Introduction
Microtubule-based motor proteins are utilized to move and position a wide range of organelles and protein complexes in cells [1-3]. The members of the kinesin superfamily are responsible for movement of cargo toward microtubule plus-ends. Cytoplasmic dynein is the major microtubule-based motor protein for cargo transport toward microtubule minus ends [4]. Cytoplasmic dynein is a large protein complex (~1.5 MDa) composed of six subunits, all of which are present as dimers [5, 6]. The largest subunit, the heavy chain (DYNC1H) has distinct head and tail domains. The head contains the motor with the microtubule binding and ATP hydrolysis domains that generate movement along microtubules [7]. The other five subunits, the intermediate chain (DYNC1I, IC), the light intermediate chains (DYNC1LI, LIC), and three light chains, roadblock (DYNC1LRB), LC8 (DYNC1LL), and Tctex (DYNC1LT) and bind directly or indirectly to the heavy chain tails and comprise the cargo binding domain [6, 8, 9].
One important characteristic of cytoplasmic microtubule based transport is that there are many kinesin motor genes but only one gene for the cytoplasmic dynein motor. Mammals contain approximately forty five kinesin genes that can be grouped into fourteen classes [1]. This provides a large choice of motors for the transport of specific cargos toward microtubule plus ends. In contrast there is a single gene for the cytoplasmic dynein motor (although there are approximately sixteen genes for the axonemal dynein motors that move flagella) [9, 10]. The cargos cytoplasmic dynein moves toward microtubule minus ends include viruses, protein complexes such as mitotic check point proteins, cytoskeletal filaments including intermediate filaments and microtubules, and most membrane bounded organelles including endosomes, lysosomes, mitochondria and nuclei [4, 6]. Since there is a single microtubule-minus end based motor, it is important to identify the mechanisms that allow cytoplasmic dynein to be differentially regulated to accomplish its many diverse functions. One hypothesis is that isoforms of the non-motor subunits contribute to dynein cargo specific regulation. There are two genes for each of those five subunits in vertebrates [9]. The light chains were hypothesized to link various proteins to cytoplasmic dynein, but structural studies showed that most of the putative binding partners bind to the light chains at their intermediate chain binding domains and thus compete for binding to the intermediate chains [6, 11]. There is also evidence that the light intermediate chains and intermediate chains specify individual dynein roles in cultured cells [6]. This review will focus on the role of different isoforms of the cytoplasmic dynein intermediate chain in dynein function and regulation.
Domains of the vertebrate intermediate chains
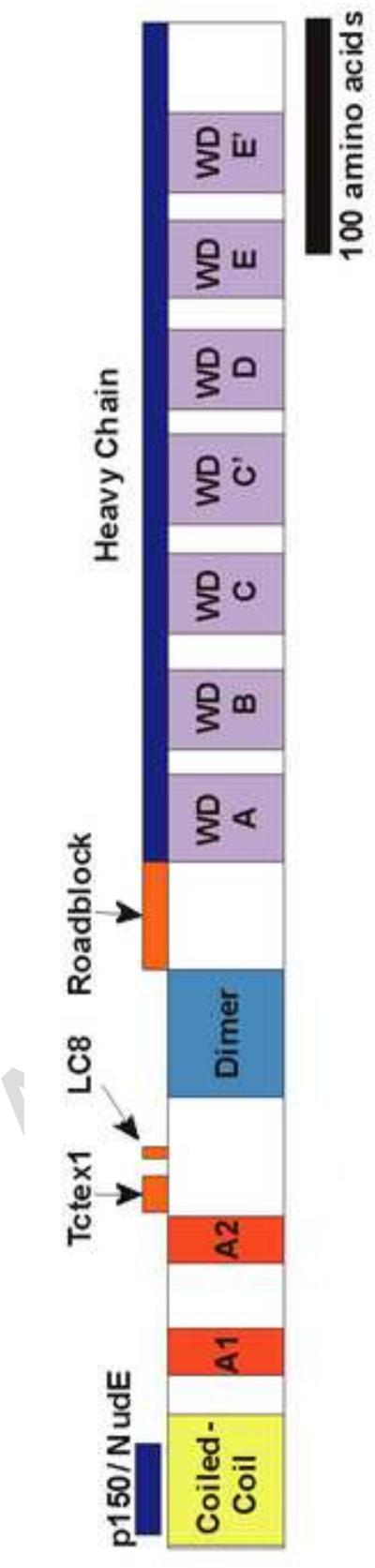
The intermediate chain (DYNC1I) has a scaffold role in the dynein complex (Figure 1). It binds directly to four of the other five subunits, the heavy chain and the three light chains. The intermediate chain also binds components of several dynein regulators, including the p150 subunit of dynactin, NUDE (which interacts with Lis1), Huntington, ZW-10, and the La RNA chaperone protein [3, 12]. The intermediate chain's C-terminus contains seven WD repeats which are thought to form a beta propeller structure, and this region is necessary for binding to the tail of the heavy chain. Mapping studies using heavy chain fragments suggest that the binding regions for the light intermediate chains and intermediates are close to one another or overlap [13]. A point mutation in the intermediate chain binding region of the mouse heavy chain results in age dependent motor neuron neurodegeneration and other neurological defects [14, 15]. The mutation lowers the affinity of the heavy chain for the intermediate chain and alters dynein motor function [16].
