Abstract
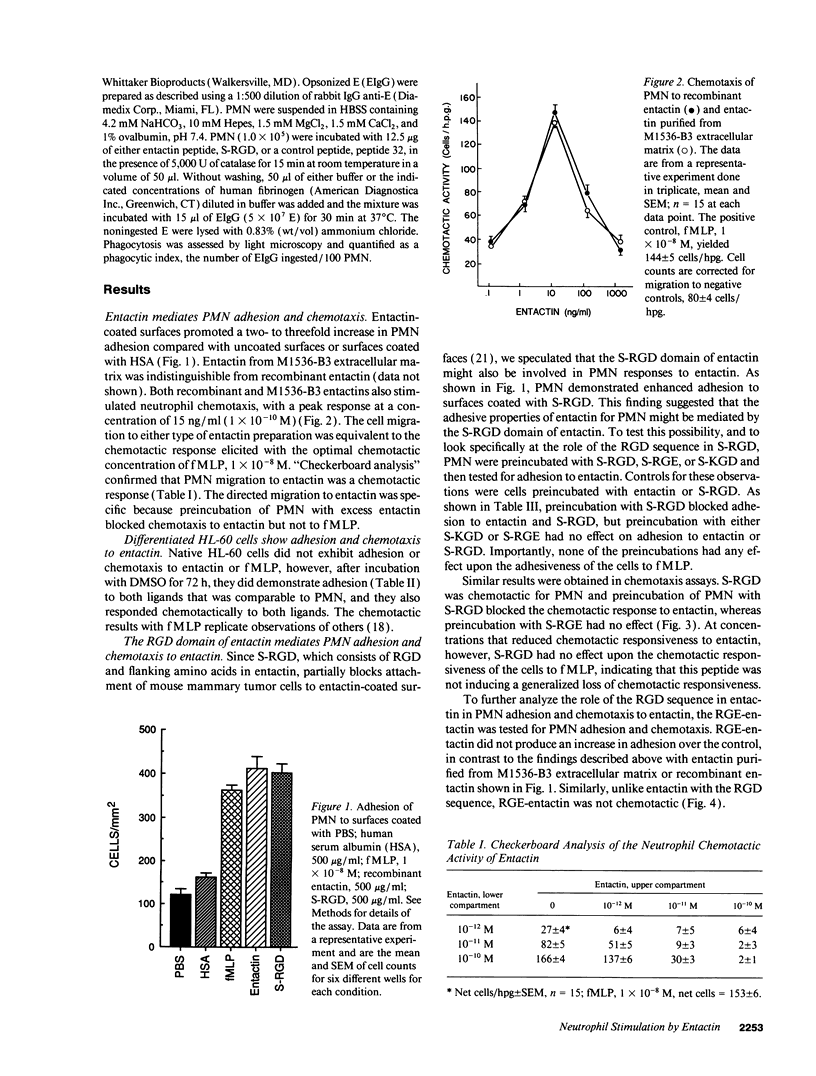
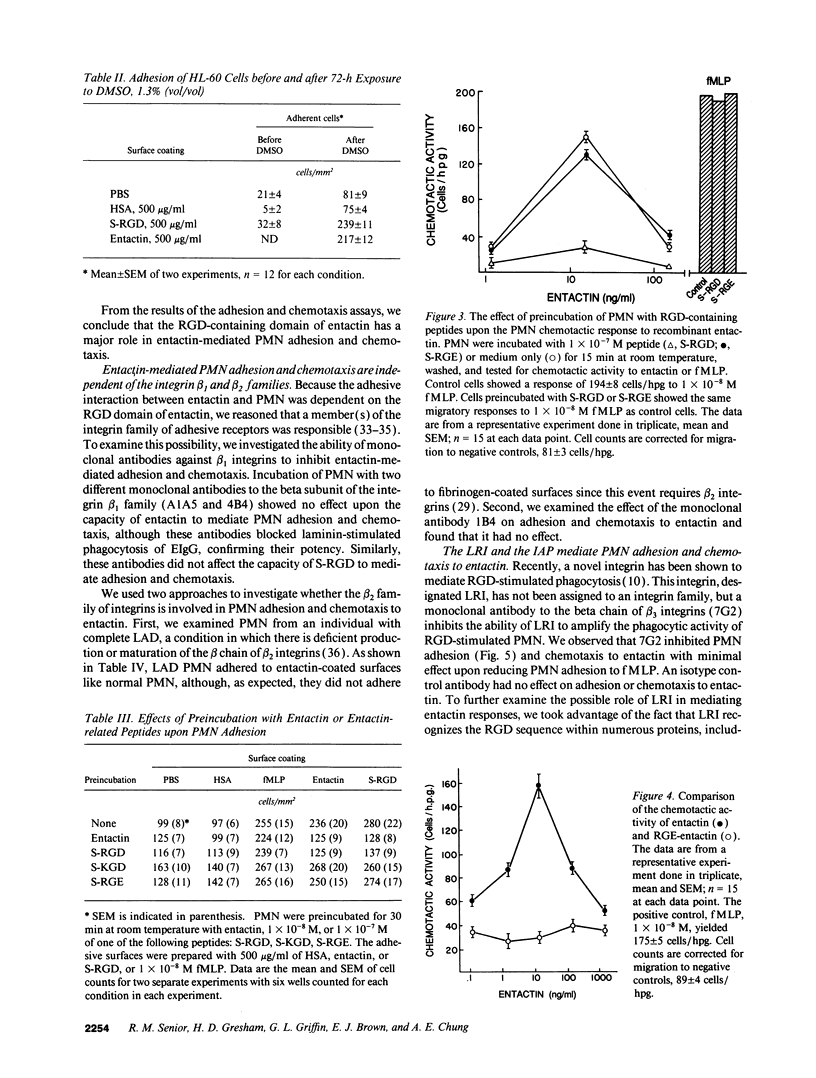
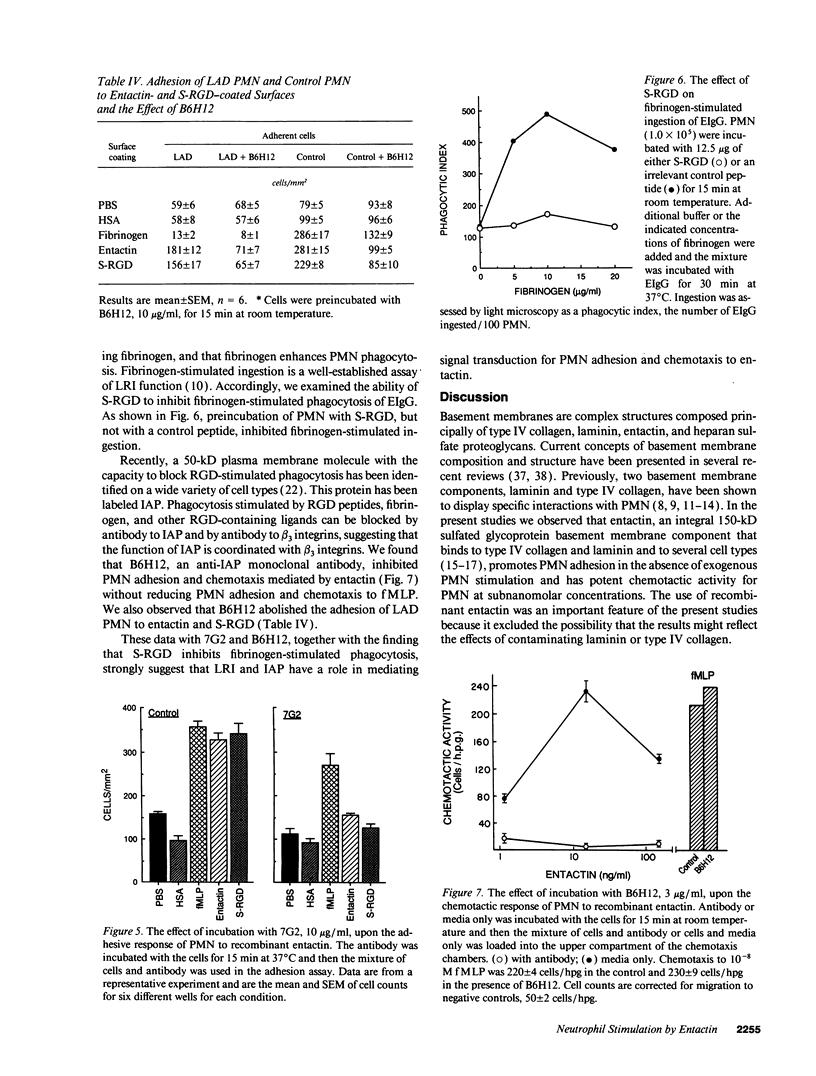
Entactin is an integral component of basement membranes that plays a major role in basement membrane assembly through its ability to bind avidly to both laminin and type IV collagen. Because neutrophil (PMN) interactions with entactin have not been examined, we investigated the ability of natural and recombinant entactin to mediate PMN adhesion and chemotaxis. With both forms of entactin, we observed that entactin-coated surfaces promoted PMN adhesion and that entactin stimulated PMN chemotaxis. The increase in adhesion to entactin over control was two to threefold whereas the chemotactic response to 15 ng/ml (1 x 10(-10) M) entactin was equivalent to the chemotactic response elicited with 1 x 10(-8) M formyl-methionyl-leucyl-phenylalanine (fMLP). HL-60 cells, after differentiation with dimethylsulfoxide, also demonstrated adhesion and chemotaxis to entactin. A synthetic peptide of the Arg-Gly-Asp (RGD) domain in entactin, SIGFRGDGQTC (S-RGD), mediated PMN adhesion and chemotaxis, and preexposure of PMN to S-RGD blocked PMN adhesion and chemotaxis induced by entactin without diminishing the adhesive and chemotactic activities of fMLP. In contrast, preexposure to peptides SIGFRGEGQTCA or SIGFKGDGQTCA had no effect. The findings with synthetic peptides were confirmed with a recombinant entactin mutant in which aspartic acid at residue 674 was replaced with glutamic acid, thus converting the RGD sequence of entactin to RGE. RGE-entactin was neither adhesive nor chemotactic for neutrophils. Monoclonal antibodies to the leukocyte response integrin (LRI) and the integrin-associated protein blocked entactin-mediated adhesion and chemotaxis whereas monoclonal antibodies to beta 1 and beta 2 integrins had no effect and PMN from an individual with leukocyte-adhesion deficiency adhered normally to entactin-coated surfaces. These data demonstrate that entactin mediates biologically and pathologically important functions of PMN through its RGD domain and that LRI, which has been shown previously to mediate RGD-stimulated phagocytosis, is also capable of mediating RGD-stimulated PMN adhesion and chemotaxis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Anderson D. C., Springer T. A. