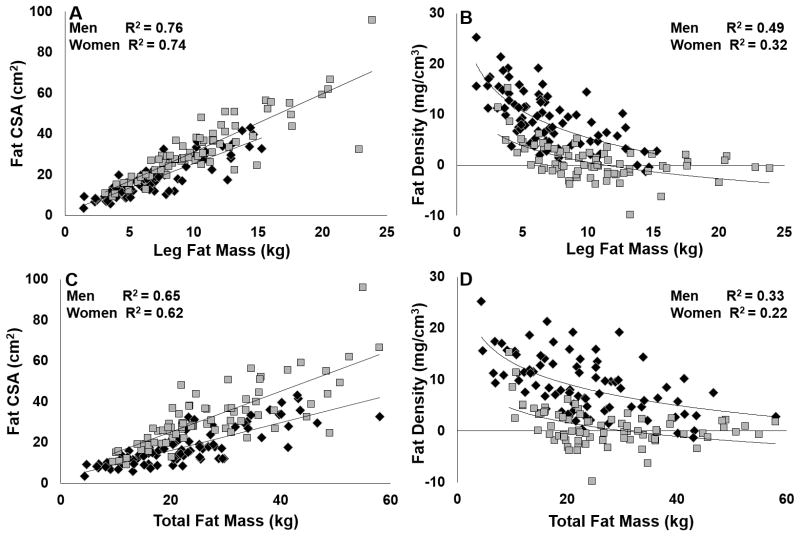
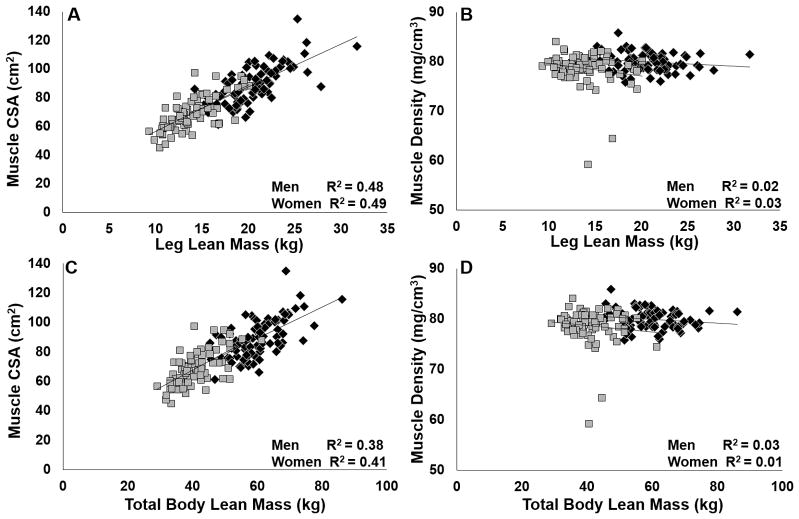
Table 2 presents the best-fit models for explaining the variability in total and leg BFLM and total and leg FM. Sex did not explain the variability in total or leg FM. However, in the lean mass models, sex was a significant predictor along with BMI and MCSA (R2=0.80, p<0.01 for both total and leg BFLM). When age was removed from the FM models, total FM was only predicted by BMI and fat density (R2 = 0.85). Figure 1 shows the significant relationships between fat density and FCSA and total and leg FM for men and women. FCSA was linearly related to FM, and correlations were similar between women and men. Inverse curvilinear associations between fat density and FCSA or FM were stronger in men. MCSA was also linearly related to lean mass, but there was no association between muscle density and MCSA or lean mass (Figures 2 and 3).
Table 2.
Stepwise regression models for total and leg bone-free lean (BFLM) and fat mass (FM).
| Dependent Variable | Independent Variables | β | SEE | R2 |
|---|---|---|---|---|
| Total BFLM | MCSA | 0.804 | 4.24e-4 | 0.65 |
| Muscle Density | 0.147 | 0.199 | ||
|
| ||||
| Leg BFLM | MCSA | 0.836 | 1.56e-4 | 0.70 |
| Muscle Density | 0.114 | 0.069 | ||
|
| ||||
| Total FM | BMI | 0.790 | 0.068 | 0.86 |
| Fat Density | −0.351 | 0.054 | ||
| Age | 0.122 | 0.027 | ||
|
| ||||
| Leg FM | FCSA | 0.444 | 1.79e−4 | 0.87 |
| BMI | 0.430 | 0.035 | ||
| Fat Density | −0.260 | 0.033 | ||
MCSA: Muscle Cross-Sectional Area; FCSA: Fat Cross-Sectional Area. All p<0.01.
