Abstract
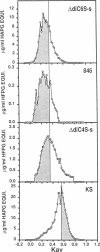
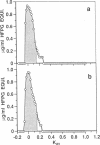
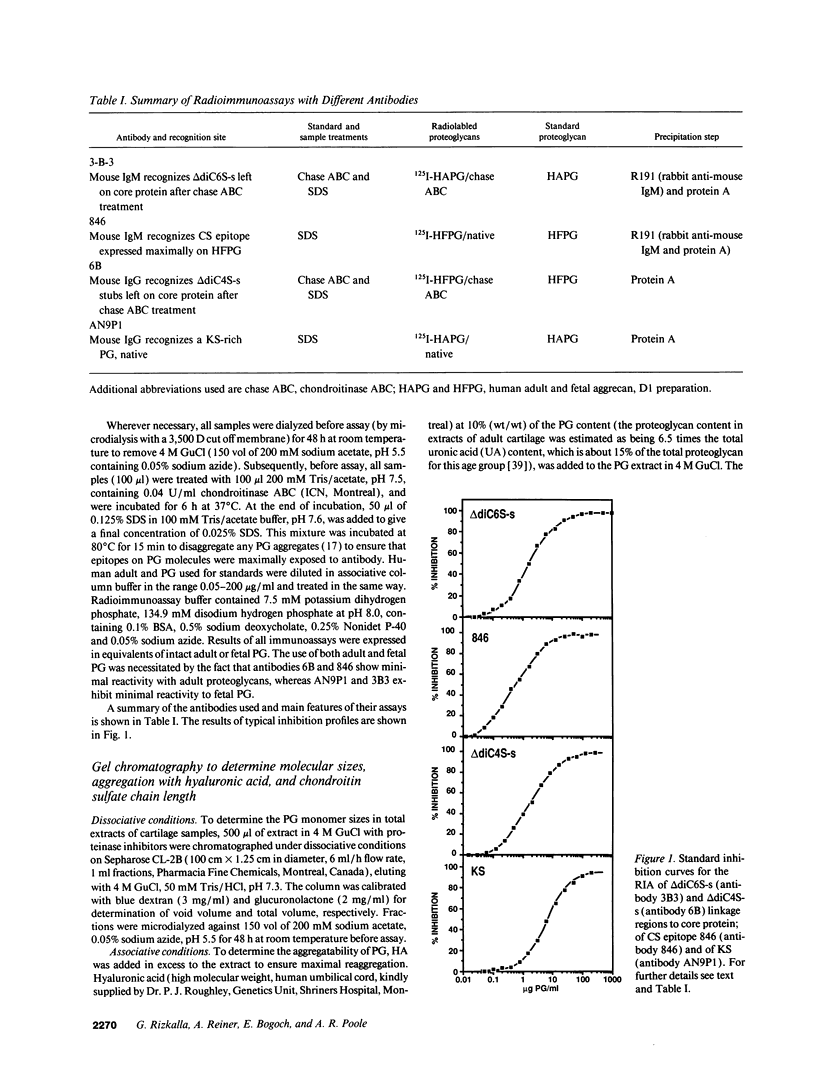
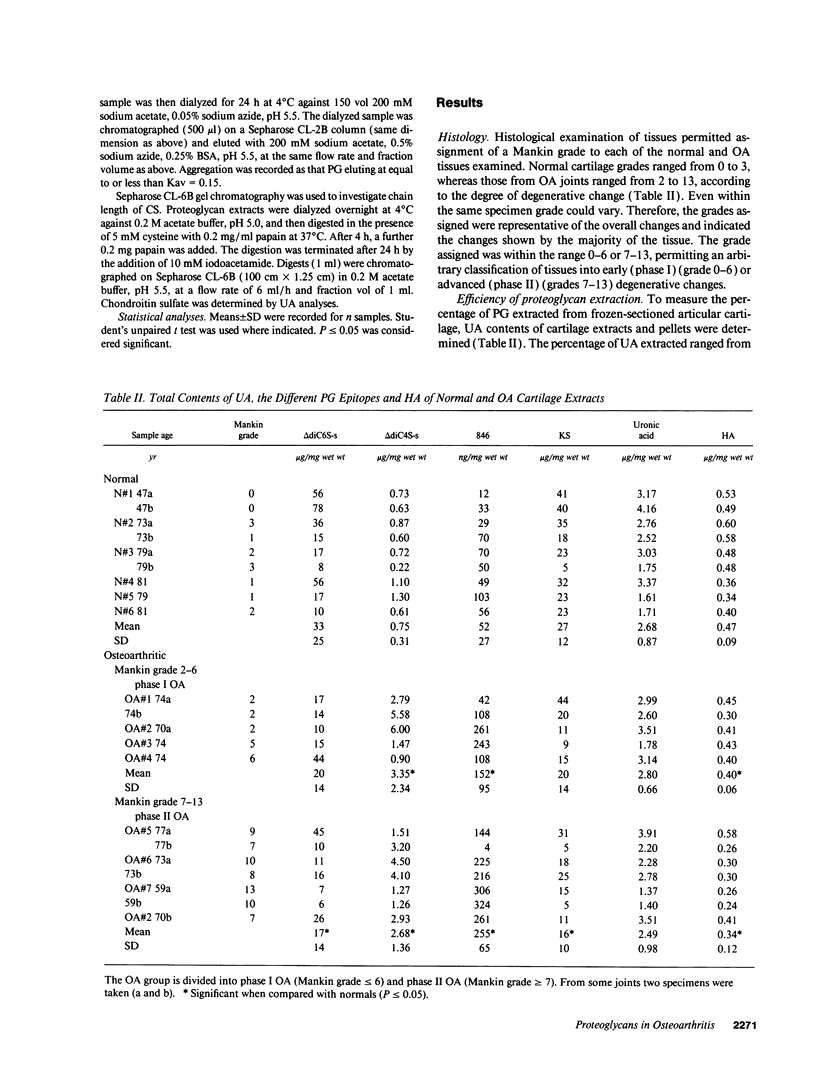
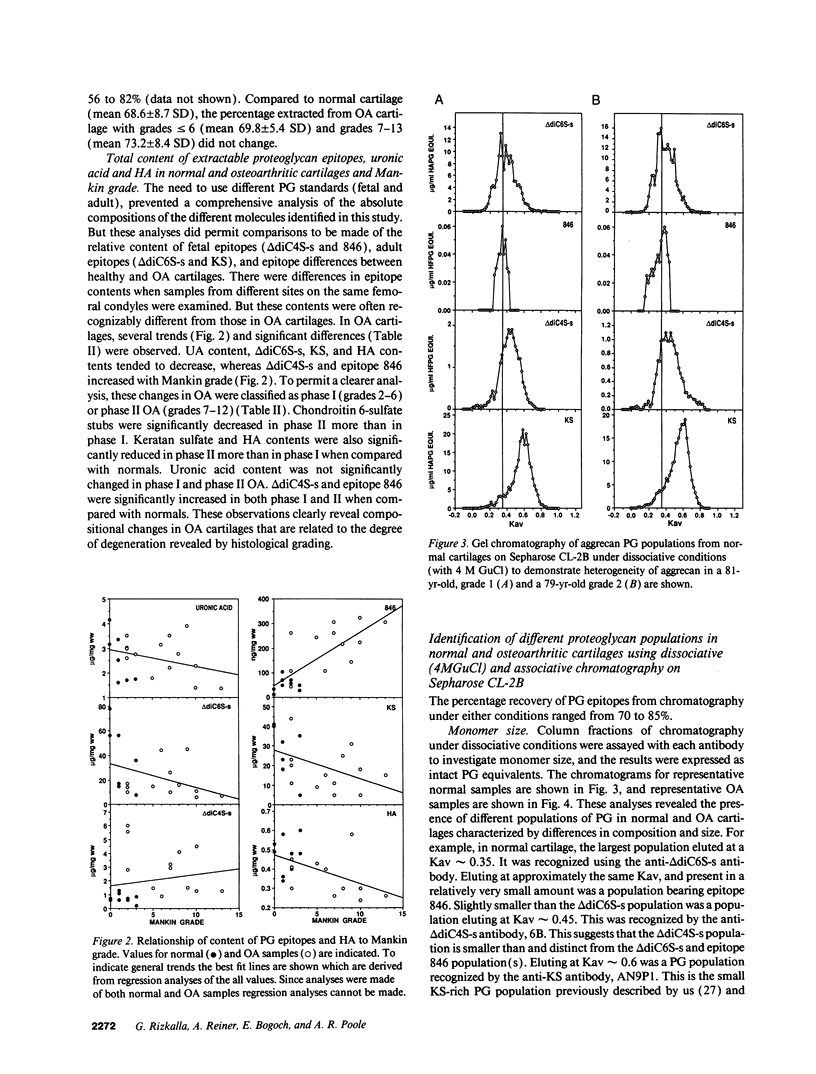
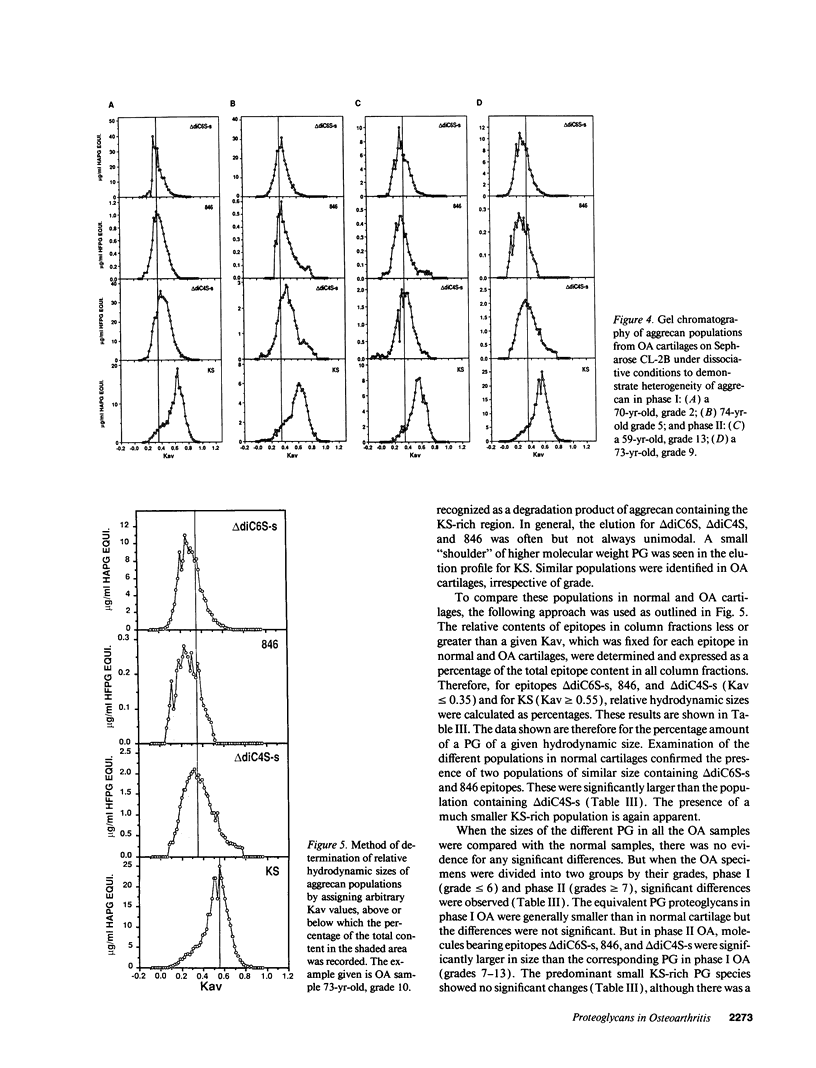
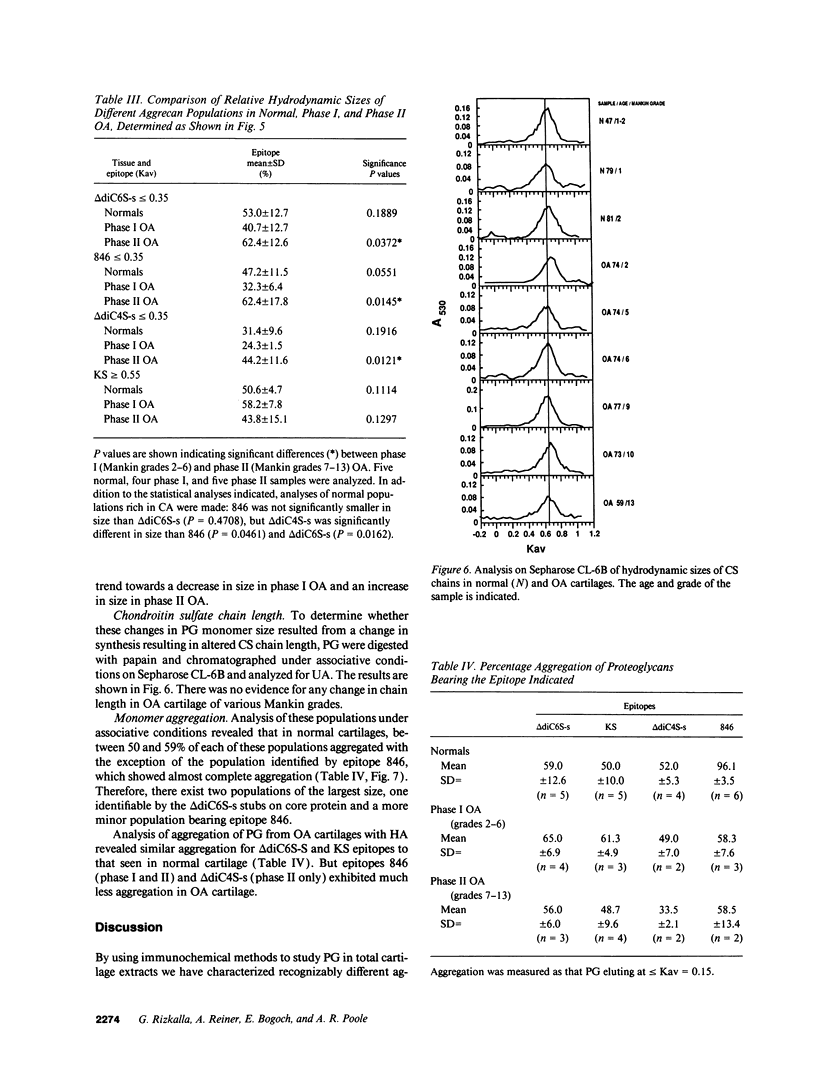
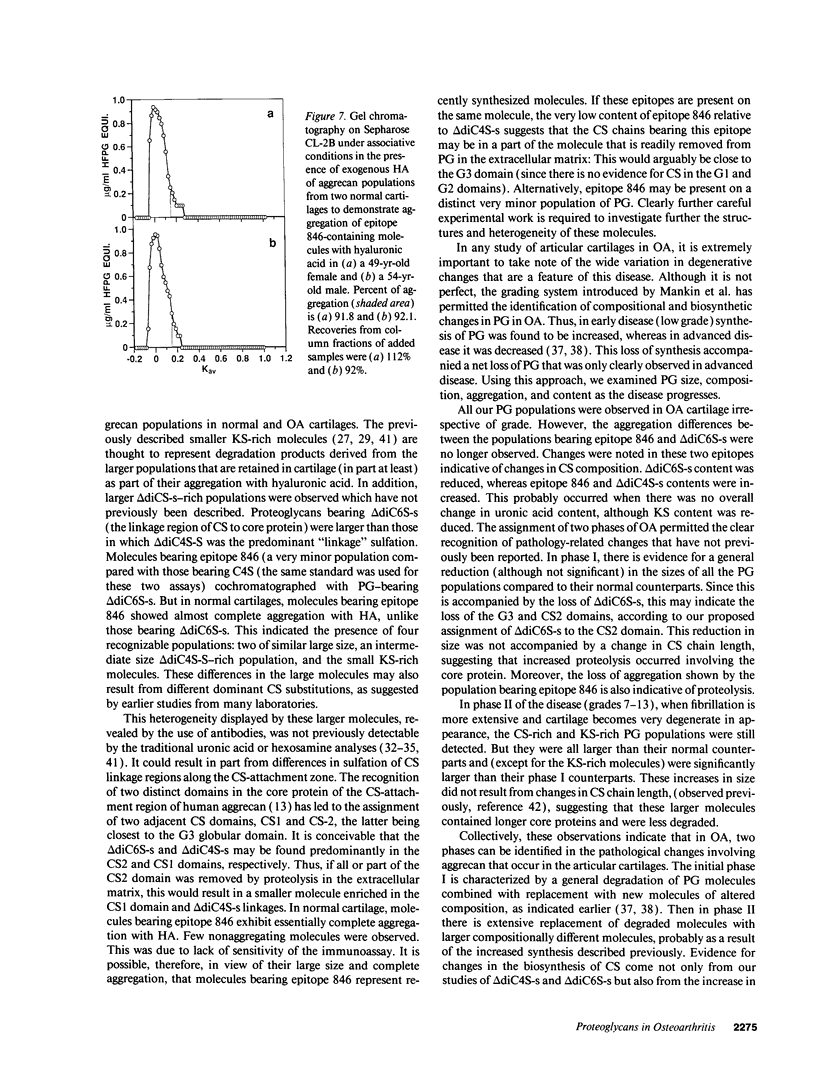
Changes in the structure of the proteoglycan aggrecan (PG) of articular cartilage were determined immunochemically by RIA and gel chromatography and related to cartilage degeneration documented histologically by the Mankin grading system. Monoclonal antibodies to glycosaminoglycan epitopes were used. In all cartilages, three chondroitin sulfate (CS)-rich populations of large size were observed in addition to a smaller keratan sulfate (KS)-rich population. In grades 7-13 OA cartilages (phase II), molecules were significantly larger than the equivalent molecules of grades 2-6 (phase I). CS chain lengths remained unchanged. In most OA cartilages, a CS epitope 846 was elevated in content, this being most marked in phase II (mean: fivefold). Loss of uronic acid, KS, and hyaluronic acid were only pronounced in phase II OA because of variations in normal contents. Aggregation of PG was unchanged (50-60%) or reduced in OA cartilages, but molecules bearing epitope 846 exhibited almost complete aggregation in normal cartilages. This study provides evidence for the capacity of OA cartilage to synthesize new aggrecan molecules to replace those