Figure 1. Domain map of the rat intermediate chain (IC).
The N-terminus is to the left. The coiled-coil is yellow; The two alternative splicing regions are red (A1 & A2); the dimerization domain is blue, the WD repeats are purple. The binding regions of the other dynein subunits and the subunits of dynein regulators, NudE and p150 (dynactin), are show above the map.
The N-terminus of the intermediate chain contains a coiled-coil region involved in binding to the p150 subunit of dynactin [17]. NUDE(L)/Lis1 also bind to this region. Current data indicate that the binding of the two regulator complexes to the intermediate chain is mutually exclusive, with binding to the p150 subunit of dynactin being favored [18]. This region also contains the binding site for a pan IC antibody (74.1), and the antibody competes with p150 for binding to the intermediate chain. This provides the basis for the ability of the antibody to block some dynein functions [18-20].
Just downstream of the intermediate chain coiled-coil are two domains that can be removed by alternative splicing and a serine rich region. Next are the binding sites for two of the light chain families, the LC8 (DYNLL) and Tctex1 (DYNLT) families, which bind as homodimers to an intermediate chain dimer [21-23]. The binding region for the roadblock light chain family is more C-terminal and is located just upstream of the first WD repeat domain [24, 25]. The dimerization domain of the rat intermediate chain was identified using independent biochemical and structural analytical techniques and it was found to be immediately N-terminal to the roadblock light chain binding region [11, 24]. Studies using over-expression of intermediate chain truncation mutations indicated that mammalian intermediate chain dimerization did not require the binding of any light chains. However, structural studies of the Drosophila intermediate chain in vitro found that dimerization required light chain binding and that the roadblock binding site partially overlapped with the proposed intermediate chain dimerization domain [26]. These results, and differences in the alternative splicing pattern of Drosophila mRNA, suggest that that some subdomains of the intermediate chain may not be well conserved among higher and lower eukaryotes.
Alternative splicing generates intermediate chain isoforms
There are two intermediate chain genes in vertebrates and both undergo alternative splicing at two conserved splicing sites in their N-termini. In the rat this results in three protein isoforms from each gene [17, 27-29]. In Drosophila, at least ten isoforms are produced by the single gene [30]. Recently a systematic survey of vertebrate alternative splicing was performed in the mouse [31]. This study identified eleven potential intermediate chain protein isoforms, five IC-1 isoforms and six IC-2 isoforms. Splicing in the mouse intermediate chain genes occurs at the same locations as that observed in rat intermediate chains, but in the mouse the size of the two spliced regions is variable. Interestingly, eleven different IC-2 mRNAs were identified. The IC-2 gene has two different promoters and first exons, but since the first exon does not contribute to the protein coding sequence, only six different IC-2 protein isoforms are produced. The presence of two different promoters suggests another potential point for regulation of the IC-2 isoform expression.
The intermediate chain isoforms have different tissue and cell expression patterns [28, 29, 31, 32]. One IC-2 isoform, IC-2C, is found almost in all cell types and tissues and it is often the only isoform found in cultured cells such as fibroblasts [27]. Although the IC-2B isoform is commonly found in most rat organs, to date it has only been found in cultured neurons and neuroblastoma cell lines [27, 28, 33]. The IC-1 isoforms are found primarily in mouse and rat brain tissues, although IC-1C is also detected in ovary and testis [28, 31]. IC-1 and IC-2 mRNAs that encode the full length proteins were found only in brain, and then only at very low levels, suggesting that there is little full length intermediate chain protein in cells. Four intermediate chain isoforms are found in cultured rat embryonic hippocampal and cortical neurons [29].
The expression of the intermediate chain isoforms is regulated during brain development [27-29]. At ages earlier than embryonic day 13 in the rat (E13) only IC-2C, the sole isoform found in fibroblasts, is observed. With increased time in development the presence of IC-2B is observed. Later in embryonic development, the IC-1 isoforms are also expressed. However 2 dimensional gel analyses of the isoforms show that it is not until five days after birth that adult protein levels are observed. Interestingly, a similar, albeit simpler, pattern is observed during nerve growth factor induced differentiation of rat pheochromocytoma, PC12, cells. Upon addition of nerve growth factor, cell division stops and the cells extend long neurite like processes. Within 24 hours of nerve growth factor addition, the intermediate chain isoform expression pattern changes from primarily IC-2C to predominantly IC-2B [33].