Figure 1.
Associations between fat cross-sectional area (CSA) and density (pQCT) and leg and total fat mass (DXA) in women (grey) and men (black) all p<0.003.
Figure 2.
Associations between muscle cross-sectional area (CSA) and density (pQCT) and leg and total lean mass (DXA) in women (grey) and men (black). A,C: p<0.0001; B, D: p>0.11.
Figure 3.
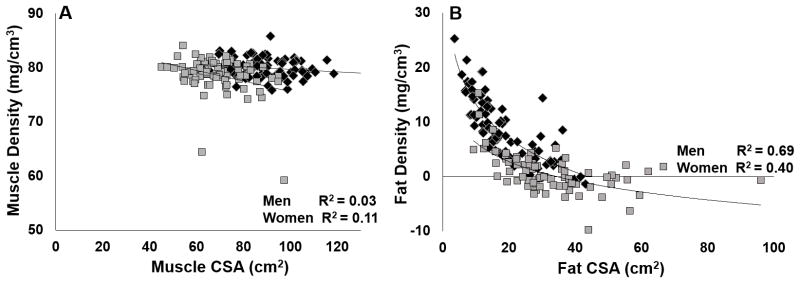
Associations between cross-sectional area (CSA) and density (pQCT) of muscle and fat in women (grey) and men (black). A: women p=0.004, men p=0.13; B: both p<0.001.
Discussion
The primary novel findings are significant sex differences in calf fat and muscle density and the independent contributions of tissue area and density in regressing on DXA-based soft tissue measures in adults. Women in our study had greater FM and FCSA values than men, whereas men had higher fat density values. There was a strong inverse relationship between fat density values and FCSA and FM values that appeared to be stronger in men than women. We expected strong associations between calf measures and leg measures, but the association between pQCT-based measures and total body measures were greater than expected. This may be due to the wide representation of body proportions represented in our sample (e.g., height and weight relationships, leanness, upper body muscularity relative to lower body muscularity, and gynoid vs. android fat pattern).
To our knowledge, this is the first study to associate body composition from DXA with pQCT-based measures in adults. Our approach was different from the approach used by Ducher et al. when they were able to use FCSA and muscle density from pQCT with height to predict DXA-based body fat % in children(8). Associations between calf fat measures and leg and total body fat measures were stronger than associations between calf muscle measures and leg and total lean mass measures, presumably because of differences in fat and muscle distributions(21–23). Interestingly, CSA and volumetric density independently contributed to the prediction of DXA-based mass measures. This seems intuitive in theory; however, in practice, CSA and density values are interpreted separately. Muscle density has been used as a measure of muscle quality(8, 16, 24–27), which is neither mutually inclusive nor exclusive of MCSA. Considering that each outcome has its own predictive value for BFLM, these outcomes could also be combined to better explain changes in muscle force production resulting from aging, disease, or intervention(24, 25, 27, 28).
A plausible explanation for the sex differences in fat density are sex differences in adipocyte cellularity. If adipocytes are larger because of greater lipid content, or if there is less blood flow or extracellular fluid, then fat density values may decrease(14, 15). Although sex differences in the levels of inflammation or issues related to partial volume effects are may influence sex differences in fat density or the associations between fat density and fat mass and area measures, we do not believe these to be the primary causes. The sex difference in muscle density corroborates the interpretation of the greater ‘fatness’ in women. However, sex differences in muscle density may have been driven by the postmenopausal subgroup. Our participants were generally healthy, were in normal hydration ranges, and did not have any pathological conditions that would automatically cause greater fluid retention. Subcutaneous adipose tissue was thicker than the resolution of the scan. Density/mass relationships would be less likely to be consistent with density/area relationships in the case of a spurious finding.