damaged and lost by disease-related changes. It also defines two phases of PG change in OA: an early predominantly degenerate phase I followed by a net reparative phase II accompanied by net loss of these molecules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonsson P., Heinegård D., Oldberg A. The keratan sulfate-enriched region of bovine cartilage proteoglycan consists of a consecutively repeated hexapeptide motif. J Biol Chem. 1989 Sep 25;264(27):16170–16173. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Age-related changes in the composition and structure of human articular-cartilage proteoglycans. Biochem J. 1978 Dec 15;176(3):683–693. doi: 10.1042/bj1760683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Isolation of proteoglycans from human articular cartilage. Biochem J. 1978 Jan 1;169(1):123–132. doi: 10.1042/bj1690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss M. T., Venn M., Maroudas A., Ali S. Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983 Feb 1;209(2):387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollet A. J., Nance J. L. Biochemical Findings in Normal and Osteoarthritic Articular Cartilage. II. Chondroitin Sulfate Concentration and Chain Length, Water, and Ash Content. J Clin Invest. 1966 Jul;45(7):1170–1177. doi: 10.1172/JCI105423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst R., Bayliss M. T., Maroudas A., Coysh H. L., Freeman M. A., Revell P. A., Ali S. Y. The composition of normal and osteoarthritic articular cartilage from human knee joints. With special reference to unicompartmental replacement and osteotomy of the knee. J Bone Joint Surg Am. 1984 Jan;66(1):95–106. [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R., Couchman J. R. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc. 1985 Feb;44(2):386–393. [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 1983 Jul 25;258(14):8848–8854. [PubMed] [Google Scholar]
- Caterson B., Mahmoodian F., Sorrell J. M., Hardingham T. E., Bayliss M. T., Carney S. L., Ratcliffe A., Muir H. Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci. 1990 Nov;97(Pt 3):411–417. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- Dodge G. R., Poole A. R. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989 Feb;83(2):647–661. doi: 10.1172/JCI113929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Doege K., Sasaki M., Horigan E., Hassell J. R., Yamada Y. Complete primary structure of the rat cartilage proteoglycan core protein deduced from cDNA clones. J Biol Chem. 1987 Dec 25;262(36):17757–17767. [PubMed] [Google Scholar]
- Eyre D. R., Apon S., Wu J. J., Ericsson L. H., Walsh K. A. Collagen type IX: evidence for covalent linkages to type II collagen in cartilage. FEBS Lett. 1987 Aug 17;220(2):337–341. doi: 10.1016/0014-5793(87)80842-6. [DOI] [PubMed] [Google Scholar]
