Although recognition of an association among hyperuricemia, kidney disease and cardiovascular disease dates to the late 19th century (1), there has been a recent resurgence in investigating the association between uric acid levels and these outcomes (2-7). Data from these and other physiological experiments and observational studies suggest that there may be causal relationships interspersed within the associations among uric acid, chronic kidney disease (CKD) and cardiovascular disease; however, convincing evidence remains elusive due to methodologic and statistical issues that complicate the study of uric acid, its determinants and its confounders. In this issue of the American Journal of Kidney Diseases, two cohort studies examine the relationship of hyperuricemia in the absence of clinical gout with CKD (8) and cardiovascular disease (9), respectively, and support the hypothesis that asymptomatic hyperuricemia is a CKD and cardiovascular disease risk factor.
This hypothesis well grounded in animal models. For example, in rats given a uricase inhibitor to promote mild hyperuricemia, systemic hypertension developed within three weeks. In this model, achieved blood pressure was highly correlated with the serum uric acid level, and, following administration of a xanthine oxidase inhibitor with resultant lowering of serum uric acid, blood pressure decreased. In this study, uric acid mediated hypertension was attributed to renal vasoconstriction, with this vasoconstriction resulting in upregulation of the renin–angiotensin system (6). Consistent with these observations, hyperuricemia in humans correlates with both endothelial dysfunction and increases in plasma renin activity, both of which over time may result in a microvascular kidney disease phenotype (10, 11). Given the established relationship among endothelial dysfunction, high renin-angiotensin-aldosterone system activity and chronic kidney disease (CKD), it is reasonable to then postulate a biological link between hyperuricemia and both incident hypertension and CKD (12). In addition, an association between CKD and increased cardiovascular disease risk is well recognized, suggesting that CKD could be on a mediating pathway linking hyperuricemia to cardiovascular disease.
However, in both epidemiologic studies and direct patient care, where elevations in serum uric acid level tend to be relatively modest and hyperuricemia often occurs in conjunction with other CKD and cardiovascular disease risk factors, the clinical significance and independence of these associations remain uncertain. This uncertainty may also reflect the fact that uric acid is cleared by the kidney: following glomerular filtration, most urate is initially reabsorbed in the early proximal tubule. Thereafter, half the filtered load is secreted in the mid-proximal tubule with subsequent post secretory reabsorption occurring in the late proximal tubule (13, 14). Overall, there is a high correlation between serum uric acid and glomerular filtration rate (GFR), strengthening the possibility that CKD status or severity may be a statistical confounder in the relationship between uric acid and cardiovascular disease rather than the mediator.
Understanding the complex relationship among uric acid, CKD and cardiovascular disease is necessary to quantify the magnitude of uric acid as a risk factor for cardiovascular disease and forces researchers and clinicians to balance evidence supporting causality versus association. Adding to the complexity, the clinical association between uric acid and subsequent cardiovascular disease remains uncertain, with several recent epidemiological studies relating increased uric acid levels to hypertension, diabetes, metabolic syndrome, kidney disease and cardiovascular disease (15) while other studies have found that, following adjustment for cardiovascular risk factors and estimated GFR, uric acid is not independently associated with cardiovascular disease (16).