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- Bohnsack J. F., Akiyama S. K., Damsky C. H., Knape W. A., Zimmerman G. A. Human neutrophil adherence to laminin in vitro. Evidence for a distinct neutrophil integrin receptor for laminin. J Exp Med. 1990 Apr 1;171(4):1221–1237. doi: 10.1084/jem.171.4.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Smolen J. E. Neutrophil granule constituents and their release in health and disease. Hematol Oncol Clin North Am. 1988 Mar;2(1):101–134. [PubMed] [Google Scholar]
- Brown E., Hooper L., Ho T., Gresham H. Integrin-associated protein: a 50-kD plasma membrane antigen physically and functionally associated with integrins. J Cell Biol. 1990 Dec;111(6 Pt 1):2785–2794. doi: 10.1083/jcb.111.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant G., Rao C. N., Brentani M., Martins W., Lopes J. D., Martin S. E., Liotta L. A., Schiffmann E. A role for the laminin receptor in leukocyte chemotaxis. J Leukoc Biol. 1987 Mar;41(3):220–227. doi: 10.1002/jlb.41.3.220. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Warner-Lambert/Parke-Davis Award lecture. Cellular and molecular mechanisms that direct leukocyte traffic. Am J Pathol. 1990 Jan;136(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- Chakravarti S., Tam M. F., Chung A. E. The basement membrane glycoprotein entactin promotes cell attachment and binds calcium ions. J Biol Chem. 1990 Jun 25;265(18):10597–10603. [PubMed] [Google Scholar]
- Chandrasekaran S., Dean J. W., 3rd, Giniger M. S., Tanzer M. L. Laminin carbohydrates are implicated in cell signaling. J Cell Biochem. 1991 Jun;46(2):115–124. doi: 10.1002/jcb.240460205. [DOI] [PubMed] [Google Scholar]
- Chung A. E., Durkin M. E. Entactin: structure and function. Am J Respir Cell Mol Biol. 1990 Oct;3(4):275–282. doi: 10.1165/ajrcmb/3.4.275. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J Exp Med. 1979 Apr 1;149(4):969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Plow E. F. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991 Jul;16(7):246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Durkin M. E., Chakravarti S., Bartos B. B., Liu S. H., Friedman R. L., Chung A. E. Amino acid sequence and domain structure of entactin. Homology with epidermal growth factor precursor and low density lipoprotein receptor. J Cell Biol. 1988 Dec;107(6 Pt 2):2749–2756. doi: 10.1083/jcb.107.6.