- Franzén A., Inerot S., Hejderup S. O., Heinegård D. Variations in the composition of bovine hip articular cartilage with distance from the articular surface. Biochem J. 1981 Jun 1;195(3):535–543. doi: 10.1042/bj1950535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Roughley P. J., Buzás E., Poole A. R. Age-related changes in protein-related epitopes of human articular-cartilage proteoglycans. Biochem J. 1986 May 15;236(1):71–75. doi: 10.1042/bj2360071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg D. F., Proulx G., Doege K., Yamada Y., Drickamer K. A segment of the cartilage proteoglycan core protein has lectin-like activity. J Biol Chem. 1988 Jul 5;263(19):9486–9490. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Kempson G. E., Muir H., Swanson S. A., Freeman M. A. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970 Jul 21;215(1):70–77. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- Lai W. M., Hou J. S., Mow V. C. A triphasic theory for the swelling and deformation behaviors of articular cartilage. J Biomech Eng. 1991 Aug;113(3):245–258. doi: 10.1115/1.2894880. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Matsui Y., Alini M., Webber C., Poole A. R. Characterization of aggregating proteoglycans from the proliferative, maturing, hypertrophic, and calcifying zones of the cartilaginous physis. J Bone Joint Surg Am. 1991 Aug;73(7):1064–1074. [PubMed] [Google Scholar]