The six isoforms of the rat intermediate chain are capable of forming all combinations of homo-and hetero-dimers when they are over-expressed in cultured cells [24]. Further evidence that the intermediate chains can heterodimerize was obtained from studies on dynein isolated from a mouse model which has a “knock-in” of IC-1 with GFP (green fluorescent protein) and the FLAG epitope tag located at the C-terminus [34]. When dynein complexes containing IC-1 were immune-purified with antibodies to FLAG, IC-2 was also detected in the purified dynein sample. The functional significance of intermediate chain heterodimer formation is unclear. It has been hypothesized that heterodimer formation of by a dynein subunit will increase the number of potential dynein motor subtypes available for cargo-binding specificity. It could also allow for multiple forms of dynein regulation on an individual cargo. Structural and biochemical studies indicate that homodimers of the light chains bind to dimers of the intermediate chains and that all six light chains can bind the six rat intermediate chains [11, 23].
Dynein complexes with different intermediate chain isoforms have distinct functions in neurons
The mRNA and protein expression patterns indicate that dyneins with different intermediate chain isoforms cannot be important for differential dynein regulation or cargo binding in fibroblasts, as these cells express only one isoform, but the various intermediate chain isoforms may be important for regulation of dynein function in neurons or other differentiated cells. Neurons are highly polarized cells and their long axons are particularly dependent on motor protein transport of material for axon growth and survival. Neurological defects are often the first phenotypes to be observed when dynein or kinesin is mutated in mouse and Drosophila Suggesting that the long distance transport in axons pushes the motor proteins’ capacities to their limits [14, 15, 35-39].
Anterograde transport
Axons have their microtubule plus ends pointing to axon terminals, and the microtubule plus end directed kinesins are responsible for delivering the cargos necessary for growth and maintenance of the axons. Dynein complexes must be transported into the axon in order to be positioned at the terminals to move material back to the cell bodies in retrograde transport. Axonal dynein cargos include the signaling endosomes necessary for survival as well as lysosomes and other organelles targeted for degradation. The transport of dynein complexes in the anterograde direction in axons was studied in optic nerves of adult rats. Cytoplasmic dynein complexes containing IC-2C were found in anterograde fast axonal transport, in association with membrane bounded organelle cargos transported by members of the kinesin family [20, 40, 41]. Interestingly, dynein complexes containing the other three intermediate chain isoforms were found in slow component b with the “cytosolic” proteins [40, 41]. These data indicate that neurons can distinguish dynein complexes with different intermediate chain isoforms and target them in different ways, most likely for different functions. One attractive hypothesis is that dynein with IC-2C is found on membrane bounded organelle cargos moving rapidly in the anterograde direction in order to provide those organelles with a reverse motor in case obstructions or other obstacles to movement are encountered. Dynein complexes containing the other isoforms would be recruited for regulated retrograde transport such as endocytosis.
Cargo specificity
Live cell imaging of fluorescent protein-tagged intermediate chain isoforms was used to characterize the properties of dynein complexes with different intermediate chains in neurons. When fluorescent protein-tagged IC-1B and IC-2C were transfected into cultured embryonic hippocampal neurons and imaged with fluorescence microscopy the intermediate chains were concentrated in small (1-2 pixels) puncta indistinguishable from puncta observed with markers for membranous organelles [27, 42, 43]. Two experiments demonstrated that the puncta observed with fluorescent intermediate chains identify dynein complexes. When dynein complexes were isolated from cell lines with stable expression of the IC-2C GFP, all the IC-GFP co-purified with the dynein complex [43]. Second, when the motility properties of the fluorescent puncta in axons of hippocampal neurons transfected with fluorescent intermediate chains were analyzed, they were comparable to those of fluorescent puncta in axons of cultured neurons from the mouse model with knock in of IC-1 tagged with GFP and FLAG [34].
When the distributions of two fluorescent intermediate chains, IC-1B and IC-2C, were compared to those of organelle markers, it was observed that dynein complexes with different intermediate chains co-localized with different organelles. Dynein complexes containing IC-1B were more likely to co-localize with organelles containing the transmembrane neurotrophin receptor kinase, TrkB (signaling endosomes), and Rab7 containing endosomes than dynein complexes containing IC-2C [42, 43] Dynein complexes containing IC-2C were more likely to co-localize with mitochondria [42]. A low level of co-localization of the three organelles with the other intermediate chain was observed. This could be due to over-expression/transfection artifacts. Alternatively, it maybe evidence for a small level of heterodimerization of the intermediate chains. The co-localization of IC-1 with Trk containing signaling endosomes was also verified by biochemical methods. No IC-2 was found when the intermediate chain composition of Trk containing organelles immuno-purified from rat brain was characterized. These data demonstrate that dynein complexes with different intermediate chains have responsibility for different cargo transport in neurons.