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. W., Mayer U., Nischt R., Aumailley M., Reinhardt D., Wiedemann H., Mann K., Timpl R., Krieg T., Engel J. Recombinant nidogen consists of three globular domains and mediates binding of laminin to collagen type IV. EMBO J. 1991 Nov;10(11):3137–3146. doi: 10.1002/j.1460-2075.1991.tb04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham I. L., Gresham H. D., Brown E. J. An immobile subset of plasma membrane CD11b/CD18 (Mac-1) is involved in phagocytosis of targets recognized by multiple receptors. J Immunol. 1989 Apr 1;142(7):2352–2358. [PubMed] [Google Scholar]
- Gresham H. D., Clement L. T., Lehmeyer J. E., Griffin F. M., Jr, Volanakis J. E. Stimulation of human neutrophil Fc receptor-mediated phagocytosis by a low molecular weight cytokine. J Immunol. 1986 Aug 1;137(3):868–875. [PubMed] [Google Scholar]
- Gresham H. D., Goodwin J. L., Allen P. M., Anderson D. C., Brown E. J. A novel member of the integrin receptor family mediates Arg-Gly-Asp-stimulated neutrophil phagocytosis. J Cell Biol. 1989 May;108(5):1935–1943. doi: 10.1083/jcb.108.5.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groebe D. R., Chung A. E., Ho C. Cationic lipid-mediated co-transfection of insect cells. Nucleic Acids Res. 1990 Jul 11;18(13):4033–4033. doi: 10.1093/nar/18.13.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A. R., Weiss S. J. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 1989 Apr;83(4):1122–1136. doi: 10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Mann K., Deutzmann R., Aumailley M., Timpl R., Raimondi L., Yamada Y., Pan T. C., Conway D., Chu M. L. Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV and cells. EMBO J. 1989 Jan;8(1):65–72. doi: 10.1002/j.1460-2075.1989.tb03349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield P. J., Boxer L. A., Suchard S. J. Thrombospondin stimulates motility of human neutrophils. J Cell Biol. 1990 Dec;111(6 Pt 2):3077–3086. doi: 10.1083/jcb.111.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzner Y., Vlodavsky I., Michaeli R. I., Eldor A. Selective inhibition of neutrophil activation by the subendothelial extracellular matrix: possible role in protection of the vessel wall during diapedesis. Exp Cell Res. 1990 Aug;189(2):233–240. doi: 10.1016/0014-4827(90)90241-2. [DOI] [PubMed] [Google Scholar]
- McClary J. A., Witney F., Geisselsoder J. Efficient site-directed in vitro mutagenesis using phagemid vectors. Biotechniques. 1989 Mar;7(3):282–289. [PubMed] [Google Scholar]