- Mehmet H., Scudder P., Tang P. W., Hounsell E. F., Caterson B., Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 1986 Jun 2;157(2):385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Mendler M., Eich-Bender S. G., Vaughan L., Winterhalter K. H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol. 1989 Jan;108(1):191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Poole A. R., Roughley P. J. Age-related changes in the structure of proteoglycan link proteins present in normal human articular cartilage. Biochem J. 1983 Jul 15;214(1):269–272. doi: 10.1042/bj2140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mow V. C., Zhu W., Lai W. M., Hardingham T. E., Hughes C., Muir H. The influence of link protein stabilization on the viscometric properties of proteoglycan aggregate solutions. Biochim Biophys Acta. 1989 Aug 18;992(2):201–208. doi: 10.1016/0304-4165(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Neame P. J., Christner J. E., Baker J. R. Cartilage proteoglycan aggregates. The link protein and proteoglycan amino-terminal globular domains have similar structures. J Biol Chem. 1987 Dec 25;262(36):17768–17778. [PubMed] [Google Scholar]
- Nguyen Q., Murphy G., Roughley P. J., Mort J. S. Degradation of proteoglycan aggregate by a cartilage metalloproteinase. Evidence for the involvement of stromelysin in the generation of link protein heterogeneity in situ. Biochem J. 1989 Apr 1;259(1):61–67. doi: 10.1042/bj2590061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmoski M., Brandt K. Hyaluronate-binding by proteoglycans. Comparison of mildly and severely osteoarthritic regions of human femoral cartilage. Clin Chim Acta. 1976 Jul 1;70(1):87–95. doi: 10.1016/0009-8981(76)90008-5. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Rosenberg L. An immunoelectron microscope study of the organization of proteoglycan monomer, link protein, and collagen in the matrix of articular cartilage. J Cell Biol. 1982 Jun;93(3):921–937. doi: 10.1083/jcb.93.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Webber C., Reiner A., Roughley P. J. Studies of a monoclonal antibody to skeletal keratan sulphate. Importance of antibody valency. Biochem J. 1989 Jun 15;260(3):849–856. doi: 10.1042/bj2600849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R. Identification of a hyaluronic acid-binding protein that interferes with the preparation of high-buoyant-density proteoglycan aggregates from adult human articular cartilage. Biochem J. 1985 Oct 1;231(1):129–138. doi: 10.1042/bj2310129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell J. M., Lintala A. M., Mahmoodian F., Caterson B. Epitope-specific changes in chondroitin sulfate/dermatan sulfate proteoglycans as markers in the lymphopoietic and granulopoietic compartments of developing bursae of Fabricius. J Immunol. 1988 Jun 15;140(12):4263–4270. [PubMed] [Google Scholar]
- Sweet M. B., Thonar E. J., Immelman A. R., Solomon L. Biochemical changes in progressive osteoarthrosis. Ann Rheum Dis. 1977 Oct;36(5):387–398. doi: 10.1136/ard.36.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet M. B., Thonar E. J., Immelman A. R., Solomon L. Biochemical changes in progressive osteoarthrosis. Ann Rheum Dis. 1977 Oct;36(5):387–398. doi: 10.1136/ard.36.5.