Dynactin is a large protein complex that binds dynein and microtubules and is an important adaptor linking dynein to many cargos. Several proteins have been identified that bind to various dynactin subunits and thus recruit dynactin to different organelles, including RILP, Bicaudal, Milton/Trak, and ZW10 reviewed in [3]. Two over-expression assays have been developed to dissect the role of dynein binding to dynactin for transport in vivo. Over-expression of the dynamitin (p50) subunit of dynactin causes the release the p150 subunit from dynactin. Since the dynein intermediate chains bind to p150, dynein is released from its cargo and dynein based motility is inhibited [44]. CC1 (coliled-coil1) is the region of p150 responsible for binding the intermediate chain [45]. Over-expressed CC1 competes with the full length p150 subunit for binding to the intermediate chain and thus prevents dynein from binding to an intact dynactin complex and thus to cargo, inhibiting dynein based motility. Over-expression of CC1 in neurons has different effects on the movement of dynein containing the IC-1B and IC-2C isoforms (Smiley et al. Abstracts of the Annual Meeting of the American Society for Cell Biology, 2014). The motility of dynein containing IC-2C is inhibited in the presence of CC1. However, there is no inhibition of the motility of dynein containing IC-1B. These results confirm the established model that binding to dynactin is required for the motility when dynein contains IC-2C. However the data further suggest that dynein complexes containing IC-1B do not require intermediate chain binding to p150 to associate with cargo or for motility.
Functional roles of phospho-intermediate chain isoforms
Phosphorylation is a common protein regulatory mechanism. There is considerable evidence that multiple dynein subunits are phosphorylated in vivo, and that phosphorylation is a component of dynein regulation [12, 20, 46-49]. Two dimensional gel analyses demonstrate that the neuronal intermediate chain isoforms are phosphorylated [28, 29, 33]. Nerve growth factor (NGF) triggers a differentiation pathway in PC12 cells, which then extend long neurite processes, and NGF also induces increased phosphorylation of PC12 cell dynein intermediate chains [33]. Neurotrophins, such as nerve growth factor, are also required for the survival of some neurons. The neurotrophin binds to its receptor tyrosine kinase, Trk, and the receptor and ligand are internalized as a signaling endosome. The endosome is transported toward microtubule minus ends and the nucleus where signal transduction pathways promote gene expression [50].
Neurotrophin signaling and endosome formation were used as a model system to investigate the role of intermediate chain phospho-isoforms for dynein regulation [42]. A phosphorylation site on dynein containing IC-2 (S81) from PC12 cells and a conserved site on IC-1 (S80) on dynein from neurons were identified by mass spectrometry. Upon addition of nerve growth factor, a time dependent increase in IC-2 S81 phosphorylation was observed with a phospho-site specific antibody. Increased phosphorylation of IC-1 S80 was also in cultured cortical neurons after addition of brain derived neurotrophic factor (BDNF). An inhibitor of the Trk kinase activity blocked IC phosphorylation. The intermediate chain kinase was identified as a member of the MAP kinase family, ERK1/2, a major downstream effector of the Trk kinase [50]. These observations demonstrated that intermediate chain phosphorylation levels are modulated by extracellular signals.
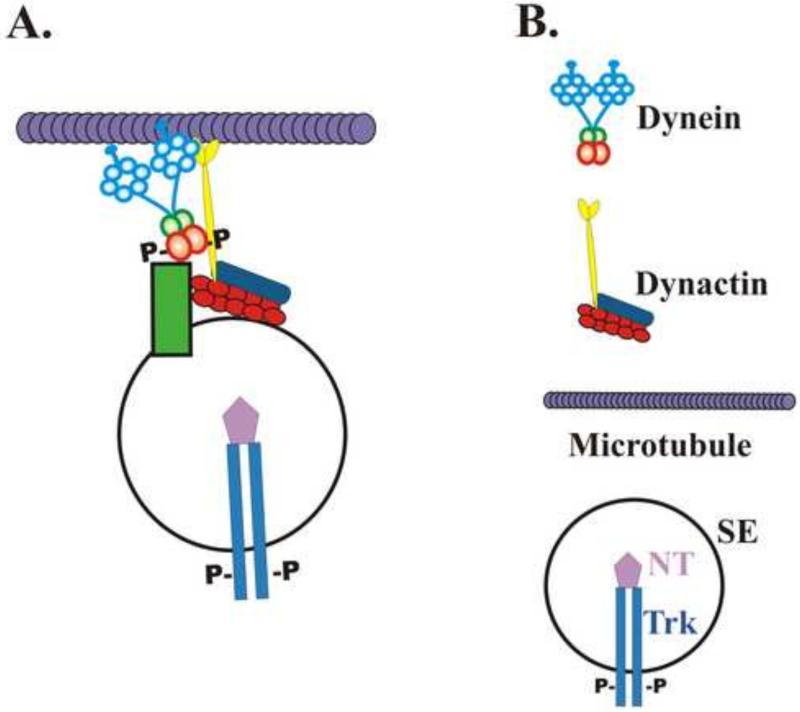
When phospho- and dephospho-mimetic mutants of IC-1B S80 were transfected into cultured hippocampal neurons, the dephospho-mutant showed significantly reduced co-localization with Trk containing signaling endosomes, and the same result was obtained with IC-2C S81 mutants in PC12 cells. In biochemical studies it was found that inhibition of the ERK kinase led to decreased levels of intermediate chain (and less phospho-IC) co-purifying with Trk containing organelles. These data are consistent with the hypothesis that intermediate chain phosphorylation on this serine increases dynein binding to the organelle. Interestingly, the IC-2C S81 phospho- and dephospho-mimetic mutants co-localized with mitochondria to the same extent as wild type IC-2C suggesting that this phosphorylation site is specific for endosomes. ERK is a major downstream effector of the Trk kinase necessary for cell survival. When the dephospho-mimic IC-1B mutant was over-expressed in in cultured sympathetic neurons there was decreased cell survival. This supports the hypothesis that there is decreased dynein binding to and transport of signaling endosomes in the absence of IC phosphorylation. Importantly, phosphorylation of S81 of IC-2C had no effect on intermediate chain binding to p150, either in a solid phase assay or in a solution assay [47, 51]. This suggests the presence of a yet to be identified component of the organelle that discerns the phosphorylation state of the intermediate chain and enhances the binding of phospho-IC to the signaling endosome in addition to, or independent of, dynactin see Figure 2. [42].