In multivariable regression aimed at exploring the relationship between a risk factor (such as hyperuricemia) and an outcome (such as cardiovascular disease), adjustment for a covariate is appropriate if the covariate is considered a confounder and is inappropriate if the covariate is a mediator in the biological pathway (17). The questions therefore become:
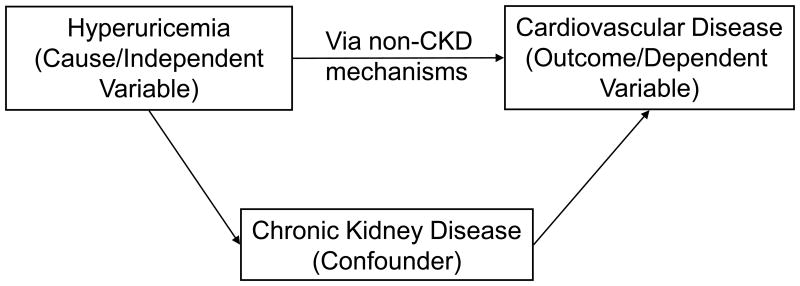
Does kidney disease mediate the relationship between uric acid and cardiovascular disease (figure 1a);
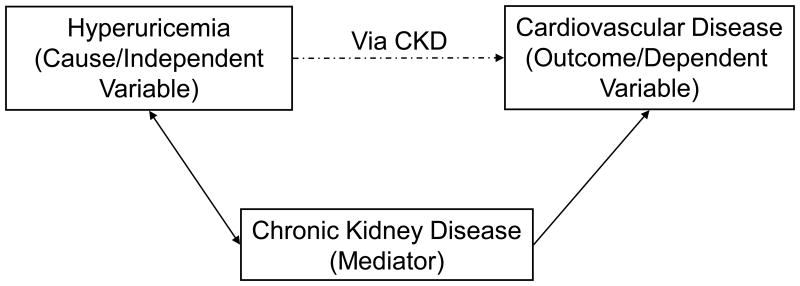
Does kidney disease confound the relationship between uric acid and cardiovascular disease (figure 1b); or
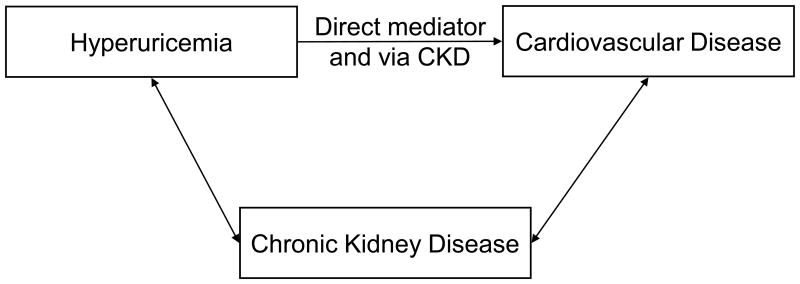
Is kidney disease, in varying degrees, both a mediator and a confounder in a relationship where hyperuricemia, CKD and cardiovascular disease each impact the other (Figure 1c)?
Figure 1a.
A confounder is associated with both the exposure and the outcome, but does not comprise the causal pathway between the exposure and the outcome. In this case, Hyperuricemia and CKD are associated and CKD and cardiovascular disease are associated but the relationship between hyperuricemia and cardiovascular disease is not mediated by the relationships with CKD.
Figure 1b.
A mediator is associated with both the exposure and the outcome, and comprises the causal pathway between the exposure and the outcome. In this case, hyperuricemia causes CKD which causes cardiovascular disease.
Figure 1c.
A causal diagram where the association between hyperuricemia and cardiovascular disease is both independently of CKD as well as mediated via CKD.
In support of CKD as a mediator are extensive animal data, prospective studies linking elevated uric acid levels temporally to the development of incident CKD, and the established association between CKD and cardiovascular disease. In support of CKD as a confounder is the fact that reductions in GFR can result in both increased uric acid levels and increased cardiovascular disease risk, and that adjustment for GFR attenuates the strength of the uric acid → cardiovascular disease association. Muddying this picture entirely is the recognition that incident cardiovascular disease can lead to development and worsening of CKD (18). Teasing out this relationship is important as, if kidney disease is indeed a confounder in the relationship between uric acid and cardiovascular disease, then the attenuated estimate for uric acid that occurs following adjustment for GFR may be appropriate (19). However, if kidney disease mediates the effect of uric acid on cardiovascular disease, then point estimates relating uric acid to cardiovascular disease should not be adjusted for GFR (and perhaps should not be adjusted for hypertension either), and uric acid may represent an extremely potent therapeutic target for both CKD and cardiovascular disease risk reduction.