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L. H., Rosenberg L., Reiner A., Poole A. R. Proteoglycans from bovine nasal cartilage. Properties of a soluble form of link protein. J Biol Chem. 1979 Oct 25;254(20):10523–10531. [PubMed] [Google Scholar]
- Thompson R. C., Jr, Oegema T. R., Jr Metabolic activity of articular cartilage in osteoarthritis. An in vitro study. J Bone Joint Surg Am. 1979 Apr;61(3):407–416. [PubMed] [Google Scholar]
- Thonar E. J., Sweet M. B., Immelman A. R., Lyons G. Hyaluronate in articular cartilage: age-related changes. Calcif Tissue Res. 1978 Nov 10;26(1):19–21. doi: 10.1007/BF02013228. [DOI] [PubMed] [Google Scholar]
- Vasan N. Proteoglycans in normal and severely osteoarthritic human cartilage. Biochem J. 1980 Jun 1;187(3):781–787. doi: 10.1042/bj1870781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber C., Glant T. T., Roughley P. J., Poole A. R. The identification and characterization of two populations of aggregating proteoglycans of high buoyant density isolated from post-natal human articular cartilages of different ages. Biochem J. 1987 Dec 15;248(3):735–740. doi: 10.1042/bj2480735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Rest M., Mayne R. Type IX collagen proteoglycan from cartilage is covalently cross-linked to type II collagen. J Biol Chem. 1988 Feb 5;263(4):1615–1618. [PubMed] [Google Scholar]