Figure 2. Model for Phospho-intermediate Chain Binding to Signaling.
A. Dynein binding to the signaling endosomes requires a component in addition to dynactin to detect changes in intermediate chain phosphorylation state (green box). B. Symbol index: NT is neurotrophin; SE is signaling endosome. Modified from Figure 10 in [42].
Similar increases in IC-2C S81 phosphorylation were observed when epidermal growth factor was added to PC12 cells (Blasier and Pfister unpublished data). In addition, IC-2C S81 is phosphorylated is response to epidermal growth factor stimulation of mouse fibroblasts [51]. These data suggest that phosphorylation of this intermediate chain serine by ERK kinases may be a generic mechanism to recruit dynein for the transport of many receptor kinase containing signaling endosomes.
Serine and threonine phosphorylation of other IC-2C intermediate chain amino acids has been implicated in regulating dynein function on other cargos [12]. On kinetochores, phosphorylation of the IC-2 T89 stimulated dynein transport to spindle poles mitosis [52]. Phosphorylation reduced binding of IC-2C to p150 in a solid phase assay, but it increased IC-2C binding to ZW10. The intermediate chain is also phosphorylated on tyrosine during mitosis [52]. Phosphorylation of IC-2C on S84 also decreased binding of p150 in the solid phase assay and this was proposed to be the basis for the disrupted membrane bounded organelle transport observed in vivo [47], but see also [53]. Live cell imaging of S84 phospho- and dephosphomimetic mutants suggests that neither of the two mutants inhibits dynein, rather they modulate dynein motor activity [54].
A comprehensive study to identify the phosphorylation sites on the dynein intermediate chains isolated from brain, cultured cortical neurons, and PC12 cells by mass spectrometry has been performed (Zhang et al in preparation). Six phosphorylation sites (serine and threonine) were found on IC-2 all of which were in the serine rich region near to the second alternative splicing site. Eight sites were identified in IC-1. Four of the IC-1 sites, in the serine rich region, are conserved with those found in IC-2. However, four of the IC-1 phosphorylation sites are not shared with IC-2, including 1 near the LC8 binding region, 1 in the IC dimerization region, and 1 at the C-terminus. It remains to be determined if the identification of unique phosphorylation sites for both IC-1 and IC-2 are evidence of differential regulation of IC-1 and IC-2 by phosphorylation. Interestingly none of the phosphorylation sites found in vertebrate neuronal tissue are conserved in the intermediate chain sequences of non-vertebrates, although the N-terminal domains of invertebrate intermediate chains do have serine rich regions.
Summary
The cytoplasmic dynein intermediate chain plays a key scaffold role in the complex. It is also has a major role in communicating with other protein complexes and for dynein regulation. In neurons cytoplasmic dynein alternative splicing isoforms are developmentally regulated, transported in different anterograde transport compartments and are involved in binding to specific classes of organelles for retrograde transport. The cytoplasmic dynein intermediate chain isoforms are phosphorylation by ERK kinase on a conserved serine in response to external signals such as growth factors. This intermediate chain phosphorylation enhances dynein binding to singling endosomes and their transport to the nucleus for cell survival. These data support the hypothesis that the intermediate chain isoforms contribute to the regulation of dynein for specific transport functions.