Limitations of this study are that we did not acquire any soft tissue samples that would enable us to characterize the cellularity of the adipose tissue. Further research will be needed to determine if changes in adipose cell size can be detected by changes in fat density or if muscle density values are indicative of the level of fatty infiltration. Thus, interpreting changes in muscle and fat density should be made cautiously, and these data are hypothesis-generating. We only tested younger, healthier adults; we do not know if our associations are generalizable to older, frail, or to clinical populations.
Conclusions
Sex differences in fat and muscle density were found in the lower limb. Calf soft tissue area and density measures from pQCT independently predict leg soft tissue mass. Although further studies are needed to determine the mechanisms that underpin sex differences in fat density, pQCT-derived analyses may provide insight to the metabolic status of tissue when more invasive methods are not available to investigators.
Acknowledgments
The authors are grateful to the participants who volunteered for this study. This study was funded by the University of Oklahoma Graduate Student Senate. VDS received support from NIH T32 DK007658.
References
- 1.Ferretti JL. Perspectives of pQCT technology associated to biomechanical studies in skeletal research employing rat models. Bone. 1995;17:353S–64S. doi: 10.1016/8756-3282(95)00313-3. [DOI] [PubMed] [Google Scholar]
- 2.Gasser JA. Assessing bone quantity by pQCT. Bone. 1995;17:145S–54S. doi: 10.1016/8756-3282(95)00287-n. [DOI] [PubMed] [Google Scholar]
- 3.Grampp S, Lang P, Jergas M, Gluer CC, Mathur A, Engelke K, et al. Assessment of the skeletal status by peripheral quantitative computed tomography of the forearm: short-term precision in vivo and comparison to dual X-ray absorptiometry. J Bone Miner Res. 1995;10:1566–76. doi: 10.1002/jbmr.5650101019. [DOI] [PubMed] [Google Scholar]
- 4.Louis O, Willnecker J, Soykens S, Van den Winkel P, Osteaux M. Cortical thickness assessed by peripheral quantitative computed tomography: accuracy evaluated on radius specimens. Osteoporos Int. 1995;5:446–9. doi: 10.1007/BF01626606. [DOI] [PubMed] [Google Scholar]
- 5.Coupaud S, Jack LP, Hunt KJ, Allan DB. Muscle and bone adaptations after treadmill training in incomplete Spinal Cord Injury: a case study using peripheral Quantitative Computed Tomography. J Musculoskelet Neuronal Interact. 2009;9:288–97. [PubMed] [Google Scholar]
- 6.Sherk VD, Bemben MG, Bemben DA. Interlimb muscle and fat comparisons in persons with lower-limb amputation. Arch Phys Med Rehabil. 2010;91:1077–81. doi: 10.1016/j.apmr.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 7.DeFreitas JM, Beck TW, Stock MS, Dillon MA, Sherk VD, Stout JR, et al. A comparison of techniques for estimating training-induced changes in muscle cross-sectional area. J Strength Cond Res. 2010;24:2383–9. doi: 10.1519/JSC.0b013e3181ec86f3. [DOI] [PubMed] [Google Scholar]
- 8.Ducher G, Daly RM, Hill B, Eser P, Naughton GA, Gravenmaker KJ, et al. Relationship between indices of adiposity obtained by peripheral quantitative computed tomography and dual-energy X-ray absorptiometry in pre-pubertal children. Ann Hum Biol. 2009;36:705–16. doi: 10.3109/03014460903055139. [DOI] [PubMed] [Google Scholar]
- 9.Rittweger J, Frost HM, Schiessl H, Ohshima H, Alkner B, Tesch P, et al. Muscle atrophy and bone loss after 90 days’ bed rest and the effects of flywheel resistive exercise and pamidronate: results from the LTBR study. Bone. 2005;36:1019–29. doi: 10.1016/j.bone.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Sherk VD, Bemben MG, Bemben DA. Comparisons of bone mineral density and bone quality in adult rock climbers, resistance-trained men, and untrained men. J Strength Cond Res. 2010;24:2468–74. doi: 10.1519/JSC.0b013e3181b60407. [DOI] [PubMed] [Google Scholar]