- Mecham R. P. Laminin receptors. Annu Rev Cell Biol. 1991;7:71–91. doi: 10.1146/annurev.cb.07.110191.000443. [DOI] [PubMed] [Google Scholar]
- Murphy G., Cockett M. I., Ward R. V., Docherty A. J. Matrix metalloproteinase degradation of elastin, type IV collagen and proteoglycan. A quantitative comparison of the activities of 95 kDa and 72 kDa gelatinases, stromelysins-1 and -2 and punctuated metalloproteinase (PUMP). Biochem J. 1991 Jul 1;277(Pt 1):277–279. doi: 10.1042/bj2770277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike M. C., Wicha M. S., Yoon P., Mayo L., Boxer L. A. Laminin promotes the oxidative burst in human neutrophils via increased chemoattractant receptor expression. J Immunol. 1989 Mar 15;142(6):2004–2011. [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Fliszar C. J., Shapiro S. D., Goldberg G. I., Welgus H. G. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991 Apr 25;266(12):7870–7875. [PubMed] [Google Scholar]
- Senior R. M., Hinek A., Griffin G. L., Pipoly D. J., Crouch E. C., Mecham R. P. Neutrophils show chemotaxis to type IV collagen and its 7S domain and contain a 67 kD type IV collagen binding protein with lectin properties. Am J Respir Cell Mol Biol. 1989 Dec;1(6):479–487. doi: 10.1165/ajrcmb/1.6.479. [DOI] [PubMed] [Google Scholar]
- Sham R. L., Packman C. H., Abboud C. N., Lichtman M. A. Signal transduction and the regulation of actin conformation during myeloid maturation: studies in HL60 cells. Blood. 1991 Jan 15;77(2):363–370. [PubMed] [Google Scholar]
- Singer I. I., Scott S., Kawka D. W., Kazazis D. M. Adhesomes: specific granules containing receptors for laminin, C3bi/fibrinogen, fibronectin, and vitronectin in human polymorphonuclear leukocytes and monocytes. J Cell Biol. 1989 Dec;109(6 Pt 1):3169–3182. doi: 10.1083/jcb.109.6.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Kishimoto T. K., Abbassi O., Hughes B., Rothlein R., McIntire L. V., Butcher E., Anderson D. C., Abbass O. Chemotactic factors regulate lectin adhesion molecule 1 (LECAM-1)-dependent neutrophil adhesion to cytokine-stimulated endothelial cells in vitro. J Clin Invest. 1991 Feb;87(2):609–618. doi: 10.1172/JCI115037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spertini O., Kansas G. S., Munro J. M., Griffin J. D., Tedder T. F. Regulation of leukocyte migration by activation of the leukocyte adhesion molecule-1 (LAM-1) selectin. Nature. 1991 Feb 21;349(6311):691–694. doi: 10.1038/349691a0. [DOI] [PubMed] [Google Scholar]