Acknowledgements
This work was funded by the National Institutes of Health, The National Institute for General Medical Science
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirokawa N, Niwa S, Tanaka Y. Molecular motors in neurons: transport mechanisms and roles in brain function, development, and disease. Neuron. 2010;68:610–638. doi: 10.1016/j.neuron.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 2.Akhmanova A, Hammer JA., 3rd Linking molecular motors to membrane cargo. Current opinion in cell biology. 2010;22:479–487. doi: 10.1016/j.ceb.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu MM, Holzbaur EL. Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends in cell biology. 2014;24:564–574. doi: 10.1016/j.tcb.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: An ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- 5.Pfister KK, Fisher EM, Gibbons IR, Hays TS, Holzbaur EL, McIntosh JR, Porter ME, Schroer TA, Vaughan KT, Witman GB, King SM, Vallee RB, Cytoplasmic dynein nomenclature J. Cell Biol. 2005;171:411–413. doi: 10.1083/jcb.200508078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfister KK, Lo KW-H. Cytoplasmic Dynein Function Defined by Subunit Composition. In: King SM, editor. Dyneins. Academic Press; London: 2012. pp. 424–439. [Google Scholar]
- 7.Koonce MP. Identification of a microtubule-binding domain in a cytoplasmic dynein heavy chain. The Journal of biological chemistry. 1997;272:19714–19718. doi: 10.1074/jbc.272.32.19714. [DOI] [PubMed] [Google Scholar]
- 8.Steffen W, Hodgkinson JL, Wiche G. Immunogold localisation of the intermediate chain within the protein complex of cytoplasmic dynein. Journal of structural biology. 1996;117:227–235. doi: 10.1006/jsbi.1996.0087. [DOI] [PubMed] [Google Scholar]
- 9.Pfister KK, Shah PR, Hummerich H, Russ A, Cotton J, Annuar AA, King SM, Fisher EM. Genetic analysis of the cytoplasmic dynein subunit families. PLoS genetics. 2006;2:e1. doi: 10.1371/journal.pgen.0020001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hom EF, Witman GB, Harris EH, Dutcher SK, Kamiya R, Mitchell DR, Pazour GJ, Porter ME, Sale WS, Wirschell M, Yagi T, King SM. A unified taxonomy for ciliary dyneins. Cytoskeleton (Hoboken) 2011;68:555–565. doi: 10.1002/cm.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams JC, Roulhac PL, Roy AG, Vallee RB, Fitzgerald MC, Hendrickson WA. Structural and thermodynamic characterization of a cytoplasmic dynein light chain-intermediate chain complex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:10028–10033. doi: 10.1073/pnas.0703614104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whyte J, Bader JR, Tauhata SB, Raycroft M, Hornick J, Pfister KK, Lane WS, Chan GK, Hinchcliffe EH, Vaughan PS, Vaughan KT. Phosphorylation regulates targeting of cytoplasmic dynein to kinetochores during mitosis. J Cell Biol. 2008;183:819–834. doi: 10.1083/jcb.200804114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tynan SH, Gee MA, Vallee RB. Distinct but overlapping sites within the cytoplasmic dynein heavy chain for dimerization and for intermediate chain and light intermediate chain binding. The Journal of biological chemistry. 2000;275:32769–32774. doi: 10.1074/jbc.M001537200. [DOI] [PubMed] [Google Scholar]
- 14.Hafezparast M, Klocke R, Ruhrberg C, Marquardt A, Ahmad-Annuar A, Bowen S, Lalli G, Witherden AS, Hummerich H, Nicholson S, Morgan PJ, Oozageer R, Priestley JV, Averill S, King VR, Ball S, Peters J, Toda T, Yamamoto A, Hiraoka Y, Augustin M, Korthaus D, Wattler S, Wabnitz P, Dickneite C, Lampel S, Boehme F, Peraus G, Popp A, Rudelius M, Schlegel J, Fuchs H, Hrabe de Angelis M, Schiavo G, Shima DT, Russ AP, Stumm G, Martin JE, Fisher EM. Mutations in dynein link motor neuron degeneration to defects in retrograde transport. Science. 2003;300:808–812. doi: 10.1126/science.1083129. [DOI] [PubMed] [Google Scholar]
- 15.Ilieva HS, Yamanaka K, Malkmus S, Kakinohana O, Yaksh T, Marsala M, Cleveland DW. Mutant dynein (Loa) triggers proprioceptive axon loss that extends survival only in the SOD1 ALS model with highest motor neuron death. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12599–12604. doi: 10.1073/pnas.0805422105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ori-McKenney KM, Xu J, Gross SP, Vallee RB. A cytoplasmic dynein tail mutation impairs motor processivity. Nature cell biology. 2010;12:1228–1234. doi: 10.1038/ncb2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaughan KT, Vallee RB. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995;131:1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKenney RJ, Weil SJ, Scherer J, Vallee RB. Mutually exclusive cytoplasmic dynein regulation by NudE-Lis1 and dynactin. The Journal of biological chemistry. 2011;286:39615–39622. doi: 10.1074/jbc.M111.289017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leopold PL, Kreitzer G, Miyazawa N, Rempel S, Pfister KK, Rodriguez-Boulan E, Crystal RG. Dynein- and microtubule-mediated translocation of adenovirus serotype 5 occurs after endosomal lysis. Hum Gene Ther. 2000;11:151–165. doi: 10.1089/10430340050016238. [DOI] [PubMed] [Google Scholar]