In this issue of the American Journal of Kidney Diseases, Bellomo et al. examined the effect of hyperuricemia on subsequent GFR and Wen et al. evaluated the relationship between uric acid level and cardiovascular disease events. Both studies support the hypotheses that uric acid may directly affect both kidney function and cardiovascular disease. In the first study, Bellomo et al. quantified the association between uric acid and change in estimated GFR in a prospective cohort of 900 healthy normotensive adult blood donors in Italy and demonstrated an association between higher uric acid levels and subsequent worsening kidney function (8). Their findings, which remained significant following adjustment for theorized confounders, suggest that higher uric acid levels may lead to a progressive decline in kidney function in healthy normotensive individuals; additionally, they establish a temporal relationship between uric acid levels and GFR decline with a dose response effect such that higher uric acid levels are associated with greater reductions in estimated GFR. In the second study, Wen et al. evaluated a cohort of 484,568 individuals participating in a medical screening program in Taiwan (9). In this very large cohort, higher serum uric acid level was a risk factor for both all-cause and cardiovascular disease mortality, with this association persisting even in individuals at very low cardiovascular risk. However, the authors also found that the relationship between uric acid and cardiovascular disease is significantly attenuated by adjustment for proteinuria and estimated GFR.
The results of these two studies complement each other and improve our understanding of the association among uric acid, CKD and cardiovascular disease. Specifically, in the study of Wen and colleagues, the attenuation of the association between uric acid and cardiovascular disease following adjustment for kidney function, when considered in conjunction with the findings of Bellomo et al. that temporally link uric acid to progressive GFR decline, are not surprising and may support a role for CKD in the intermediary pathway linking hyperuricemia and cardiovascular disease. Ultimately, whether this relationship reflects a direct association between CKD and cardiovascular disease, an indirect relationship whereby CKD is a risk state for other traditional and non-traditional risk factors that result in cardiovascular disease, or the effect of reduced GFR on uric acid clearance remains unknown. There are limitations to a primary uric acid → CKD → cardiovascular disease mediation hypothesis. The studies by Bellomo et al and Wen et al., along with most epidemiological reports examining uric acid as a risk factor, have used single time point measurements and therefore ignored physiological and pathological changes over time. Accordingly, these studies cannot truly determine causality, but rather merely support the hypothesis. Additionally, these studies rely on estimated GFR as a marker of kidney function; given the imprecision associated with creatinine-based estimated GFR at more normal GFR levels and the high degree of correlation between GFR and uric acid levels, it is possible that higher levels of uric acid may identify individuals with lower initial GFR at baseline for whom there is greater imprecision with creatinine-based estimation alone.
Hyperuricemia is a readily modifiable risk factor, rendering considerable clinical importance to a construct linking uric acid → CKD → cardiovascular disease. Unfortunately, clinical trials evaluating this construct remain limited, with the most notable randomized trial to date demonstrating improved blood pressure control associated with allopurinol use for uric acid lowering in 30 adolescents (20). Other studies in patients with CKD demonstrated slower progression in early stage CKD with allopurinol treatment and more rapid CKD progression in individuals where allopurinol was withdrawn (21, 22). Larger studies examining the effect of allopurinol on hypertension and endothelial dysfunction are currently underway, and the results are expected to further delineate the association between uric acid and cardiovascular disease (23). Critically, there are insufficient randomized clinical trial data evaluating the effect of uric acid reduction in generalizable populations, and those that exist have largely focused on the surrogate outcome of hypertension rather than hard kidney disease and cardiovascular events.
In caring for the growing number patients with CKD, practitioners will continue to encounter hyperuricemia in the absence of clinical gout. The studies by Bellomo et al and Wen et al suggest that hyperuricemia may represent a therapeutic target for CKD progression and cardiovascular disease risk reduction, rather than an incidental finding related to CKD severity. Ultimately, large randomized trials evaluating major kidney disease and cardiovascular disease outcomes are needed to fully understand the association among uric acid, kidney disease and cardiovascular disease and to determine whether therapy for hyperuricemia may play a role in preventing CKD and cardiovascular disease.
Acknowledgments
Dr. Weiner participated in a scientific advisory board in 2009 for Takeda Pharmaceuticals, the manufacturer of febuxostat.