- Suchard S. J., Burton M. J., Dixit V. M., Boxer L. A. Human neutrophil adherence to thrombospondin occurs through a CD11/CD18-independent mechanism. J Immunol. 1991 Jun 1;146(11):3945–3952. [PubMed] [Google Scholar]
- Terranova V. P., DiFlorio R., Hujanen E. S., Lyall R. M., Liotta L. A., Thorgeirsson U., Siegal G. P., Schiffmann E. Laminin promotes rabbit neutrophil motility and attachment. J Clin Invest. 1986 Apr;77(4):1180–1186. doi: 10.1172/JCI112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. 1989 Apr 1;180(3):487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x. [DOI] [PubMed] [Google Scholar]
- Tsao T., Hsieh J. C., Durkin M. E., Wu C. Y., Chakravarti S., Dong L. J., Lewis M., Chung A. E. Characterization of the basement membrane glycoprotein entactin synthesized in a baculovirus expression system. J Biol Chem. 1990 Mar 25;265(9):5188–5191. [PubMed] [Google Scholar]
- Vedder N. B., Winn R. K., Rice C. L., Chi E. Y., Arfors K. E., Harlan J. M. A monoclonal antibody to the adherence-promoting leukocyte glycoprotein, CD18, reduces organ injury and improves survival from hemorrhagic shock and resuscitation in rabbits. J Clin Invest. 1988 Mar;81(3):939–944. doi: 10.1172/JCI113407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Weitz J. I., Huang A. J., Levin S. M., Silverstein S. C., Loike J. D. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chung A. E. Potential role of entactin in hemostasis. Specific interaction of entactin with fibrinogen A alpha and B beta chains. J Biol Chem. 1991 Oct 5;266(28):18802–18807. [PubMed] [Google Scholar]
- Wu C., Reing J., Chung A. E. Entactin forms a complex with fibronectin and co-localizes in the extracellular matrix of the embryonal carcinoma-derived 4CQ cell line. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1219–1225. doi: 10.1016/0006-291x(91)91023-6. [DOI] [PubMed] [Google Scholar]
- Yoon P. S., Boxer L. A., Mayo L. A., Yang A. Y., Wicha M. S. Human neutrophil laminin receptors: activation-dependent receptor expression. J Immunol. 1987 Jan 1;138(1):259–265. [PubMed] [Google Scholar]
- Yurchenco P. D., Schittny J. C. Molecular architecture of basement membranes. FASEB J. 1990 Apr 1;4(6):1577–1590. doi: 10.1096/fasebj.4.6.2180767. [DOI] [PubMed] [Google Scholar]