- 20.Dillman JF, 3rd, Pfister KK. Differential phosphorylation in vivo of cytoplasmic dynein associated with anterogradely moving organelles. J Cell Biol. 1994;127:1671–1681. doi: 10.1083/jcb.127.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo KW, Naisbitt S, Fan JS, Sheng M, Zhang M. The 8-kDa dynein light chain binds to its targets via a conserved (K/R)XTQT motif. The Journal of biological chemistry. 2001;276:14059–14066. doi: 10.1074/jbc.M010320200. [DOI] [PubMed] [Google Scholar]
- 22.Mok YK, Lo KW, Zhang M. Structure of Tctex-1 and its interaction with cytoplasmic dynein intermediate chain. The Journal of biological chemistry. 2001;276:14067–14074. doi: 10.1074/jbc.M011358200. [DOI] [PubMed] [Google Scholar]
- 23.Lo KW, Kogoy JM, Rasoul BA, King SM, Pfister KK. Interaction of the DYNLT (TCTEX1/RP3) light chains and the intermediate chains reveals novel intersubunit regulation during assembly of the dynein complex. The Journal of biological chemistry. 2007;282:36871–36878. doi: 10.1074/jbc.M705991200. [DOI] [PubMed] [Google Scholar]
- 24.Lo KW, Kan HM, Pfister KK. Identification of a novel region of the cytoplasmic Dynein intermediate chain important for dimerization in the absence of the light chains. The Journal of biological chemistry. 2006;281:9552–9559. doi: 10.1074/jbc.M511721200. [DOI] [PubMed] [Google Scholar]
- 25.Susalka SJ, Nikulina K, Salata MW, Vaughan PS, King SM, Vaughan KT, Pfister KK. The roadblock light chain binds a novel region of the cytoplasmic Dynein intermediate chain. The Journal of biological chemistry. 2002;277:32939–32946. doi: 10.1074/jbc.M205510200. [DOI] [PubMed] [Google Scholar]
- 26.Nyarko A, Barbar E. Light chain-dependent self-association of dynein intermediate chain. The Journal of biological chemistry. 2011;286:1556–1566. doi: 10.1074/jbc.M110.171686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myers KR, Lo KW, Lye RJ, Kogoy JM, Soura V, Hafezparast M, Pfister KK. Intermediate chain subunit as a probe for cytoplasmic dynein function: Biochemical analyses and live cell imaging in PC12 cells. Journal of neuroscience research. 2007;85:2640–2647. doi: 10.1002/jnr.21213. [DOI] [PubMed] [Google Scholar]
- 28.Pfister KK, Salata MW, Dillman JF, 3rd, Torre E, Lye RJ. Identification and developmental regulation of a neuron-specific subunit of cytoplasmic dynein. Molecular biology of the cell. 1996;7:331–343. doi: 10.1091/mbc.7.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfister KK, Salata MW, Dillman JF, 3rd, Vaughan KT, Vallee RB, Torre E, Lye RJ. Differential expression and phosphorylation of the 74-kDa intermediate chains of cytoplasmic dynein in cultured neurons and glia. The Journal of biological chemistry. 1996;271:1687–1694. doi: 10.1074/jbc.271.3.1687. [DOI] [PubMed] [Google Scholar]
- 30.Nurminsky DI, Nurminskaya MV, Benevolenskaya EV, Shevelyov YY, Hartl DL, Gvozdev VA. Cytoplasmic dynein intermediate-chain isoforms with different targeting properties created by tissue-specific alternative splicing. Molecular and cellular biology. 1998;18:6816–6825. doi: 10.1128/mcb.18.11.6816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuta A, Deng W, Morsi El-Kadi A, Banks GT, Hafezparast M, Pfister KK, Fisher EM. Mouse cytoplasmic dynein intermediate chains: identification of new isoforms, alternative splicing and tissue distribution of transcripts. PloS one. 2010;5:e11682. doi: 10.1371/journal.pone.0011682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paschal BM, Mikami A, Pfister KK, Vallee RB. Homology of the 74-kD cytoplasmic dynein subunit with a flagellar dynein polypeptide suggests an intracellular targeting function. J Cell Biol. 1992;118:1133–1143. doi: 10.1083/jcb.118.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salata MW, Dillman JF, 3rd, Lye RJ, Pfister KK. Growth factor regulation of cytoplasmic dynein intermediate chain subunit expression preceding neurite extension. Journal of neuroscience research. 2001;65:408–416. doi: 10.1002/jnr.1168. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Twelvetrees AE, Lazarus JE, Blasier KR, Yao X, Inamdar NA, Holzbaur EL, Pfister KK, Xiang X. Establishing a novel knock-in mouse line for studying neuronal cytoplasmic dynein under normal and pathologic conditions. Cytoskeleton (Hoboken) 2013;70:215–227. doi: 10.1002/cm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puls I, Oh SJ, Sumner CJ, Wallace KE, Floeter MK, Mann EA, Kennedy WR, Wendelschafer-Crabb G, Vortmeyer A, Powers R, Finnegan K, Holzbaur EL, Fischbeck KH, Ludlow CL. Distal spinal and bulbar muscular atrophy caused by dynactin mutation. Annals of neurology. 2005;57:687–694. doi: 10.1002/ana.