Dr. Tangri is supported by the KRESCENT Post Doctoral Fellowship, A joint initiative of the Kidney Foundation of Canada, the Canadian Institute of Health Research and the Canadian Society of Nephrology
References
- 1.Davis N. The cardio-vascular and renal relations and manifestations of gout. JAMA. 1897;29(6):261–262. [Google Scholar]
- 2.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008;359(17):1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25(1):39–42. doi: 10.1016/j.semnephrol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13(12):2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 5.Lehto S, Niskanen L, Ronnemaa T, Laakso M. Serum uric acid is a strong predictor of stroke in patients with non-insulin-dependent diabetes mellitus. Stroke. 1998;29(3):635–639. doi: 10.1161/01.str.29.3.635. 1998. [DOI] [PubMed] [Google Scholar]
- 6.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38(5):1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 7.Niskanen LK, Laaksonen DE, Nyyssonen K, et al. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: a prospective cohort study. Arch Intern Med. 2004;164(14):1546–1551. doi: 10.1001/archinte.164.14.1546. [DOI] [PubMed] [Google Scholar]
- 8.Bellomo G, Venanzi S, Verdura C, Saronio P, Esposito A, Timio M. Association of Uric Acid with Change in Kidney Function in Healthy Normotensive Individuals. Am J Kidney Dis. 2010 Apr 10; doi: 10.1053/j.ajkd.2010.01.019. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Wen CP, Cheng TY, Chan HT, Tsai MK, Chung IW, Tsai SP, Wu SB, Wen SF. Is High Serum Uric Acid a Risk Marker or a Target for Treatment? Examination of Individuals with Low Cardiovascular Risk in a Large Cohort. Am J Kidney Dis. 2010 doi: 10.1053/j.ajkd.2010.01.024. In press. [DOI] [PubMed] [Google Scholar]
- 10.Doehner W, Schoene N, Rauchhaus M, et al. Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: results from 2 placebo-controlled studies. Circulation. 2002;105(22):2619–2624. doi: 10.1161/01.cir.0000017502.58595.ed. [DOI] [PubMed] [Google Scholar]
- 11.Mercuro G, Vitale C, Cerquetani E, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol. 2004;94(7):932–935. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 12.Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int. 2005;68(Suppl 99):S57–65. doi: 10.1111/j.1523-1755.2005.09911.x. [DOI] [PubMed] [Google Scholar]
- 13.Puig JG, Anton FM, Alonso-Vega GG. Renal handling of uric acid: hypouricemia and tubular urate secretion. Arch Intern Med. 1986;146(9):1865. [PubMed] [Google Scholar]
- 14.Rose BD, Post TW. Clinical Physiology of Acid-Base and Electrolyte Disorders. 5th. New York, NY: McGraw-Hill; 2001. pp. 96–99. [Google Scholar]
- 15.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS. Uric acid and incident kidney disease in the community. J Am Soc Nephrol. 2008;19(6):1204–1211. doi: 10.1681/ASN.2007101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med. 1999;131(1):7–13. doi: 10.7326/0003-4819-131-1-199907060-00003. [DOI] [PubMed] [Google Scholar]
- 17.McNamee R. Confounding and confounders. Occup Environ Med. 2003;60(3):227–234. doi: 10.1136/oem.60.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elsayed EF, Tighiouart H, Griffith J, et al. Cardiovascular disease and subsequent kidney disease. Arch Intern Med. 2007;167(11):1130–1136. doi: 10.1001/archinte.167.11.1130. [DOI] [PubMed] [Google Scholar]
- 19.Christenfeld NJ, Sloan RP, Carroll D, Greenland S. Risk factors, confounding, and the illusion of statistical control. Psychosom Med. 2004;66(6):868–875. doi: 10.1097/01.psy.0000140008.70959.41. 2004. [DOI] [PubMed] [Google Scholar]
- 20.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300(8):924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siu YP, Leung KT, Tong MK, Kwan TH. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis. 2006;47(1):51–59. doi: 10.1053/j.ajkd.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Talaat KM, el-Sheikh AR. The effect of mild hyperuricemia on urinary transforming growth factor beta and the progression of chronic kidney disease. Am J Nephrol. 2007;27(5):435–440. doi: 10.1159/000105142. [DOI] [PubMed] [Google Scholar]
- 23.Segal S. Uric Acid and Hypertension in African Americans: NCT00241839. 2010 [Google Scholar]