- 11.Farr JN, Chen Z, Lisse JR, Lohman TG, Going SB. Relationship of total body fat mass to weight-bearing bone volumetric density, geometry, and strength in young girls. Bone. 2010;46:977–84. doi: 10.1016/j.bone.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sherk VD, Bemben MG, Palmer IJ, Bemben DA. Effects of filtering methods on muscle and fat cross-sectional area measurement by pQCT: a technical note. Physiol Meas. 2011;32:N65–72. doi: 10.1088/0967-3334/32/12/N01. [DOI] [PubMed] [Google Scholar]
- 13.Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18–24. doi: 10.1111/j.1749-6632.2000.tb06416.x. [DOI] [PubMed] [Google Scholar]
- 14.Cavallini I, Marino MA, Tonello C, Marzola P, Nicolato E, Fabene PF, et al. The hydrolipidic ratio in age-related maturation of adipose tissues. Biomed Pharmacother. 2006;60:139–43. doi: 10.1016/j.biopha.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Laaksonen DE, Nuutinen J, Lahtinen T, Rissanen A, Niskanen LK. Changes in abdominal subcutaneous fat water content with rapid weight loss and long-term weight maintenance in abdominally obese men and women. Int J Obes Relat Metab Disord. 2003;27:677–83. doi: 10.1038/sj.ijo.0802296. [DOI] [PubMed] [Google Scholar]
- 16.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89:104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 17.Warden SJ, Bogenschutz ED, Smith HD, Gutierrez AR. Throwing induces substantial torsional adaptation within the midshaft humerus of male baseball players. Bone. 2009;45:931–41. doi: 10.1016/j.bone.2009.07.075. [DOI] [PubMed] [Google Scholar]
- 18.Pahor M, Manini T, Cesari M. Sarcopenia: clinical evaluation, biological markers and other evaluation tools. J Nutr Health Aging. 2009;13:724–8. doi: 10.1007/s12603-009-0204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Practice Committee of the American Society for Reproductive Medicine. The menopausal transition. Fertil Steril. 2008;90:S61–5. doi: 10.1016/j.fertnstert.2008.08.095. [DOI] [PubMed] [Google Scholar]
- 20.Pietrobelli A, Wang Z, Formica C, Heymsfield SB. Dual-energy X-ray absorptiometry: fat estimation errors due to variation in soft tissue hydration. Am J Physiol. 1998;274:E808–16. doi: 10.1152/ajpendo.1998.274.5.E808. [DOI] [PubMed] [Google Scholar]
- 21.Ross R, Leger L, Morris D, de Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol. 1992;72:787–95. doi: 10.1152/jappl.1992.72.2.787. [DOI] [PubMed] [Google Scholar]
- 22.Ross R, Shaw KD, Rissanen J, Martel Y, de Guise J, Avruch L. Sex differences in lean and adipose tissue distribution by magnetic resonance imaging: anthropometric relationships. Am J Clin Nutr. 1994;59:1277–85. doi: 10.1093/ajcn/59.6.1277. [DOI] [PubMed] [Google Scholar]
- 23.Lee SJ, Janssen I, Heymsfield SB, Ross R. Relation between whole-body and regional measures of human skeletal muscle. Am J Clin Nutr. 2004;80:1215–21. doi: 10.1093/ajcn/80.5.1215. [DOI] [PubMed] [Google Scholar]
- 24.Conroy MB, Kwoh CK, Krishnan E, Nevitt MC, Boudreau R, Carbone LD, et al. Muscle strength, mass, and quality in older men and women with knee osteoarthritis. Arthritis Care Res (Hoboken) 2012;64:15–21. doi: 10.1002/acr.20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauretani F, Bandinelli S, Bartali B, Di Iorio A, Giacomini V, Corsi AM, et al. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging. 2006;27:1145–54. doi: 10.1016/j.neurobiolaging.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farr JN, Funk JL, Chen Z, Lisse JR, Blew RM, Lee VR, et al. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;26:2217–25. doi: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, et al. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105:1498–503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57:1411–9. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]