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dupuis L, Fergani A, Braunstein KE, Eschbach J, Holl N, Rene F, Gonzalez De Aguilar JL, Zoerner B, Schwalenstocker B, Ludolph AC, Loeffler JP. Mice with a mutation in the dynein heavy chain 1 gene display sensory neuropathy but lack motor neuron disease. Experimental neurology. 2009;215:146–152. doi: 10.1016/j.expneurol.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Martin M, Iyadurai SJ, Gassman A, Gindhart JG, Jr., Hays TS, Saxton WM. Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Molecular biology of the cell. 1999;10:3717–3728. doi: 10.1091/mbc.10.11.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gindhart JG, Jr., Desai CJ, Beushausen S, Zinn K, Goldstein LS. Kinesin light chains are essential for axonal transport in Drosophila. J Cell Biol. 1998;141:443–454. doi: 10.1083/jcb.141.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGrail M, Hays TS. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differentiation in Drosophila. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- 40.Dillman JF, 3rd, Dabney LP, Karki S, Paschal BM, Holzbaur EL, Pfister KK. Functional analysis of dynactin and cytoplasmic dynein in slow axonal transport. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:6742–6752. doi: 10.1523/JNEUROSCI.16-21-06742.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dillman JF, 3rd, Dabney LP, Pfister KK. Cytoplasmic dynein is associated with slow axonal transport. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:141–144. doi: 10.1073/pnas.93.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell DJ, Blasier KR, Jeffery ED, Ross MW, Pullikuth AK, Suo D, Park J, Smiley WR, Lo KW, Shabanowitz J, Deppmann CD, Trinidad JC, Hunt DF, Catling AD, Pfister KK. Trk Activation of the ERK1/2 Kinase Pathway Stimulates Intermediate Chain Phosphorylation and Recruits Cytoplasmic Dynein to Signaling Endosomes for Retrograde Axonal Transport. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:15495–15510. doi: 10.1523/JNEUROSCI.5599-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ha J, Lo KW, Myers KR, Carr TM, Humsi MK, Rasoul BA, Segal RA, Pfister KK. A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J Cell Biol. 2008;181:1027–1039. doi: 10.1083/jcb.200803150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quintyne NJ, Gill SR, Eckley DM, Crego CL, Compton DA, Schroer TA. Dynactin is required for microtubule anchoring at centrosomes. J Cell Biol. 1999;147:321–334. doi: 10.1083/jcb.147.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin SX, Ferro KL, Collins CA. Cytoplasmic dynein undergoes intracellular redistribution concomitant with phosphorylation of the heavy chain in response to serum starvation and okadaic acid. J Cell Biol. 1994;127:1009–1019. doi: 10.1083/jcb.127.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaughan PS, Leszyk JD, Vaughan KT. Cytoplasmic dynein intermediate chain phosphorylation regulates binding to dynactin. The Journal of biological chemistry. 2001;276:26171–26179. doi: 10.1074/jbc.M102649200. [DOI] [PubMed] [Google Scholar]
- 48.Kumar S, Lee IH, Plamann M. Cytoplasmic dynein ATPase activity is regulated by dynactin-dependent phosphorylation. The Journal of biological chemistry. 2000;275:31798–31804. doi: 10.1074/jbc.M000449200. [DOI] [PubMed] [Google Scholar]
- 49.Niclas J, Allan VJ, Vale RD. Cell cycle regulation of dynein association with membranes modulates microtubule-based organelle transport. J Cell Biol. 1996;133:585–593. doi: 10.1083/jcb.133.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Current opinion in neurobiology. 2002;12:268–274. doi: 10.1016/s0959-4388(02)00326-4. [DOI] [PubMed] [Google Scholar]
- 51.Pullikuth AK, Ozdemir A, Cardenas D, Bailey E, Sherman NE, Pfister KK, Catling AD. Epidermal growth factor stimulates extracellular-signal regulated kinase phosphorylation of a novel site on cytoplasmic Dynein intermediate chain 2. International journal of molecular sciences. 2013;14:3595–3620. doi: 10.3390/ijms14023595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang CY, Chang CP, Huang CL, Ferrell JE., Jr. M phase phosphorylation of cytoplasmic dynein intermediate chain and p150(Glued) The Journal of biological chemistry. 1999;274:14262–14269. doi: 10.1074/jbc.274.20.14262. [DOI] [PubMed] [Google Scholar]
- 53.King SJ, Brown CL, Maier KC, Quintyne NJ, Schroer TA. Analysis of the dynein dynactin interaction in vitro and in vivo. Molecular biology of the cell. 2003;14:5089–5097. doi: 10.1091/mbc.E03-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blasier KR, Humsi MK, Ha J, Ross MW, Smiley WR, Inamdar NA, Mitchell DJ, Lo KW, Pfister KK. Live cell imaging reveals differential modifications to cytoplasmic dynein properties by phospho- and dephosphomimic mutations of the intermediate chain 2C S84. Journal of neuroscience research. 2014;92:1143–1154. doi: 10.1002/jnr.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]